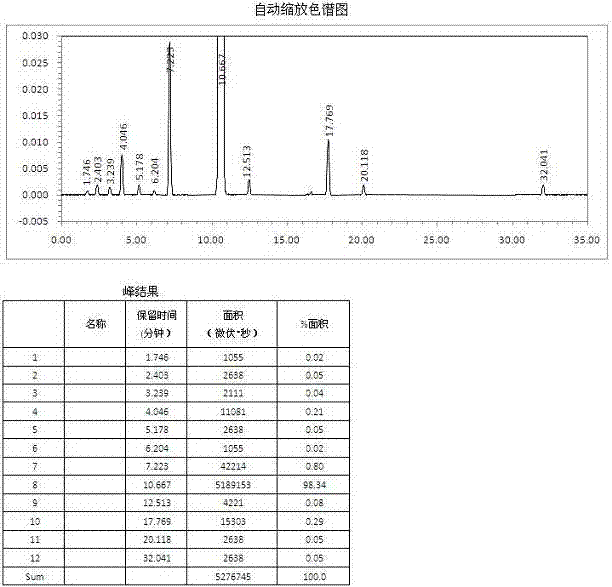
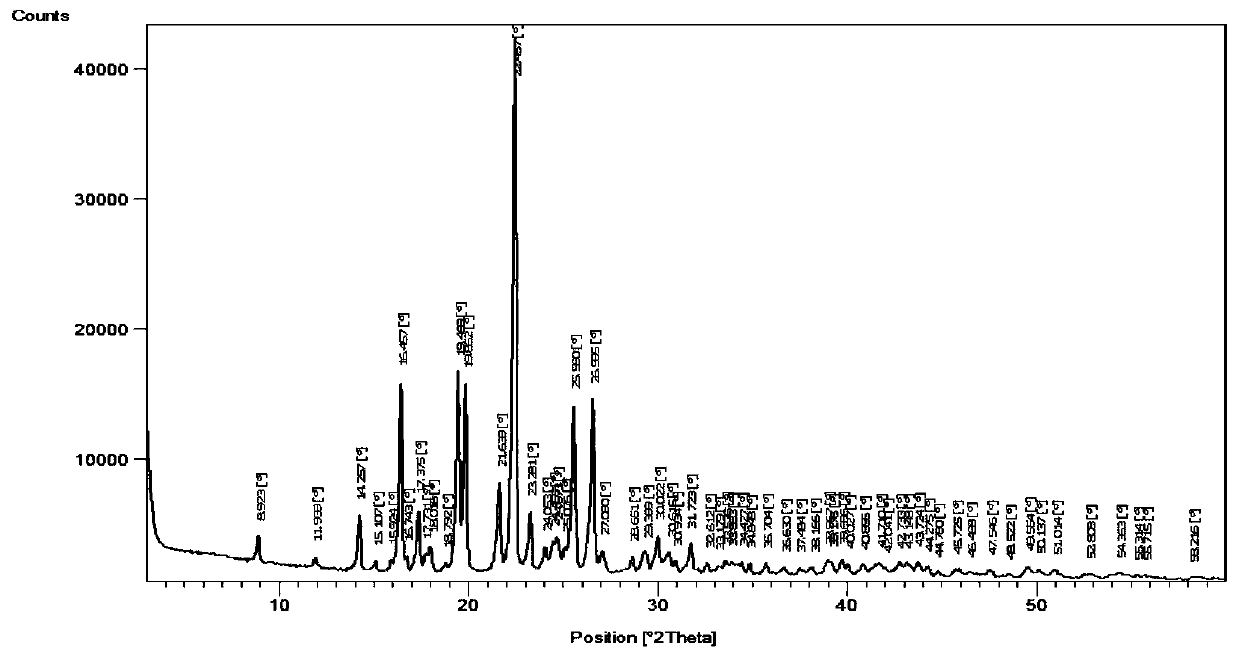
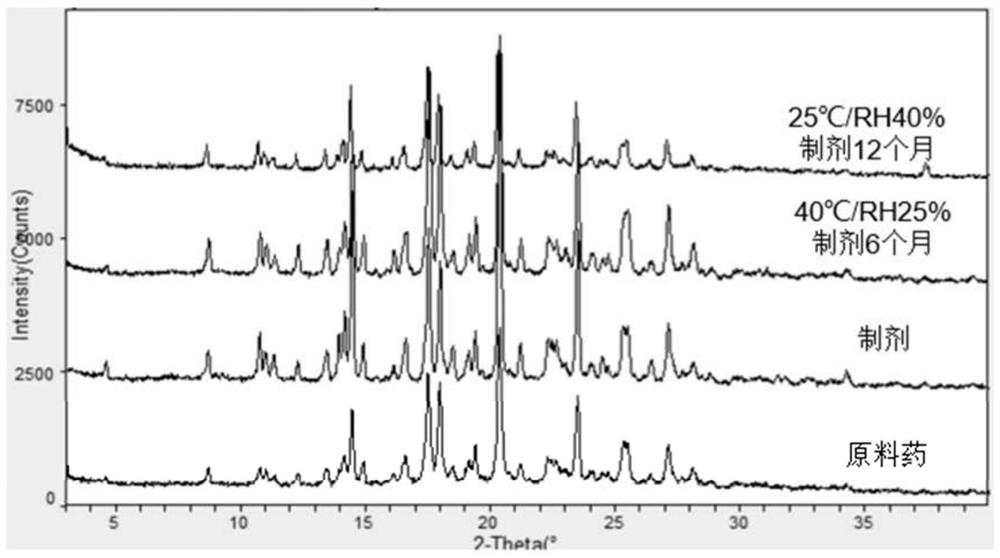
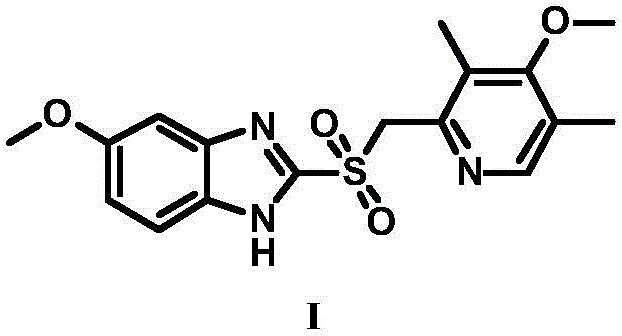
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65results about How to "Comply with medicinal requirements" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyl phthalide raw material drug product and preparation method thereof
ActiveCN105130934AGuaranteed clinical efficacyEnsure medication safetyOrganic active ingredientsNervous disorderBiomedical engineeringClinical efficacy
The present invention provides a butyl phthalide raw material drug product with the butyl phthalide content not less than 99.0%; the raw material drug is stable in quality, and can ensure the clinical curative effect and drug safety of a butyl phthalide preparation.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Butylphthalide medicine active composition and preparation method of butylphthalide medicine active composition
ActiveCN102716121AComply with medicinal requirementsQuality improvementOrganic active ingredientsOrganic chemistryButylphthalidePharmaceutical drug
The invention provides a butylphthalide medicine active composition, which comprises the following ingredients: first ingredients: the butylphthalide content is higher than or equal to 98.0 percent; second ingredients: the second ingredients are one kind of materials or several kinds of materials selected from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide, amylene phthalide, phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, in addition, the content of the second ingredients is higher than 0 but is lower than or equal to 2.0 percent, when the second ingredients comprise any one kind of materials from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide and amylene phthalide, the content of any one kind of included ingredients does not exceed 0.5 percent, and when the second ingredients comprise any one kind of materials from phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, the content of any one kind of included ingredients does not exceed 1.0 percent. The quality of the medicine active composition is stable, and the clinic curative effect and the medication safety of the butylphthalide preparation can be ensured.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Preparation method of histamine dihydrochloride
ActiveCN102477014AFacilitate industrial mass productionEasy to recycleOrganic active ingredientsOrganic chemistrySolventHistamine Hydrochloride
