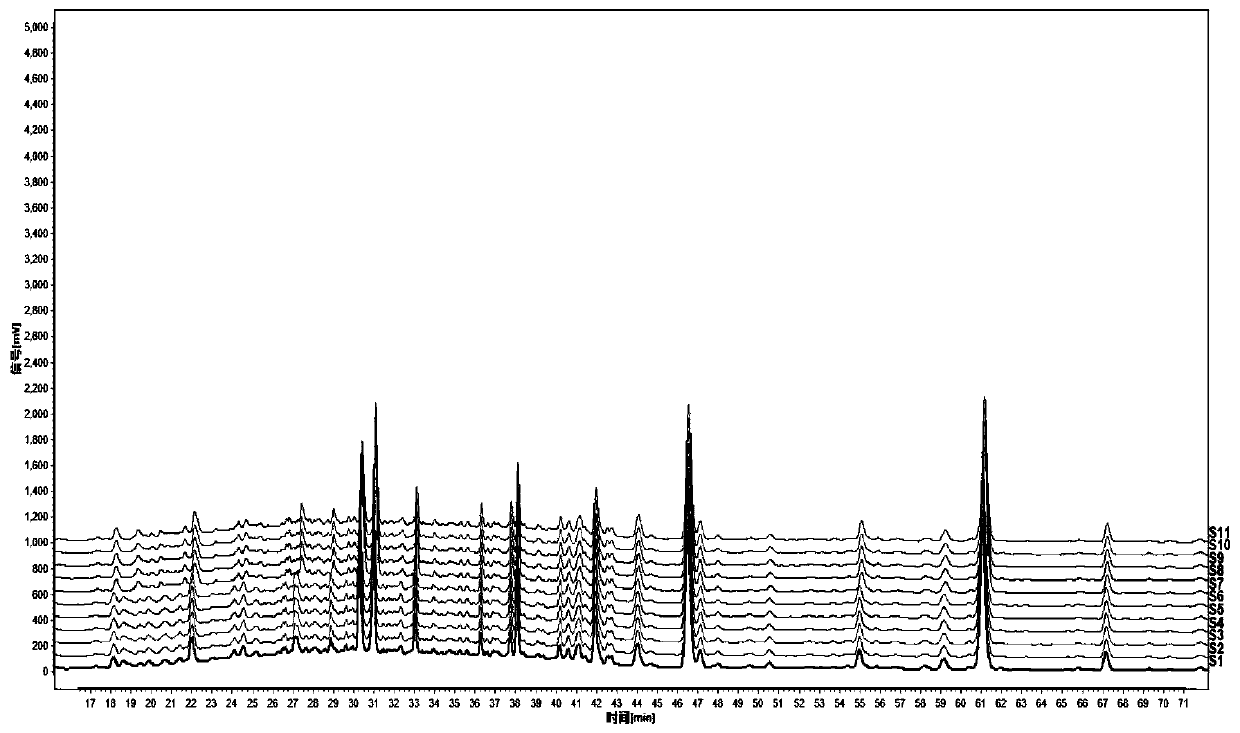
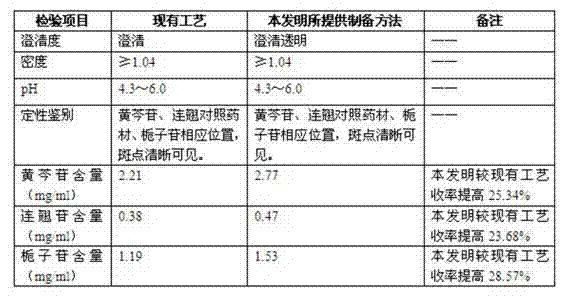
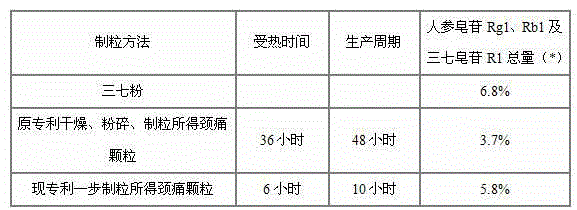
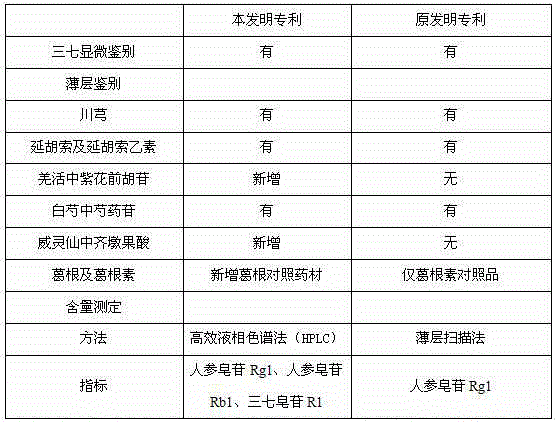
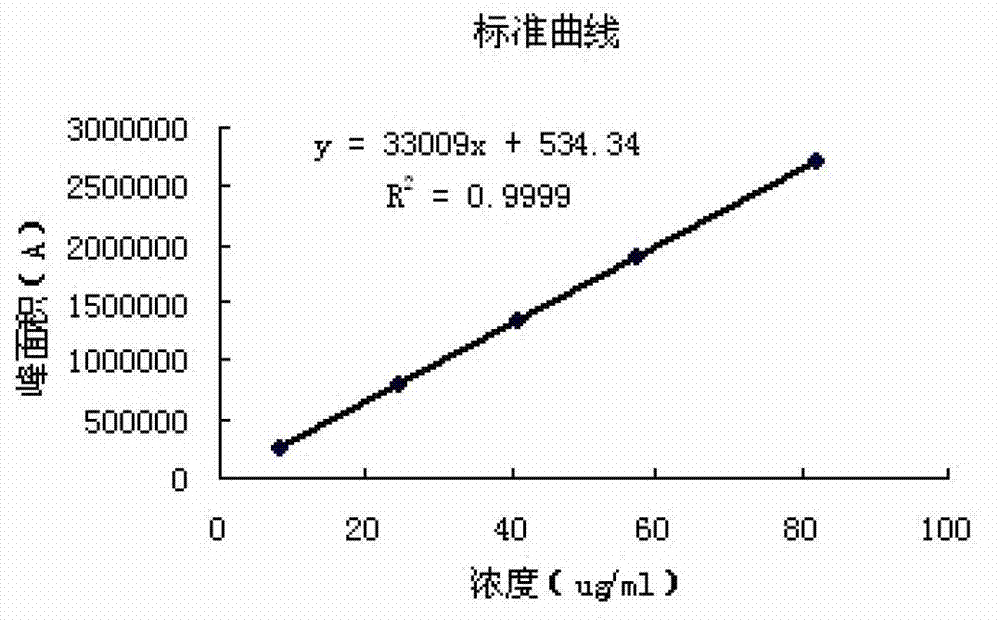
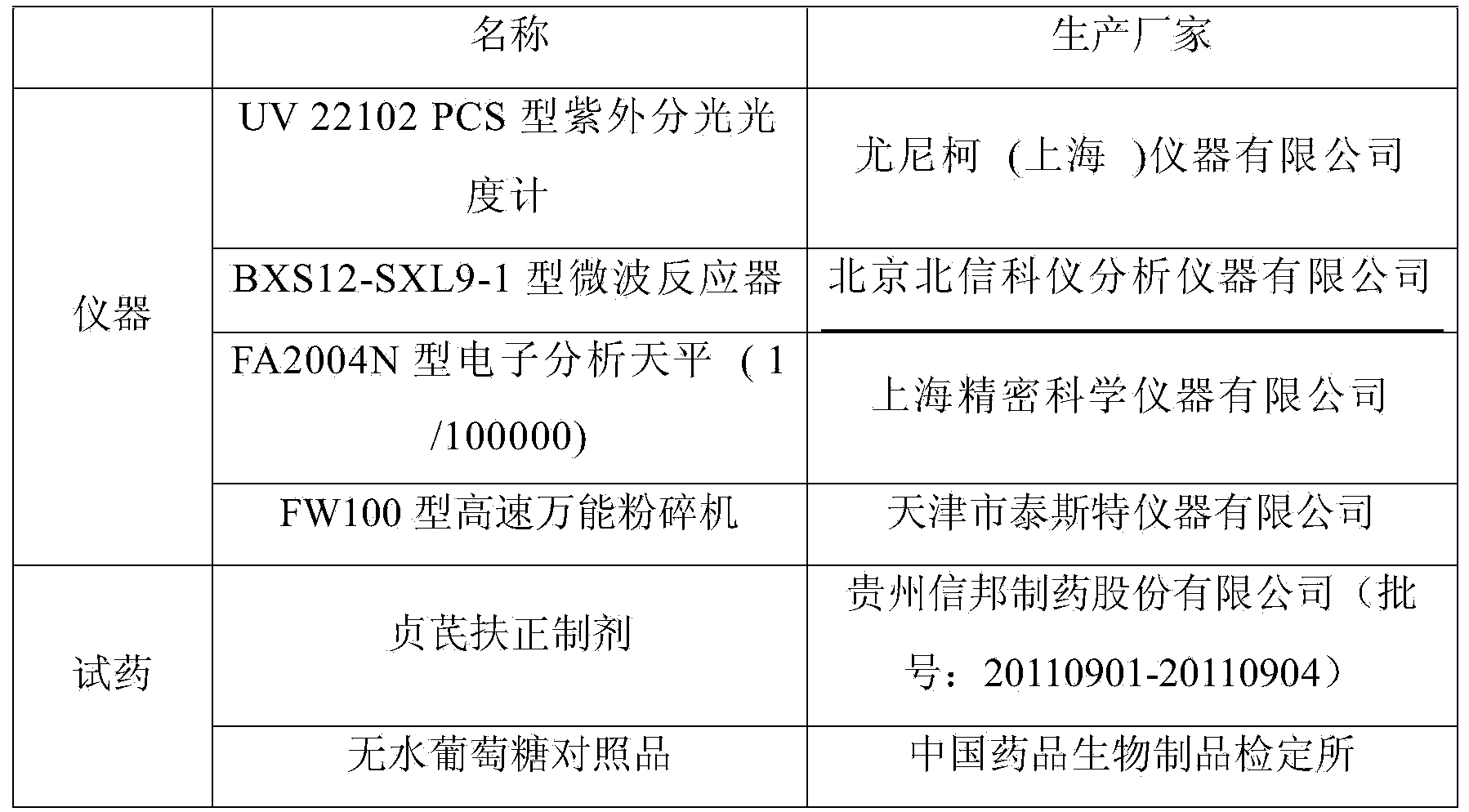
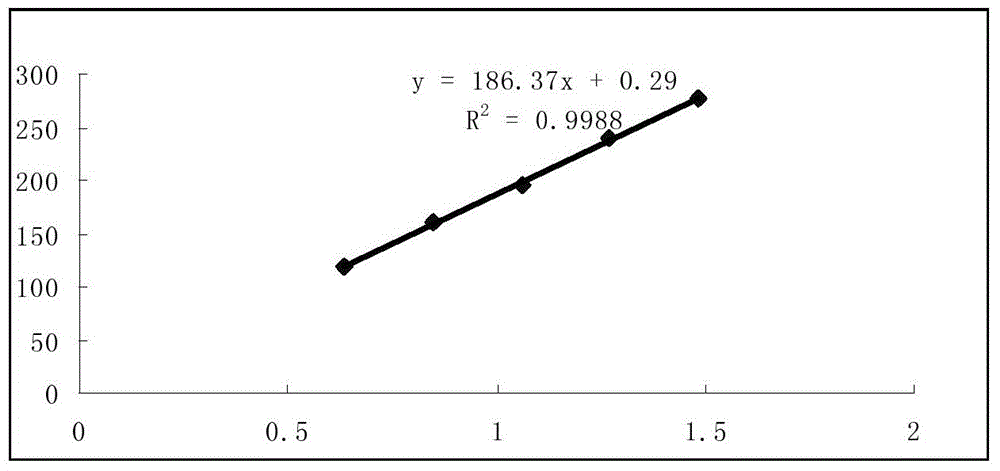
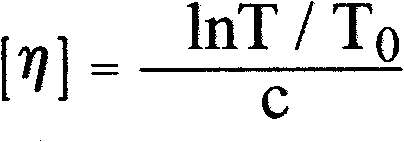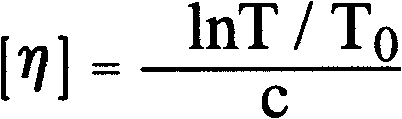Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
357results about How to "Guaranteed clinical efficacy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for controlling quality of ginkgo leaves and extract thereof
InactiveCN103175912AEasy to separateEasy CalibrationComponent separationCurative effectControl quality
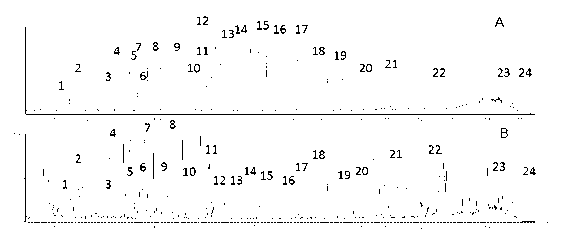
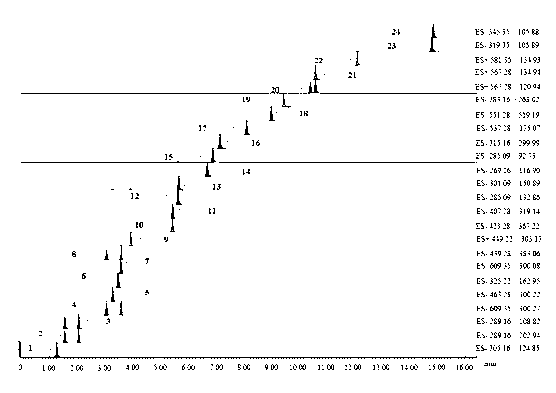
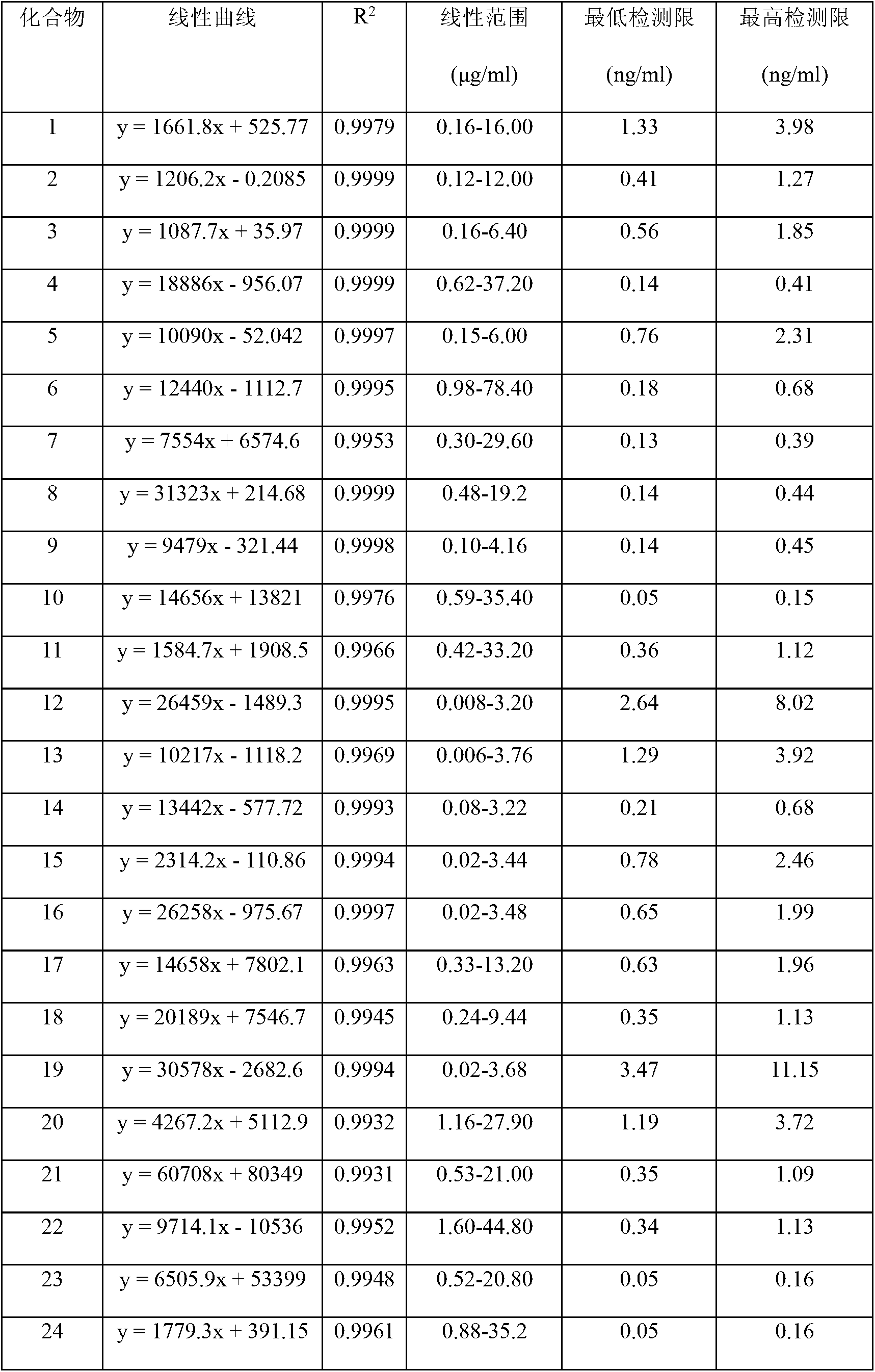
The invention discloses a method for controlling the quality of ginkgo leaves and an extract thereof. After the screening of a great quantity of experiments, the method adopts ultra-high performance liquid chromatography for detection; and