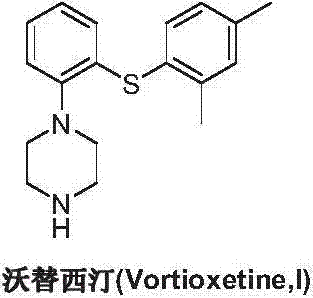
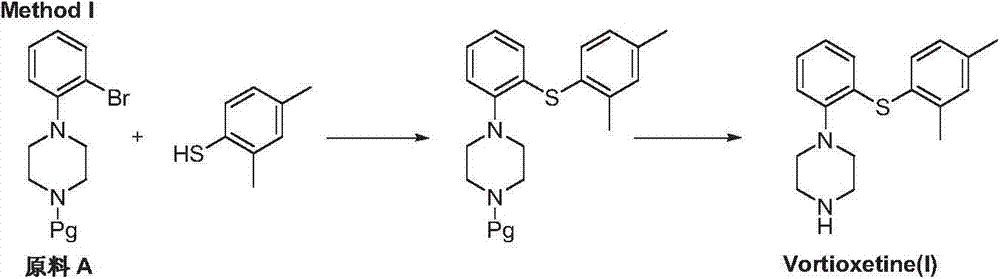
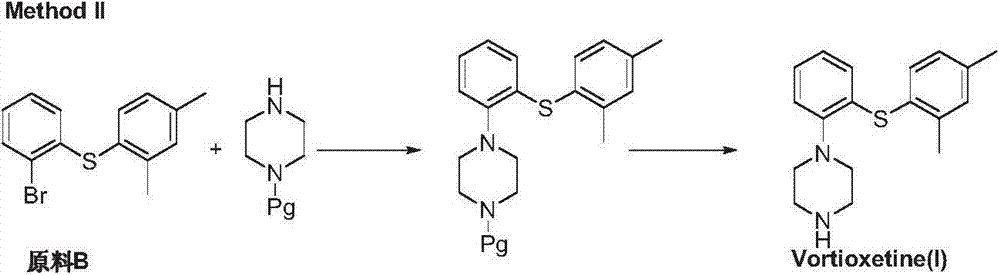
Preparation method of vortioxetine
A technology of vortioxetine and preparation steps, which is applied in the field of preparation of vortioxetine, can solve problems such as unfavorable industrialization, cumbersome steps, and difficult purification, and achieve the effects of promoting development, simple process, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a dry reaction flask under a nitrogen atmosphere, add 2-bromonitrobenzene (II) (10.0g, 0.05mol), 2,4-dimethylthiophenol (III) (7.59g, 1.1eq) and toluene 100mL, add tris(dibenzylideneacetone)dipalladium (0.46g, 0.01eq) and racemic 1,1'-binaphthyl-2,2'-diphenylphosphine (0.31g, 0.01eq) under stirring, room temperature React for 15 minutes. Potassium tert-butoxide (6.2 g, 1.1 eq) was added, the temperature was raised to reflux, and the reaction was stirred for 2 hours. The temperature was lowered to 0°C, and the reaction was continued for 2 hours. After filtration, the filtrate was distilled under reduced pressure to obtain 11.8 g of light yellow viscous liquid 2-(2,4-dimethylphenylsulfanyl)nitrobenzene with a yield of 91.1%.
Embodiment 2
[0040] In a dry glass tube reactor under a nitrogen atmosphere, add cuprous iodide (0.95g, 0.1eq), sodium tert-butoxide (4.8g, 1.0eq) and 30mL of acetonitrile, and add 2-bromonitrobenzene ( II) (10.0g, 0.05mol) and 2,4-dimethylthiophenol (III) (7.59g, 1.1eq), sealed and reacted at 0°C with a mercury lamp for 12-15 hours. Reduce to normal pressure, distill off the solvent under reduced pressure, dissolve the residue with ether, filter, and wash the filtrate with 5% dilute hydrochloric acid, saturated saline and pure water successively, and dry over anhydrous magnesium sulfate. The solvent was removed under reduced pressure to obtain 10.2 g of light yellow viscous liquid 2-(2,4-dimethylphenylsulfanyl)nitrobenzene (IV), with a yield of 78.8%.
Embodiment 3
[0042] Add 2-(2,4-dimethylphenylsulfanyl) nitrobenzene (IV) (2.59g, 10mmol), ferric chloride (0.27g, 1mmol), activated carbon 0.4g and ethanol in the reaction flask 50mL, add 80% hydrazine hydrate (1.25g, 20mmol) dropwise at room temperature, after the addition, raise the temperature to 50-60°C, react for 4-5 hours, filter, concentrate to remove the solvent ethanol, and recrystallize the residue with isopropyl ether to obtain Off-white solid 2-(2,4-dimethylphenylsulfanyl)aniline (V) 2.1 g, yield 91.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com