The invention relates to an industrial preparation method of officinal histamine dihydrochloride. The preparation method comprises the following steps of: suspending histidine in hexahydrocresol which is used as a reaction solvent; carrying out a decarboxylation reaction at the temperature of 170 DEG C-175 DEG C; and then quantitatively dropwise adding the hexahydrocresol solution containing hydrochloric acid twice so as to synthesize histamine dihydrochloride. According to the invention, the content of the related substances in histamine dihydrochloride is less than 0.5%, and the content of a single impurity in histamine dihydrochloride is less than 0.1%, thus the histamine dihydrochloride completely meets officinal grade; and the preparation method is simple in equipment, easy and simple in process operation and safe and reliable in reaction; and the solvent can be recycled, thus the preparation method is environment-friendly and is beneficial to industrial large-scale production.
Owner:SHANDONG NEWTIME PHARMA
Methods for preparing abiraterone acetate and intermediate thereof
The invention relates to a method for preparing abiraterone which is a key intermediate of abiraterone acetate serving as a medicament for treating prostatic cancer and a method for preparing the abiraterone acetate by using the intermediate thereof. The methods are simple in processes, and the products have high purity and low content of impurities.
Owner:CHONGQING PHARMA RES INST
Preparation method for pharmaceutical crystal form of febuxostat
ActiveCN106565627AImprove quality controlEasy to operateOrganic chemistry methodsAqueous solutionRepeatability
The invention discloses a preparation method for a pharmaceutical crystal form of febuxostat. The preparation method comprises the following steps: dissolving the febuxostat in an ethanol aqueous solution, and carrying out heating and dissolving under stirring; carrying out hot filtration, slowly cooling a filtrate, adding a seed crystal, carrying out stirring until a solid is separated, continuing cooling, maintaining the temperature, and carrying out crystallization; and carrying out filtering, placing a filter cake in a hot-air drying oven, and carrying out drying so as to obtain a product. The method provided by the invention has the advantages of simple and convenient operation, good repeatability, high product purity and yield, good crystal stability, and applicability to industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
Pharmaceutical composition of Silybin and preparation method thereof
ActiveCN100594898CHigh yieldSolve the problem of large-scale industrial productionOrganic active ingredientsDigestive systemPhospholipid complexDrug product
The invention provides a silybin and phosphatidy lcholine compound which is prepared into capsules with high dissolving degree. The invention also provides a process for preparing the silybin and phosphatidy lcholine compound and its capsule medicament, the preparing process is characterized by simple manufacturing method, high yield and fitting for mass production.