the method can be used for detecting three types of components including flavone, terpene lactones and phenolic acid, twenty-four active compounds in total, in the ginkgo leaves and the extract of the ginkgo leaves. The results of the experiments prove that the method is high in detection sensitivity and good in stability, and can be used for objectively, comprehensively and accurately evaluating the quality of ginkgo leaf medicines, the extract of the ginkgo leaves and ginkgo leaf preparations, thus having important significance on controlling the quality and guaranteeing the clinical treatment effect.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Colloid bismuth pectin compound and quality control method of pharmaceutical compositions thereof
InactiveCN102507381AStrong selective adhesionGood selective adhesionMaterial analysis by observing effect on chemical indicatorDirect flow property measurementClinical efficacyBismuth / pectin
The invention relates to a colloid bismuth pectin compound and a quality control method of pharmaceutical compositions of the colloid bismuth pectin compound, and specifically provides a colloid bismuth pectin compound, new quality control indexes of pharmaceutical compositions of the colloid bismuth pectin compound and a detection method of the colloid bismuth pectin compound and the pharmaceutical compositions of the colloid bismuth. In the prior art, the quality of products having poor efficacy can not be well controlled because the efficacy-related indexes are not controlled strictly in the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof. Based on the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof, the following detection items and indexes are added: intrinsic viscosity, gel property, uniformity and galacturonic acid content. The invention can effectively ensure clinical efficacy of products, make the product quality standards more scientific, reasonable and controllable, and has great significance in quality control of the colloid bismuth pectin and the pharmaceutical preparation of colloid bismuth pectin.
