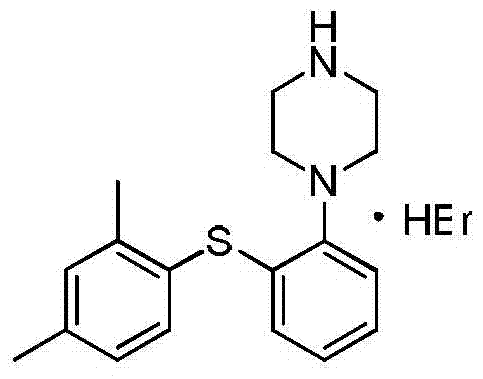
A kind of vortioxetine hydrobromide crystal and preparation method thereof
A technology of vortioxetine hydrobromide and vortioxetine, which is applied in the field of medicine and can solve the problems of cumbersome operation and great human injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 vortioxetine hydrobromide crystal
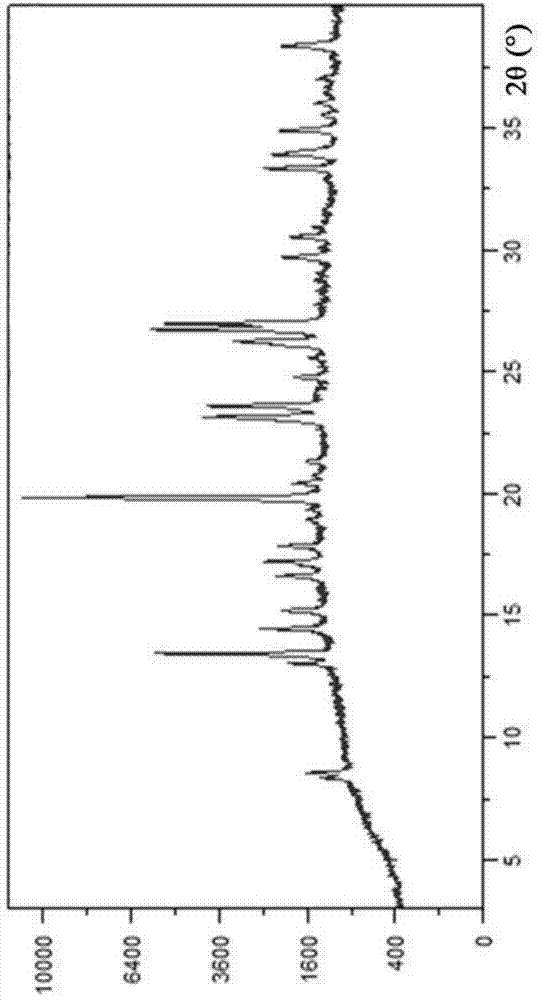
[0036] Dissolve 10 g of vortioxetine free base in 300 ml of ethyl acetate, stir and dissolve at 15°C, and then filter, and control the temperature of the filtrate at 0°C. Measure 7g of hydrobromic acid (converted based on the actual content of the 42.3% by weight aqueous solution), and add it dropwise to the free alkali solution at a constant speed, and control the temperature at 0°C. After the addition, continue stirring at 0°C for 8 hours. After filtering, the filter cake 1 was rinsed with ethyl acetate and stirred in 50 ml of ethyl acetate for 4 hours at 0°C. Filtration, filter cake 2 was vacuum-dried at 30°C to obtain 11.6 g of off-white solid, with a molar yield of 91%, and a chromatographic purity of 99.91%. The XRPD and IR figures are shown in figure 1 , figure 2 .
Embodiment 2
[0037] The preparation of embodiment 2 vortioxetine hydrobromide crystals
[0038] Dissolve 10 g of vortioxetine free base in 350 ml of ethyl acetate, stir to dissolve at 25°C and then filter, and control the temperature of the filtrate at 5°C. Measure 6.4g of hydrobromic acid (calculated based on the actual content of the 42.3% by weight aqueous solution), and add it dropwise to the free alkali solution at a constant speed, and control the temperature at 5°C. After the addition, continue stirring at 5°C for 6 hours. After filtering, the filter cake 1 was rinsed with an appropriate amount of ethyl acetate and then stirred and washed in 70 ml of ethyl acetate for 5 hours at 5°C. Filtration, filter cake 2 was vacuum-dried at 40° C. to obtain 11.8 g of an off-white solid, with a molar yield of 93%, and a chromatographic purity of 99.90%. figure 1 , figure 2 unanimous.
Embodiment 3
[0039] The preparation of embodiment 3 vortioxetine hydrobromide crystals
[0040] Dissolve 10 g of vortioxetine free base in 400 ml of ethyl acetate, stir and dissolve at 30°C, and then filter, and control the temperature of the filtrate at 15°C. Measure 7.7g of hydrobromic acid (calculated based on the actual content of the 42.3% by weight aqueous solution), and add it dropwise to the free alkali solution at a constant speed, with the temperature controlled at 15°C. After the addition, continue stirring at 15°C for 8 hours. After filtering, the filter cake 1 was rinsed with an appropriate amount of ethyl acetate and stirred in 90 ml of ethyl acetate for 6 hours at 15°C. Filtration, filter cake 2 was vacuum-dried at 50° C. to obtain 12.1 g of an off-white solid, with a molar yield of 95%, and a chromatographic purity of 99.92%. figure 1 , figure 2 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com