Owner:天津天士力圣特制药有限公司
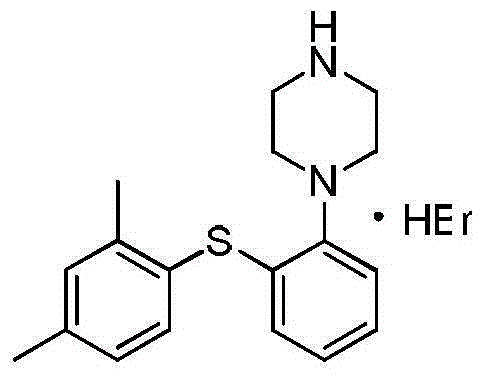
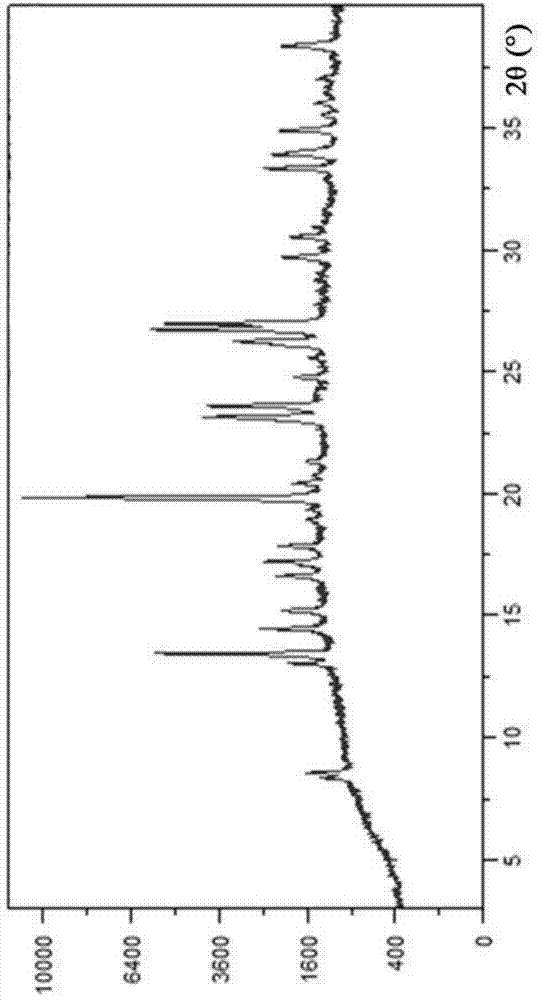
Hydrobromic acid vortioxetine crystal and preparation method therefor
ActiveCN105017176AComply with medicinal requirementsHigh yieldOrganic chemistry methodsHydrobromic acidVortioxetine
The present invention discloses a hydrobromic acid vortioxetine crystal that has diffraction peaks at 8.55+ / -0.2, 13.05+ / -0.2, 13.44+ / -0.2, 14.46+ / -0.2, 15.20+ / -0.2, 16.63+ / -0.2, 16.94+ / -0.2, 17.22+ / -0.2, 17.85+ / -0.2, 19.83+ / -0.2, 20.43+ / -0.2, 21.33+ / -0.2, 23.14+ / -0.2, 23.60+ / -0.2, 24.77+ / -0.2, 26.25+ / -0.2, 26.72+ / -0.2, 26.96+ / -0.2, 29.69+ / -0.2, 30.52+ / -0.2, 33.33+ / -0.2, 33.89+ / -0.2, 34.89+ / -0.2, 35.54+ / -0.2, 37.03+ / -0.2, and 38.33+ / -0.2 in a powder X-ray diffraction diagram represented with 2 theta. In addition, the present invention further discloses a preparation method for the crystal. The hydrobromic acid vortioxetine crystal and the preparation method therefor in the present invention have good repeatability, easy operation, good product stability, and high yield and purity, and are suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of preparation method of vortioxetine hydrobromide crystal
ActiveCN104910099BComply with medicinal requirementsHigh yieldOrganic chemistry methodsHydrobromideThermal insulation
The invention discloses a vortioxetine hydrobromide crystal preparation method. The method comprises a, dissolving vortioxetine free alkali in ethyl acetate at a temperature of 20-30 DEG C, b, carrying out filtration, cooling the filtrate to a temperature of 0-10 DEG C, dropwisely adding an ethyl acetate solution of hydrobromic acid into the filtrate along with thermal insulation and then carrying out thermal insulation stirring for 2-8h, c, filtering the mixture subjected to thermal insulation stirring in the step b to obtain filter cake 1, leaching the filter cake 1 by ethyl acetate, and carrying out stirring washing in ethyl acetate at a temperature of 0-10 DEG C for 0.5-5h, d, filtering the mixture subjected to stirring washing in the step c to obtain filter cake 2, leaching the filter cake 2 by methyl tert-butyl ether / ethyl acetate pre-cooled at a temperature of 0-10 DEG C and carrying out stirring washing in methyl tert-butyl ether at a temperature of 10-30 DEG C for 15-24h, and e, filtering the mixture subjected to stirring washing in the step d to obtain filter cake 3, leaching the filter cake 3 by methyl tert-butyl ether and carrying out vacuum drying at a temperature of 40-50 DEG C to obtain the product. The method has the advantages of good repeatability, simple processes, a high yield and high product purity and is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Maleic acid levorotation amlodipine drug active pharmaceutical composition and preparation method thereof
InactiveCN103006648AGuaranteed clinical efficacyEnsure medication safetyOrganic active ingredientsCardiovascular disorderUse medicationClinical efficacy
The invention provides a maleic acid levorotation amlodipine drug active pharmaceutical composition which comprises the following components: component I: maleic acid levorotation amlodipine; component II: dextrorotation amlodipine; component III: aspartic acid amlodipine; component IV: impurity D; and the active pharmaceutical composition does not contain impurity A, impurity B, impurity C, impurity E, impurity F, impurity G and impurity H basically. The active pharmaceutical composition is stable in quality, and can completely meet the quality requirement of maleic acid levorotation amlodipine preparation on active pharmaceutical composition; and the prepared preparation is safe, effective and controllable in quality, and ensures the clinical effect and medication safety of the maleic acid levorotation amlodipine preparation.
Owner:CSPC OUYI PHARM CO LTD
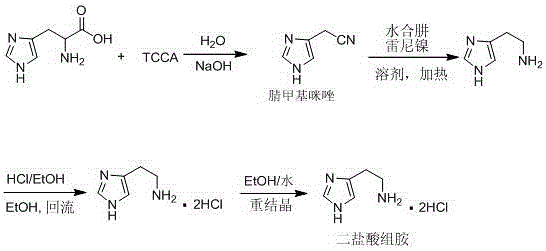
Synthetic method of histamine dichloride
The invention discloses a synthetic method of histamine dichloride, and belongs to the field of drug synthesis. The synthetic method comprises the following steps: preparing a cyano methylimidazole intermediate through oxidization of TCCA (Trichloroisocyanuric Acid) under an alkaline condition by taking L-histidine as an original raw material, and then preparing medicinal histamine dichloride through catalytic hydrogenation and one-step salifying process. In the histamine dichloride compounded through the synthetic method, the content of related substances is less than 0.5 percent, the content of a single impurity is less than 0.1 percent, and the histamine dichloride accords with medicinal level. A reagent adopted by the technology is cheap and of low-toxicity, the reaction is safe and reliable, an alcohol solvent can be recycled, after-treatment operation is simple and convenient, and the technology is environment-friendly and is beneficial for large-scale industrial production.