Owner:于学敏
Butyl phthalide raw material drug product and preparation method thereof
ActiveCN105130934AGuaranteed clinical efficacyEnsure medication safetyOrganic active ingredientsNervous disorderBiomedical engineeringClinical efficacy
The present invention provides a butyl phthalide raw material drug product with the butyl phthalide content not less than 99.0%; the raw material drug is stable in quality, and can ensure the clinical curative effect and drug safety of a butyl phthalide preparation.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD
Quality control method of sunset abelmoschus root medicinal material as well as extract and preparation of sunset abelmoschus root
ActiveCN103018371AHigh detection sensitivityImprove stabilityComponent separationChromatographic separationMedicine
The invention discloses a quality control method of a sunset abelmoschus root medicinal material, as well as an extract and a preparation of a sunset abelmoschus root. With the adoption of a liquid chromatographic separation technique, the contents of seven flavone components in the sunset abelmoschus root are calculated with the aid of a one-detection multi-evaluation method by calculating a correction factor. The method is high in detection sensitivity and good in stability, can evaluate the quality of the sunset abelmoschus root medicinal material, the extract thereof and the preparation of the sunset abelmoschus root objectively, comprehensively and accurately, can solve the problem that the quality of the medicinal material and the preparation thereof cannot be controlled objectively and reasonably due to the lack of a control, and has a great significance in controlling the quality and ensuring the curative effect.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Chinese medicinal composition for treating cardiovascular and cerebrovascular diseases, preparation method and application thereof
ActiveCN1965966AOvercoming the disadvantages of traditional dosage formsGuaranteed clinical efficacyPowder deliveryAnthropod material medical ingredientsSalvia miltiorrhizaAdjuvant
The invention provides a Chinese medicinal composition for treating cardiovascular and cerebrovascular diseases which comprises the following raw material herbs (by weight portions): astragalus root 480-660, radix salvia miltiorrhiza 60-160, red peony root 60-160, rhizome of Sichuan lovage 60-160, Chinese angelica root 60-160, safflower 60-160, leech 40-90, ground beetle 40-90, peach kernel 60-160, artificial ox gallstone 10-40, antelope's horn 10-40, buthus martensi kirsch 50-140, polygala root 60-160, acorus gramineus soland 60-160 and medicinal adjuvant. The invention also provides the method for preparation and use of the composition.
Owner:天津市石天药业有限责任公司
Butylphthalide medicine active composition and preparation method of butylphthalide medicine active composition
ActiveCN102716121AComply with medicinal requirementsQuality improvementOrganic active ingredientsOrganic chemistryButylphthalidePharmaceutical drug
The invention provides a butylphthalide medicine active composition, which comprises the following ingredients: first ingredients: the butylphthalide content is higher than or equal to 98.0 percent; second ingredients: the second ingredients are one kind of materials or several kinds of materials selected from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide, amylene phthalide, phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, in addition, the content of the second ingredients is higher than 0 but is lower than or equal to 2.0 percent, when the second ingredients comprise any one kind of materials from methylene phthalide, ethylene phthalide, propylene phthalide, butane phthalide and amylene phthalide, the content of any one kind of included ingredients does not exceed 0.5 percent, and when the second ingredients comprise any one kind of materials from phthalide, methyl phthalide, ethyl phthalide, propyl phthalide and amyl phthalide, the content of any one kind of included ingredients does not exceed 1.0 percent. The quality of the medicine active composition is stable, and the clinic curative effect and the medication safety of the butylphthalide preparation can be ensured.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Method for detecting fingerprint spectrum of Guigui zhugan decoction
ActiveCN109709251AComprehensive detection effectComprehensive evaluationComponent separationTest sampleRetention time
The invention discloses a method for detecting a fingerprint spectrum of Guigui zhugan decoction. The fingerprint spectrum is established by the following steps: S1, preparing a Guigui zhugan decoction test sample solution; S2, preparing a reference solution; S3, absorbing the test sample solution and the reference solution precisely and respectively and injecting the solutions into a liquid chromatograph, and recording a chromatogram map; and S4, outputting a Guigui zhugan decoction fingerprint spectrum obtained in the S3 into a traditional Chinese medicine chromatographic fingerprint similarity evaluation system, selecting chromatographic peaks exiting in all chromatogram maps of Guigui zhugan decoction with different batches as common peaks and generating a contrast fingerprint spectrumof the Guigui zhugan decoction based on an average value calculation method, and calculating relative retention time and relative peak areas of all common peaks. According to the invention, the detecting method having advantages of high stability and precision, and high reproducibility and the like is capable of analyzing the effective components in the Guigui zhugan decoction accurately and hasthe important significance in evaluating the quality of the Guigui zhugan decoction comprehensively and objectively.
Owner:NANJING HAICHANG CHINESE MEDICINE GRPCO LTD +2
Method for preparing veterinary heat-clearing and detoxifying oral liquid
ActiveCN103919914AHigh dissolution rateGuaranteed clinical efficacyPlant ingredientsMedicinal herbsPlant cell
The invention belongs to the technical field of preparation of veterinary Chinese medicinal oral liquid, and in particular relates to a method for preparing veterinary heat-clearing and detoxifying oral liquid. The method comprises the steps of crushing of raw medicinal materials, screening, enzymolysis, decocting, micro-filtration, ultra-filtration, reduced pressure concentration, volume setting and the like. Compared with the prior art, the method has the advantages that plant cell walls can be hydrolyzed with an enzyme more effectively, so that the dissolution rate of active ingredients of the medicinal materials is improved; and by combining the micro-filtration and ultra-filtration technologies, impurities are effectively removed from the medicinal liquid, the clarity of the solution is higher, the loss of the active ingredients of the medicinal materials is less, and the clinical treatment effect of the medicaments is well guaranteed. By reasonably matching the bio-enzyme and using the bio-enzyme to assist in extracting the active ingredients in the medicinal materials, the content of key effect ingredients in the medicinal liquid is effectively improved, so that the quality of the product is well improved, and the oral liquid has good popularization and application values.