Owner:KAIFENG PHARMA GRP +1
A kind of vortioxetine hydrobromide crystal and preparation method thereof
ActiveCN105017176BComply with medicinal requirementsHigh yieldOrganic chemistry methodsHydrobromideX-ray
The present invention discloses a hydrobromic acid vortioxetine crystal that has diffraction peaks at 8.55+ / -0.2, 13.05+ / -0.2, 13.44+ / -0.2, 14.46+ / -0.2, 15.20+ / -0.2, 16.63+ / -0.2, 16.94+ / -0.2, 17.22+ / -0.2, 17.85+ / -0.2, 19.83+ / -0.2, 20.43+ / -0.2, 21.33+ / -0.2, 23.14+ / -0.2, 23.60+ / -0.2, 24.77+ / -0.2, 26.25+ / -0.2, 26.72+ / -0.2, 26.96+ / -0.2, 29.69+ / -0.2, 30.52+ / -0.2, 33.33+ / -0.2, 33.89+ / -0.2, 34.89+ / -0.2, 35.54+ / -0.2, 37.03+ / -0.2, and 38.33+ / -0.2 in a powder X-ray diffraction diagram represented with 2 theta. In addition, the present invention further discloses a preparation method for the crystal. The hydrobromic acid vortioxetine crystal and the preparation method therefor in the present invention have good repeatability, easy operation, good product stability, and high yield and purity, and are suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method of bazedoxifene acetate polycrystalline type B
ActiveCN104370796AEasy to prepareSuitable for industrial productionOrganic chemistry methodsAcetic acidAntioxidant
The invention discloses a preparation method of bazedoxifene acetate polycrystalline type B. Bazedoxifene free alkali and glacial acetic acid are taken as raw materials and salified to obtain the bazedoxifene acetate polycrystalline type B. The operation conditions of the preparation process are simple and do not need to be carried out under the protection of inert gas; no antioxidant is used in the preparation process; the yield and purity of the prepared product are high, and therefore the product is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
A kind of butylphthalide pharmaceutical active composition and preparation method thereof
ActiveCN102716121BComply with medicinal requirementsQuality improvementOrganic active ingredientsOrganic chemistryClinical efficacyButylphthalide
The invention provides a butylphthalide pharmaceutical active composition, comprising the following components: component I: butylphthalide content ≥ 98.0%; component II: selected from methylenephthalide, vinylphthalide, propylenephthalide, butylphthalide One or more of enphthalide, pentylenephthalide, phthalide, toluene, ethylphthalide, propylphthalide, and pentylenephthalide, and the content of component II > 0 and ≤ 2.0%; when the component When any one of methylenephthalide, vinylphthalide, propylenephthalide, butylenephthalide, and amylenephthalide is contained in II, the maximum content of any one of the ingredients contained therein shall not exceed 0.5%. When component II contains When any one of phthalide, methylphthalide, ethylphthalide, propylphthalide, and pentaphthalide is used, the maximum content of any one of the ingredients contained in it shall not exceed 1.0%. The pharmaceutical active composition has stable quality and can guarantee the clinical curative effect and drug safety of the butylphthalide preparation.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Refining method of ibutilide fumarate
InactiveCN102030689AEnsure safetySimple process operationOrganic compound preparationSulfonic acid amide preparationSolventActivated carbon
The invention relates to a refining method of ibutilide fumarate raw material medicine capable of resisting arrhythmia. The method comprises the following steps of: dissolving a crude ibutilide fumarate product into a solvent with stronger polarity or medium polarity, decoloring with activated carbon and filtering; then adding a solvent with smaller polarity to an obtained filter liquor, stirring, standing still and removing the supernate; adding a solvent with medium polarity to an obtained residue; and recrystallizing to obtain high-purity ibutilide fumarate. In the invention, impurities in the crude ibutilide fumarate product are effectively removed by adopting a mixed solvent eluting method, which improves the product purity, ensures that the product conforms to the medicinal requirement and guarantees the safety of clinical medication. The invention has the advantages of stable product quality and simple process operation and is suitable for mass industrial production.
Owner:YAOPHARMA CO LTD
Synthesizing and refining methods of rivaroxaban
InactiveCN110172060AEconomical in proportion to usageLow costOrganic chemistry methodsAlcoholSynthesis methods
The invention relates to the field of pharmaceutical chemistry, and provides synthesizing and refining methods of rivaroxaban. The synthesis method comprises a step 1) of performing condensation reaction on 4-{4-[(5S)-5-(aminomethyl)-2-oxo-1,3-oxazolidin-3-yl]phenyl}morpholin-3-one(LF-II) and 5-chlorothiophene-2-carboxylic acid (SMI) under an alkaline system to prepare crude rivaroxaban; the refining method further comprises a step 2) of stirring and crystallizing the crude rivaroxaban and an alcohol solution at room temperature to obtain high-purity refined rivaroxaban which meets requirements of desired crystal form. The synthesizing and refining methods are simple in operation of preparation processes, high in yield, low in pollution, and suitable for large-scale industrial production.
Owner:SUZHOU ERYE PHARMA CO LTD
Suspension of triazole antifungal drug for atomizer
ActiveCN111658610ALarge particle sizeComply with medicinal requirementsOrganic active ingredientsAntimycoticsTriazole antifungalsAntifungal drug
The invention provides a suspension of a triazole antifungal drug for an atomizer, and a preparation method of the suspension. The pH of the suspension is 3-8. The suspension comprises the triazole antifungal drug, surfactant, osmotic pressure regulation agent, metal complexing agent, a pH regulation agent and water, wherein the D50 diameter of the triazole antifungal drug is 1-10 [mu] m, the triazole antifungal drug occupies the total mass of the suspension by 0.025-1%, and a mass ratio of the triazole antifungal drug to the surfactant is 1:1-50:1. The invention also provides a preparation method for the suspension. The suspension provided by the invention has good stability and can be placed for one year at a constant temperature without obviously increasing a particle size.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Purification method of creatine phosphate sodium
ActiveCN105153222AHigh purityImprove securityGroup 5/15 element organic compoundsPurification methodsPhosphate
The invention discloses a purification method of creatine phosphate sodium, which comprises the following steps: pretreating a creatine phosphate sodium mother solution with 40-70 vol% ethanol, loading the pretreated creatine phosphate sodium mother solution, eluting creatine by using a 0.02-0.15M electrolyte solution as an eluent, eluting the creatine phosphate sodium by using a 0.2-0.8M electrolyte solution as an eluent, collecting the eluate, crystallizing, and drying to obtain the creatine phosphate sodium. The method is simple, does not need to introduce sulfates or barium, and has high safety. The recovery rate of the prepared creatine phosphate sodium is high and up to 40%, and the purity reaches 99.80%, thereby satisfying the medicinal requirements.
Owner:XINAN PHARMA
Preparation method of atazanavir hydrosulfate crystal form H1
ActiveCN107382833AGood repeatabilitySimple and fast operationOrganic chemistry methodsAcetic acidPotential market
The invention discloses a preparation method of an atazanavir hydrosulfate crystal form H1. The preparation method comprises the following steps: mixing atazanavir hydrosulfate with a solvent, and stirring to obtain the atazanavir hydrosulfate crystal form H1, wherein the solvent is a mixed solvent of methanol, butanone and ethyl acetate. The preparation method of an atazanavir hydrosulfate crystal form H1 disclosed by the invention has the advantages of high repeatability, easiness in operation and high yield; the methanol content in the product meets the requirements for medicinal use, and the chromatographic purity is high; thus, the product is more applicable to medicament and suitable for industrial production and has a potential market value.
Owner:SHANGHAI VIWIT PHARMA CO LTD
Pharmaceutical composition containing flurbiprofen and patch
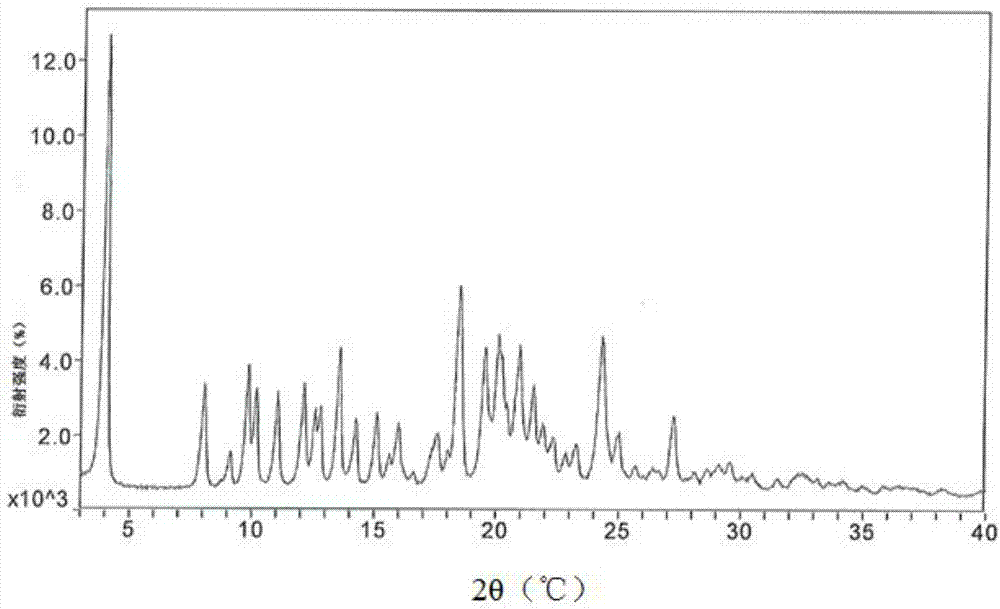
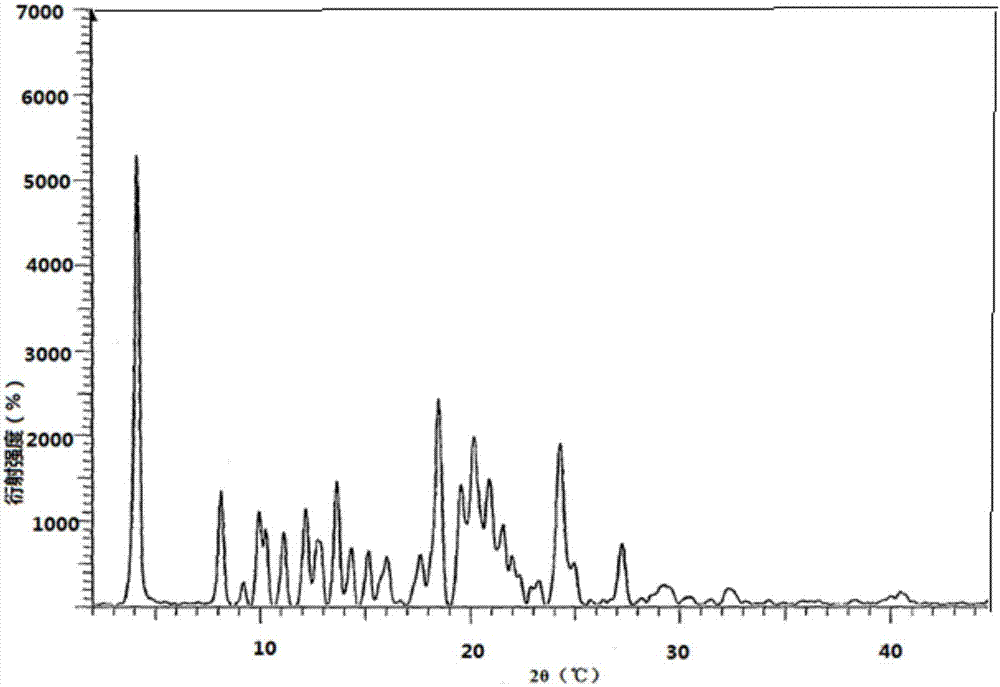
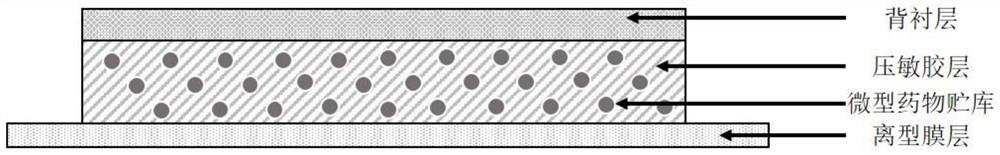
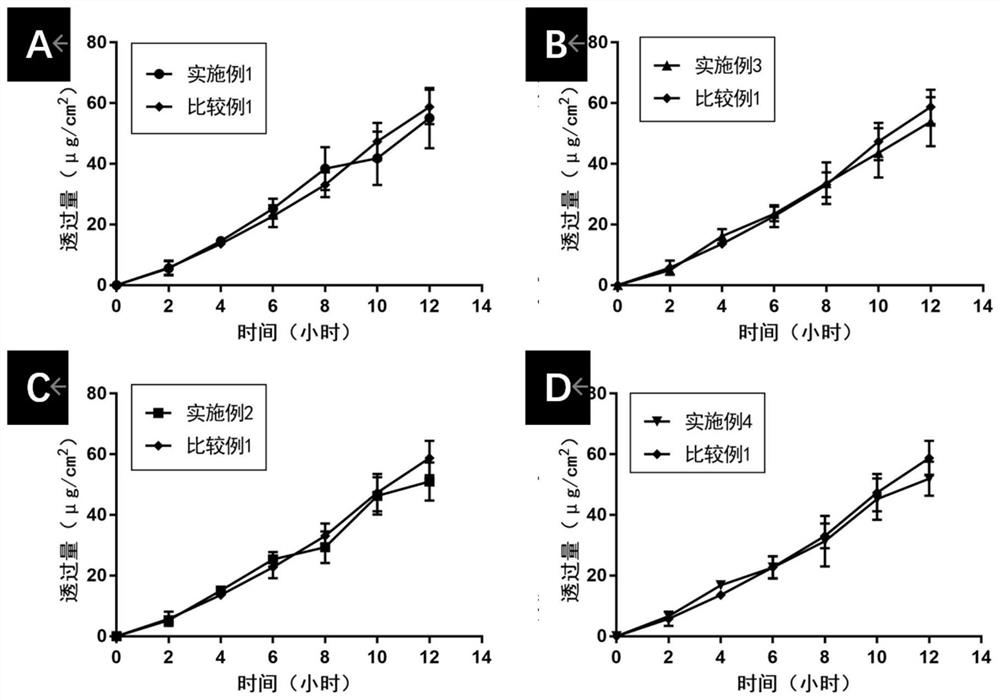