Owner:HENAN SOAR VETERINARY PHARMA
Medicinal preparation for treating nerve-root cervical spondylopathy, and preparation method and quality detection method thereof
InactiveCN104887771AMeet needsVarious dosage formsNervous disorderComponent separationClinical efficacyCervical spondylopathy
The invention relates to a medicinal preparation for treating nerve-root cervical spondylopathy, and a preparation method and a quality detection method thereof. The invention is an extension of an original invention. The medicinal preparation comprises a granule, a tablet and a capsule, the preparation method is an optimized and screened production technology based on the original invention, a modern new device, a new process and a new technique are adopted to realize industrial production; and quality standard researches are completed and improved on the basis of original standards, HPLC is adopted to simultaneously determine the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in a finished product, and thin layer discrimination of all medicines is carried out to comprehensively control the quality, so the clinic curative effects are guaranteed.
Owner:SHANDONG MINGREN FURUIDA PHARMA
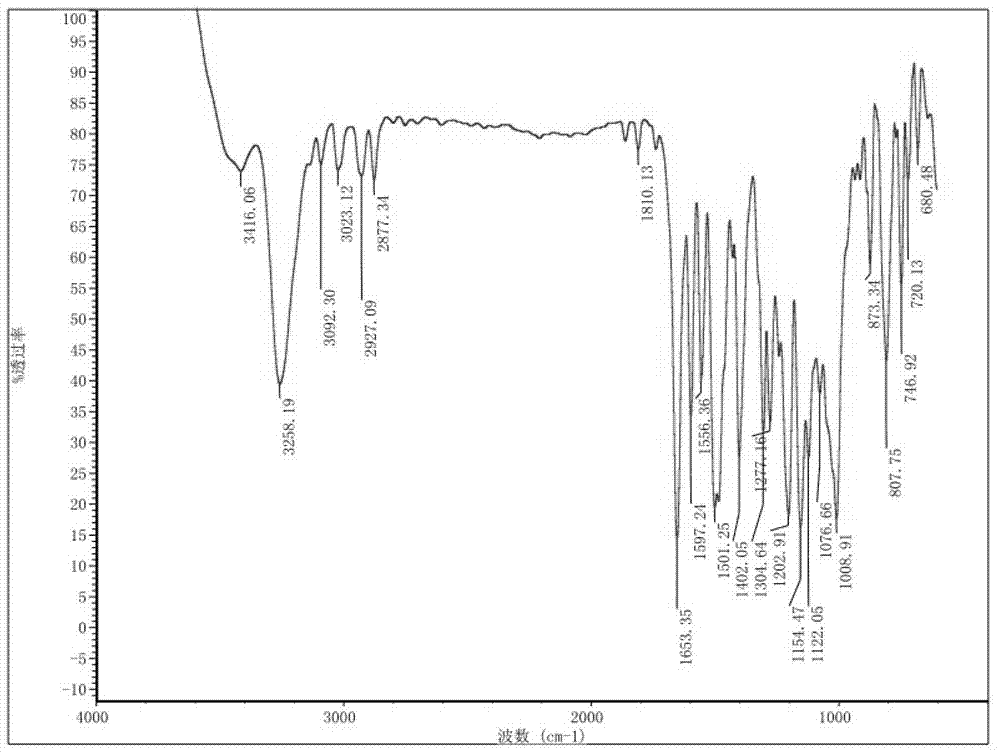
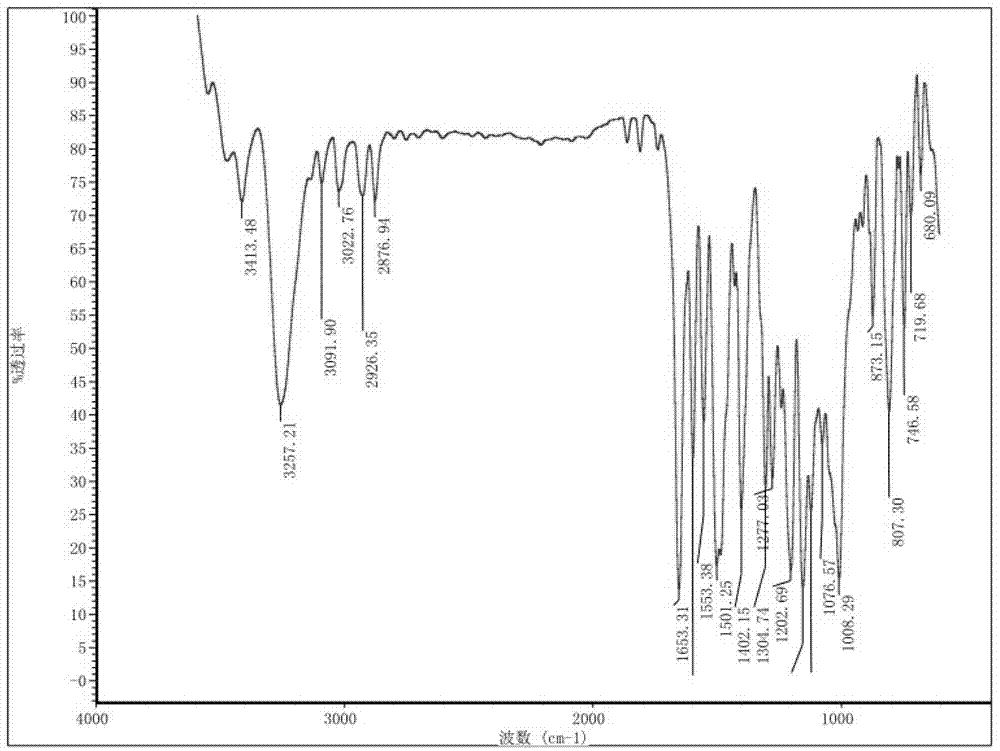
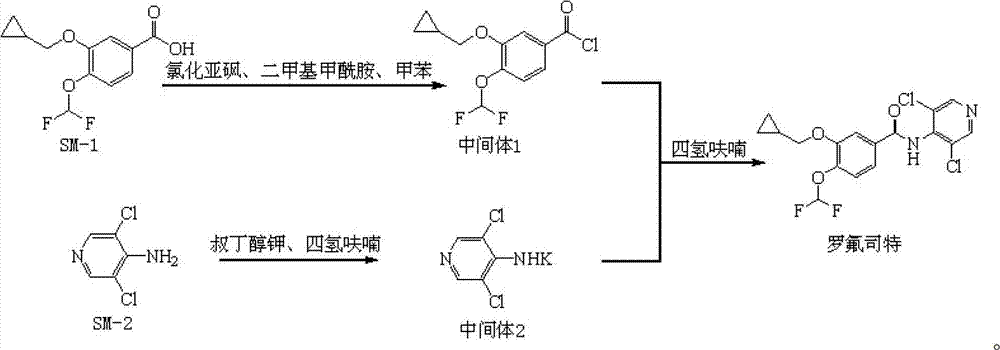
Preparation method and detection method of roflumilast material
ActiveCN102964297ASimple preparation processReduce adverse reactionsOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzoic acidDisease
The invention discloses a preparation method and a detection method of a roflumilast material. The preparation method comprises the following steps: mixing 3-cyclopropyl methoxy group-4-difluoro methoxy group benzoic acid SM-1, thionyl chloride, dimethyl formamide with toluene, and carrying out an acylating chlorination reaction to obtain a midbody 1; mixing 3,5-dichloro-4-aminopyridine SM-2, tetrahydrofuran with potassium tert-butoxide and carrying out a salt forming reaction to obtain tetrahydrofuran solution of a midbody 2; and then mixing the midbody 1 and the midbody 2 with tetrahydrofuran, carrying out amidation to obtain a crude product of roflumilast, and refining the crude product of roflumilast to prepare the roflumilast material. Aiming to overcome the shortage of the prior art, the preparation process of the roflumilast material is optimized, so that the curative effect for treating diseases such as chronic obstructive pulmonary disease (COPD) is more remarkable; and besides, a systematic, complete and effective composition identifying and content measuring method is provided, so that the quality of the medicine can be effectively controlled, and the clinical effect is ensured.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for detecting quality of medicinal preparation for curing hepatitis
ActiveCN101837072AQuality improvementQuality is easy to controlComponent separationDigestive systemThin-layer chromatographyChemistry
The invention discloses a method for detecting the quality of a medicinal preparation for curing hepatitis. The method comprises the following steps of: detecting characters, identifying, inspecting and determining content, wherein identification means identifying astragalus, red paeony root and root of red-rooted salvia in the preparation by adopting thin layer chromatography; and content determination means determining icraiin content of the preparation through high performance liquid chromatography or simultaneously determining tetrandrine content and fangichinoline content of the preparation through the high performance liquid chromatography under the same chromatographic condition. The method has the advantages of effectively controlling and ensuring stable, controllable, high-efficient and safe quality of Jinma Gantai tablets and Jinma Gantai capsules for curing the hepatitis, ensuring clinical curative effect of the preparation and meeting medical requirements, along with scientificity, reasonability, high accuracy and high reproducibility.
Owner:贵州益康制药有限公司
Callicarpa nudiflora medicine, intermediate and fingerprint detection method and standard fingerprint of preparation
ActiveCN104215698AThe pretreatment method is simpleCharacteristic ingredients remain intactComponent separationClinical efficacyCallicarpa nudiflora
The invention discloses a callicarpa nudiflora medicine, an intermediate and a fingerprint detection method of a preparation. The method comprises the following steps: (a) preparation of a reference solution; (b) preparation of a test solution; (c) chromatographic condition; (d) formulation of a standard fingerprint of isoacteoside as a reference peak; and (e) quality control of the fingerprint. According to the above method, a pretreatment method of each tested object is simple, characteristic constituents are retained completely, stability is good, precision is high, and there is certain specificity. The separation effect of each characteristic peak in the fingerprint is good. The callicarpa nudiflora medicine, the intermediate and the fingerprint of the preparation have good correlation. The method can be used for identifying quality of the callicarpa nudiflora medicine and increasing production process controllability of the callicarpa nudiflora preparation, and is helpful for guaranteeing quality stability and clinical efficacy of the callicarpa nudiflora preparation. The invention also discloses the callicarpa nudiflora medicine obtained according to the above fingerprint detection method, the intermediate and the standard fingerprint of the preparation.