ActiveCN113648297APrevent crystallizationGood stability of active ingredientsOrganic active ingredientsAntipyreticPharmaceutical medicineBULK ACTIVE INGREDIENT
The invention discloses a pharmaceutical composition containing flurbiprofen and a patch, namely a pharmaceutical composition. The pharmaceutical composition contains flurbiprofen serving as an active ingredient and pharmaceutically acceptable auxiliary materials, and the pharmaceutically acceptable auxiliary materials at least comprise a stabilizer, wherein the weight ratio of the flurbiprofen to the stabilizer is 1: (0.5-2). In the pharmaceutical composition, the flurbiprofen is dispersed in an amorphous manner, wherein all the components are matched with one another, so that the hot-melt pressure-sensitive adhesive transdermal preparation containing the composition has the characteristics of good stability of active components and excellent transdermal absorption performance.
Owner:江苏乐明医药有限公司
Production method of medicinal plastic bag
InactiveCN107199650AImprove permeabilityComply with medicinal requirementsBag making operationsPaper-makingBLENDER/MIXEREngineering
The invention discloses a production method of a medicinal plastic bag. Polyethylene 5000S, PE2426H and PE3505 are fed into a blender mixer to be mixed in proportion of 50%, 25% and 25% and then fed into a grain pulling machine for high-temperature plasticizing, particles are cut out, the particles are fed into a hopper of a film blowing machine, heated extrusion is performed to fabricate a film, and the film is sealed and cut into the bag in the length direction. The medicinal plastic bag has the advantages of being high in transparency, resistant to water, good in steam permeability, capable of performing efficient sterilization and blocking germs and the like.
Owner:广州市永乐塑料制品有限公司
Synthesis method of high-purity histamine dihydrochloride
ActiveCN112266360AImprove the coordination effectLower the decarboxylation temperatureOrganic chemistryMethylanilineDimethylaniline N-oxide
The invention discloses a synthesis method of high-purity histamine dihydrochloride, and belongs to the technical field of organic synthesis. The method comprises the steps: in a solvent A, carrying out a decarboxylation reactionon L-histidine at the temperature of 110-150 DEG C under the effect of a composite decarboxylation catalyst, and carrying out filtering after the reaction is completed, wherein the composite decarboxylation catalyst is composed of a main catalyst and an auxiliary catalyst, the main catalyst is selected from sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, pyridine, 4-methylpyridine, aniline, 4-methylaniline, ,N,N-dimethylaniline and N, N-dimethylformamide, and the auxiliary catalyst is one selected from benzophenone, acetophenone, benzophenone and p-methyl acetophenone; carrying out reduced pressure distillation on a filtrate, adding water into residues, and adjusting the pH value to 5-6 by using hydrochloric acid to obtain an aqueous solution; extracting the aqueous solution at least once by using an extracting agent to remove impurities; and evaporating to remove water in the aqueous solution, pulping with a solvent B, filtering and drying to obtain the product.