Owner:JIUZHITANG +2
Oil composition containing 1,8-cineole and flavanones extracted from Litsea lancilimba Merr. and Blumea Balsamifera DC. And use thereof
ActiveCN101301355AEasily damagedGood treatment effectOrganic active ingredientsDigestive systemLitseaChemical composition
The invention discloses an oily composition which is extracted from Litsea lancilimba and Blumea mollis and contains 1, 8-eudesmin and blumeatin. The oily composition is oily substance extracted from the Litsea lancilimba and the Blumea mollis. The main compositions by weight percentage of the oily composition are: 2.01 to 19.61 percent of the 1, 8-eudesmin, 0.2 to 9.40 percent of sabinene, 0.40 to 4.71 percent of alpha-pinene, 0.40 to 7.84 percent of lemonene, 0.21 to 8.27 percent of the blumeatin, 1.66 to 23.95 percent of L-camphol and 0.64 to 7.98 percent of beta-caryophyllene. The oily composition is definite in main active ingredients, small in content variation, stable in quality which can be effectively controlled, and reliable in healing effect. The invention also discloses use of the oily composition.
Owner:贵州宏宇药业有限公司
Quality control method of capsule preparation for treating a painful swollen joint
ActiveCN101708208AQuality improvementScientific and reasonable quality control methodsAntipyreticComponent separationClinical efficacyMedicine
The invention discloses a quality control method of a capsule preparation for treating a painful swollen joint. The quality control method of the capsule comprises the following steps of property research, identification, inspection and content determination, wherein the identification refers to identifying Yunnan gaultheria, sargentgloryvine stem and psammosilene tunicoides; and the content determination refers to determining the contents of a fumaric acid in the capsule according to high performance liquid chromatography in an appendix VI D of volume one of Chinese pharmacopoeia 2005. The quality control method of a Jingulian capsule preparation for treating the painful swollen joint is provided. The adopted quality control method has the advantages of scientificity, rationality, high accuracy, good reproducibility, comprehensive and effective quality control of the Jingulian capsule, and guaranteed clinical curative effect of the preparation.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Preparation method and detection method of febuxostat raw material
InactiveCN103030605AUniform qualityEffective quality controlOrganic chemistryComponent separationHydroxylamineThio-
The invention discloses a preparation method and a detection method of febuxostat raw material. The preparation method comprises Cyanophenol, DMF (Dimethyl Formamide) saturated with HCl, and thioacetamide are mixed and react to obtain an intermediateIII; the intermediate III, anhydrous ethyl alcohol, 2-chlorination acetoacetic ester are mixed and react to obtain an intermediate IV; the intermediate IV, hexamethylene tetramine and polyphosphoric acid are mixed and react to obtain an intermediate V; the intermediate V, DMF, anhydrous potassium carbonate and bromo-2-methylpropane are mixed and react to obtain an intermediate VI; the intermediate VI, anhydrous formic acid, oxammonium hydrochloride and sodium formate are mixed and react to obtain an intermediate VII; the intermediate VII, anhydrous ethyl alcohol and sodium hydroxide are mixed and react to obtain a febuxostat crude product; and refining and crystal transformation are performed to the febuxostat crude product to obtain the febuxostat raw material. Aiming at avoiding defects in the prior art, the preparation technology of the febuxostat raw material is optimized, and a systematic, complete and effective component identifying and content measuring method is established.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for controlling quality of Runzao Zhiyang tablet
InactiveCN101574439AQuality assuranceGuaranteed clinical efficacyComponent separationDigestive systemClinical efficacyMatrine
The invention discloses a method for controlling the quality of a traditional Chinese medicine preparation-Runzao Zhiyang tablet. The quality control method includes the steps of adopting the thin-layer chromatography to identify kuh-seng and mulberry leaves and adopting the high performance liquid chromatography to determine the content of matrine in the preparation. The quality control method is reliable and feasible, and can guarantee the product quality and the clinical efficacy.
Owner:唐弟光
A kind of traditional Chinese medicine preparation for treating stomach disease and its preparation method and detection method
ActiveCN102274401AImprove securityHigh dissolution rateComponent separationDigestive systemClinical efficacySide effect
The invention discloses a traditional Chinese medicine preparation for treating gastropathy and a preparation method and a detection method thereof. The preparation is prepared by the following herbs by weight: 40-44 parts of radix hemsleyae, 30-34 parts of wild buckwheat Rhizome, 30-34 parts of sargentgloryvine stem, 20-22 parts of beautyberry, 30-34 parts of gunny, 30-34 parts of rhizoma corydalis, 30-34 parts of herba agrimoniae, 60-66 parts of bletilla rhizome, 40-44 parts of membrana follicularis ovi, 30-34 parts of elecampane, and 38-42 parts of walnut kernels. By using elecampane instead of aristolochia debilis in Shuangjinweiyang capsules, the traditional Chinese medicine preparation prepared by the invention has the same efficacy of smoothing liver and rectifying qi, strengthening stomach and relieving pain, convergence and haemostasis, and the drug efficacy is more significant. In addition, the toxic and side effect of aristolochia debilis is removed in the formula, and the safety of the drug is greatly improved; the medicinal materials are pulverized into fine powder during preparation, which increases the dissolubility and bioavailability of the finished product, and brings the efficacy into full play; the newly-established detection method has high precision, good stability, and accurate measurement results, can effectively control the product quality, and thus can ensure the clinical curative effect.
Owner:贵州三仁堂药业有限公司
Quality detection method of traditional Chinese medicinal composition for curing diabetic retinopathy
ActiveCN104161847AGuaranteed stabilityGuaranteed clinical efficacyComponent separationMetabolism disorderAstragalosideBerberine
The invention provides a quality detection method of a traditional Chinese medicinal composition for curing diabetic retinopathy. Raw medicines of the traditional Chinese medicinal composition comprise, by weight, 30-60 parts of Radix Astragali, 9-15 parts of ligustrum lucidum, 9-30 parts of motherwort, 3-10 parts of dark plum, 2-9 parts of coptis, 1-5 parts of cortex cinnamomi and 3-9 parts of Buddleja officinalis. The quality detection method comprises steps as follows: content determination of astragaloside, berberine and linarin in the traditional Chinese medicinal composition and thin-layer chromatography of the above seven raw medicines. The quality detection method provided by the invention has advantages of high specificity, simple, accurate and reliable operation and good reappearance. By the quality detection method, clinical medication safety can better be guaranteed.
Owner:BEIJING HANDIAN PHARMA CO LTD +1
Detection method for compounded Sichuan fritillary bulb extract tablets
ActiveCN104777265AThe extraction effect is comparableEasy to operateComponent separationGlycyrrhiza uralensisDrug
The invention discloses a detection method for compounded Sichuan fritillary bulb extract tablets. The detection method comprises the steps of carrying out character detection, carrying out microscopic observation, carrying out thin-layer chromatography qualitative identification and carrying out content determination, wherein thin-layer chromatography qualitative identification comprises the steps of identifying ephedrannin and neoephedrine A in a preparation, identifying polygala root and cynanchum atratum and identifying glycyrrhiza uralensis; content determination comprises the step of determining the content of ephedrine hydrochloride in the preparation. The detection method disclosed by the invention has the advantages that the mixing of radix ephedrae and cynanchum atratum is effectively controlled, and meanwhile, the composition and content of the compounded Sichuan fritillary bulb extract tablets are effectively controlled, so that the drug can be safer and more effective.
Owner:SICHUAN FENGCHUN PHARMA
Chinese medicinal composition for treating heart failure and preparation method as well as application thereof
ActiveCN102416101AKeep active ingredientsGuaranteed clinical efficacyUnknown materialsCardiovascular disorderSalvia miltiorrhizaClinical efficacy
The invention provides a Chinese medicinal composition for treating heart failure. The composition includes the following herbal medicines in parts by weight: 65-185 parts of codonopsis pilosula, 65-185 parts of acanthopanax root, 65-185 parts of astragalus root, 65-185 parts of root of red-rooted salvia, 15-185 parts of turtle shell, 50-150 parts of tuckahoe, 30-130 parts of semen lepidii, 50-150 parts of dwarf lilyturf root, 30-130 parts of bitter orange and medicinal auxiliary materials. Meanwhile, the invention provides a preparation method of the Chinese medicinal composition for treating heart failure. The Chinese medicinal composition is used for treating the heart failure. The Chinese medicinal composition has the effects of overcoming the defects of the conventional formulations of medicinal broth and the like; by using a water extraction method, active ingredients with a clinical curative effect in the composition are remained; by using high-efficiency dynamic loop extraction equipment, the active ingredients are extracted to the greatest extent; and thus, the clinical curative effect of the composition is guaranteed, and the prevention and treatment effect of the composition on qi deficiency, blood stasis and water retention symptoms of the heart failure is obviously superior to those of similar medicaments.
Owner:天津市石天药业有限责任公司
Detection method for traditional Chinese medicine preparation compounded Sichuan fritillary bulb extract tablets
The invention discloses a detection method for traditional Chinese medicine preparation compounded Sichuan fritillary bulb extract tablets. The detection method comprises the steps of carrying out thin-layer chromatography qualitative identification and carrying out content determination, wherein thin-layer chromatography qualitative identification comprises the steps of identifying ephedrannin and neoephedrine A in the preparation, identifying polygala root and cynanchum atratum and identifying glycyrrhiza uralensis and schisandra fruit; content determination comprises the step of determining the content of ephedrine hydrochloride in the preparation. The detection method disclosed by the invention has the advantages that the mixing of radix ephedrae and cynanchum atratum is effectively controlled, and meanwhile, the composition and content of the compounded Sichuan fritillary bulb extract tablets is effectively controlled, so that the drug can be safer and more effective.