Owner:WUHAN JASON BIOTECH CO LTD
Butylphthalide drug activity composition and preparation method thereof
PendingCN107648222AComply with medicinal requirementsQuality improvementOrganic active ingredientsNervous disorderClinical efficacyButylphthalide
The invention provides a butylphthalide drug activity composition. The butylphthalide drug activity composition is composed of a component I and a component II, wherein in the component I, the contentof butylphthalide is greater than or equal to 98.0%; in the component II, one or multiple of methylene phthalide, ethylene phthalide, propylene phthalide, butylidenephthalide, pentene phthalide, phthalide, toluene phthalein, ethyl benzene phthalein, C phthalide and E phthalide is / are selected, the content of the component II is greater than 0 and is smaller than or equal to 2.0%; when the component II includes any one of methylene phthalide, ethylene phthalide, propylene phthalide, butylidenephthalide, pentene phthalide, the maximum content is no more than 0.5%; when the component II includesany one of phthalide, toluene phthalein, ethyl benzene phthalein, C phthalide and E phthalide, the maximum content is no more than 1.0%. The drug activity composition is stable in quality and is capable of guaranteeing clinical efficacy and drug safety of butylphthalide preparations.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Refining method of thymol
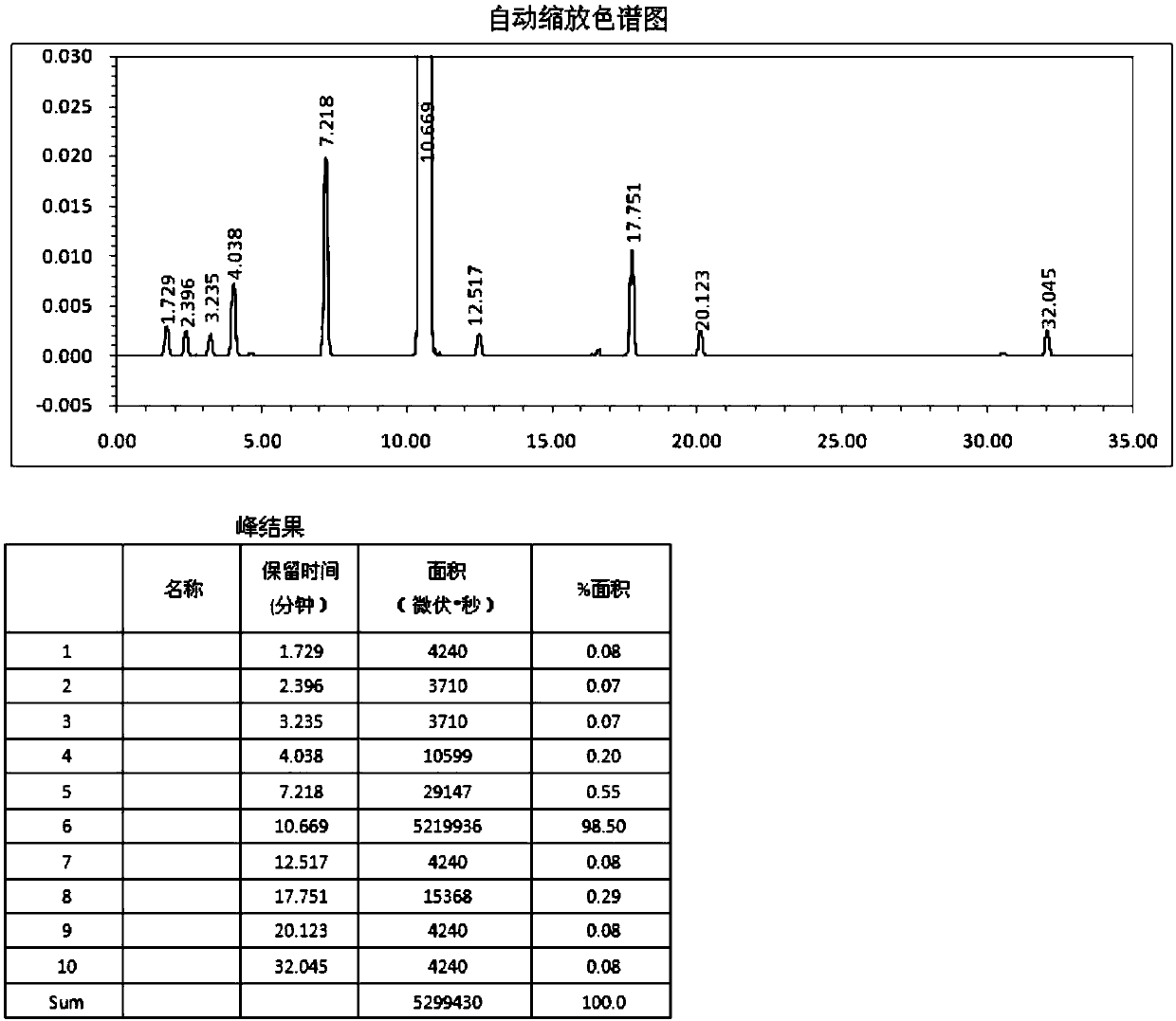
ActiveCN110668922ARapid precipitationHigh purityOrganic chemistryOrganic compound preparationSorbentThermal insulation
The invention relates to the technical field of pharmaceutical chemicals, and provides a refining method of thymol. The refining method comprises the steps that a refining solvent is added to crude thymol, and the mixture is heated and stirred sufficiently to obtain a first mixed solution; the amount of the refining solvent is 0.5-3 times the mass-volume ratio of the crude thymol; an adsorbent isadded to the first mixed solution, and thermal insulation decolorization treatment is performed to obtain second mixed liquid; and the second mixed liquid is filtered, the obtained filtrate is subjected to cooling crystallization, thermal insulation crystallization and cooling crystallization separately, then centrifugal filtering is performed, and the thymol is obtained. The refining method can refine impurities such as citronellal and isopulegol in the crude thymol to a hardly detected degree, the purity of the product is effectively improved, and the medical requirements are met; and meanwhile, compared with a traditional rectification method, the refining method is simple, and the refining cost is greatly reduced.
Owner:JIANGXI ALPHA HI TECH PHARMA
A kind of butylphthalide crude drug product and preparation method thereof
ActiveCN105130934BGuaranteed clinical efficacyEnsure medication safetyOrganic active ingredientsNervous disorderDrug utilisationClinical efficacy
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
High-purity esomeprazole sodium preparation method
InactiveCN106083818AEfficient removalPlay a role in decolorizationOrganic chemistryEsomeprazole SodiumOrganic solvent
The invention discloses a high-purity esomeprazole sodium preparation method. The method comprises the following steps: A) dissolving esomeprazole sodium in a mixed solvent of an organic solvent A and an organic solvent B; B) dropping a sodium hydroxide aqueous solution, stirring the materials for crystallization; C) filtering the material, stirring a filter cake by a mixed solvent of acetone and an organic solvent C and washing the filter cake; and D) filtering the material, and performing vacuum drying on the filter cake to obtain the product. The method has the advantages of good repeatability, simple operation, high products yield, and high purity, and is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for preparing cariprazine and intermediate thereof
PendingCN114262283AReaction raw materials are readily availableEasy to operateCarbamic acid derivatives preparationOrganic compound preparationCariprazineNucleophilic substitution
The invention relates to a method for preparing cariprazine and an intermediate thereof, and the method comprises the following steps: taking trans-4-aminocyclohexane acetate hydrochloride as a starting material, and carrying out Boc protection, reduction, halogenation, nucleophilic substitution, Boc protection removal and amidation reaction to prepare the cariprazine. The alcoholic hydroxyl group of the trans-2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl} ethanol is activated and converted into a group easy to leave, the alcoholic hydroxyl group is creatively converted into the halogen atom, the reaction condition is mild, the yield is high, the raw material medicine which is high in purity and low in impurity content and meets the medicinal requirement can be obtained, and the method is suitable for industrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD
Glucokinase preparing process
ActiveCN100564522CComply with medicinal requirementsHigh activityEnzymesEscherichia coliFusion Protein Expression
The invention discloses a method for expressing and preparing staphylokinase (Staphylokinase, SAK), comprising: 1. Construction of staphylokinase fusion protein expression engineering bacteria: constructing a recombinant plasmid containing the gene sequence of SEQ NO.1, and introducing the recombinant plasmid into Escherichia coli to obtain fermentation strains. 2. The preparation method includes: a. fermenting and expressing a soluble fusion protein having an amino acid sequence such as SEQ NO.2 by engineering bacteria; b. absorbing the fusion protein in step a with a nickel ion affinity gel column to extract the fusion protein; c. Dilute the fusion protein in step b with enterokinase, dilute appropriately, pass through the gel column in step b, and collect the permeate; d, concentrate the permeate through ultrafiltration in step c, and pass through the Q gel column to obtain the staphylokinase stock solution . The method of the invention has simple and reliable steps, and is especially suitable for large-scale production. The staphylokinase obtained by the method of the invention has high purity, high specific activity and good thrombolytic activity; it provides a new way for producing medicinal staphylokinase.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Composition of oxyclopidogrel optical isomer or salt thereof and application of composition
PendingCN111150731AComply with medicinal requirementsOrganic active ingredientsOrganic chemistry methodsClopidogrelPharmaceutical Substances
The invention discloses a composition of an oxyclopidogrel optical isomer or a salt thereof and application of the composition, and belongs to the technical field of medicines. The composition comprises an optical isomer of clopidogrel represented by a formula I or a salt thereof, and one or two compounds represented by formula IIA and formula IIB and salts thereof.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Oxazolidinone-based antibacterial drug preparation method
InactiveCN107892701AComply with medicinal requirementsProcess environmental protectionGroup 5/15 element organic compoundsChemical industryAcid catalyzed
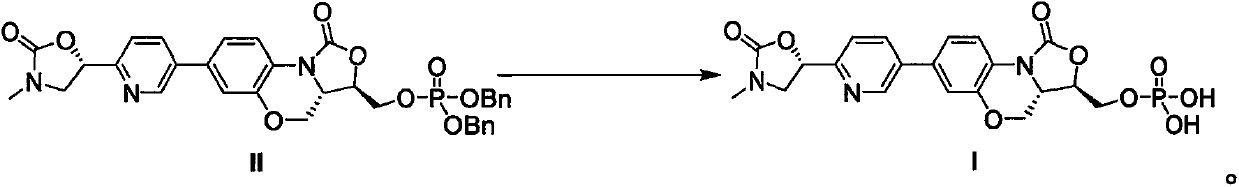
The invention discloses a method for preparing an oxazolidinone-based antibacterial drug through acid catalyzed debenzylation, and belongs to the field of medicine and chemical industry. According tothe present invention, a compound represented by a formula II is subjected to debenzylation under the action of an acid catalyst to obtain a compound represented by a formula I; and the method has advantages of inexpensive and easily-available raw material, safe and simple operation, environmental protection technology, high product quality, high yield and easily-controlled metal residue in the product, and is suitable for commercial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Preparation of benzamide histone deacetylase inhibitor with differentiation and anti-proliferation activity
InactiveCN104876857AEfficient productionEfficient qualityOrganic chemistryAntineoplastic agentsDiseaseProliferation activity
The invention discloses a preparation method of a benzamide histone deacetylase inhibitor with differentiation and anti-proliferation activity. The structure of the benzamide histone deacetylase inhibitor is as shown in a general formula (I), wherein A, Z, Y, B, R1, R2, X1, X2, X3 and X4 are defined as the specification. The compound as a histone deacetylase inhibitor can be used for curing and differentiating the diseases, such as cancer and psoriasis which are related to proliferation.
Owner:TAIZHOU EOC PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com