Owner:九寨沟天然药业股份有限公司
Traditional Chinese medicine composition with effects of clearing lung, moistening dryness and relieving sore throat as well as preparation method and application thereof
ActiveCN104587249AGood treatment effectImprove securityRespiratory disorderFood preparationBelamcanda chinensisRespiratory tract disease
The invention discloses a traditional Chinese medicine composition with the effects of clearing the lung, moistening dryness and relieving sore throat. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 5-15 parts of platycodon root, 3-10 parts of dwarf lilyturf tuber, 5-20 parts of dried tangerine peel, 5-15 parts of coastal glehnia root, 2-12 parts of gynostemma pentaphyllum, 1-8 parts of blackberrylily rhizome, 8-12 parts of ginkgo seeds, 150-300 parts of yellow-peel pears, 5-15 parts of Indian trum etflower seed, 5-15 parts of ginkgo leaf, 2-10 parts of liquorice root, 5-15 parts of mulberry leaves, 2-12 parts of honeysuckle flower, 5-15 parts of loquat leaf, 2-12 parts of corn stigma, 5-15 parts of peppermint, 1-12 parts of lotus leaf, 1-3 parts of green tea, 3-15 parts of fruit-spike of common selfheal, 0.5-6 parts of Chinese magnoliavine fruit and 3-15 parts of sea buckthorn fruit. According to the traditional Chinese medicine composition disclosed by the invention, herbal medicine formulas and dosage compatibilities can be preferably obtained by adopting treatment based on syndrome differentiation according to the theories of traditional Chinese medicines, and clinical experiment results show that the traditional Chinese medicine composition has very good effects of clearing and benefiting the throat and palate, moistening the lung, and reducing phlegm and stopping coughing, and has very good treatment effects on respiratory tract diseases such as chronic pharyngitis, pneumonia and cough.
Owner:曹成瑞
Sugar-free granule for treating chronic fatigue syndrome and preparation method and detecting method thereof
ActiveCN102488837ADissolve fastImprove bioavailabilityComponent separationAntinoxious agentsOld patientsClinical efficacy
The invention discloses a sugar-free granule for treating chronic fatigue syndrome and a preparation method and a detecting method thereof, wherein the sugar-free granule is prepared by the following ingredients in parts by weight: 75 parts of ginseng, 250 parts of dwarf lilyturf root, 150 parts of fructus schizandrae, 150 parts of angelica, 250 parts of astragalus root, 200 parts of arillus longan, 50 parts of cinnamon, 150 parts of lucid ganoderma, 150 parts of leatherleaf milletia, 125 parts of tuckahoe, 250 parts of hawthorn, 150 parts of radix salviae miltiorrhizae, 150 parts of jujube kernels, 100 parts of donkey-hide gelatin, 5 parts of steviosin and 767 parts of dextrin. The sugar-free preparation of the invention has the advantages of fast dissolution speed, high bioavailability and good stability so as to solve the drug use limit problem of diabetics and old patients, thereby expanding the adaptable crowds; besides, the detecting method of the invention has the advantages of high precision, good repeatability and accurate measurement result, thereby effectively guaranteeing the clinical treatment effect of the preparation.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Dimethyl sulfonate of compound A, crystal form of dimethyl sulfonate, and medicinal composition containing dimethyl sulfonate
InactiveCN106674202AHigh purityOptimizationOrganic active ingredientsOrganic chemistry methodsDrugDisease
The invention provides dimethyl sulfonate of a compound A, a crystal form of the dimethyl sulfonate, application of the dimethyl sulfonate of the compound A in preparation of a medicine for preventing and / or treating mammal diseases, and a medicinal composition containing the dimethyl sulfonate of the compound A, wherein the mammals comprise human beings, and the diseases comprise various cancers, preferably non-small cell lung cancer, particularly mutated non-small cell lung cancer.
Owner:HUIZHOU XINLITAI PHARMA
Medicinal smectite content measuring standard
InactiveCN101477068AGuaranteed clinical efficacyQuality assuranceDigestive systemAluminium/calcium/magnesium active ingredientsSesquioxideMontmorillonite
The invention discloses a standard for determining the content of medicinal montmorillonite, and in particular relates to a standard for controlling the content of silicon dioxide and aluminium sesquioxide in a quality standard of medicinal montmorillonite with pertinence. The standard is characterized in that the content standard of silicon dioxide and aluminium sesquioxide is as follows: the content of montmorillonite needs to be between 95.0 and 105.0 percent; according to the calculation of dry matter, the content of silicon dioxide (SiO2) needs to be between 55 and 65 percent; and the content of aluminium sesquioxide (Al2O3) needs to be between 12 and 22 percent.
Owner:JINAN KANGZHONG PHARMA TECH DEV
Method for measuring content of polysaccharides in glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation
InactiveCN103558166AQuality improvementSimple and fast operationColor/spectral properties measurementsPHENOL LIQUIDCurative effect
The invention discloses a method for measuring the content of polysaccharides in a glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation. The method takes anhydrous dextrose as a reference substance and can be used for measuring the content of the polysaccharides in the glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation at the wavelength being 480-490nm by using a phenol-sulfuric acid method or at the wavelength being 600-625nm by using an anthrone-sulfuric acid method. The method is simple and convenient to operate, good in quick reproducibility, high in recovery rate and sensitivity and accurate in measurement result, and can be used for effectively controlling the quality of the glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation so as to ensure a clinical treatment effect of the glossy privet fruit and astragalus membranaceus healthy energy-strengthening preparation.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Quality control method of medicinal preparation for treating gynecological inflammation and hysteromyoma
InactiveCN101926887AScientific and reasonable quality control methodsEffective massComponent separationColor/spectral properties measurementsChlorogenic acidClinical efficacy
The invention discloses a quality control method of a medicinal preparation for treating gynecological inflammation and hysteromyoma. The quality control method of the capsules comprises character, identification, examination and content detection, wherein the identification is to identify bittersweet herb, spreading hedyotis herb, sowthistle tasselflower herb, asiatic pennywort herb and herb of common goldernrod; the content measurement is to measure the chlorogenic acid content and total flavones content of the preparation by using high performance liquid chromatography and UV spectrophotometry respectively. The quality control method of the invention is scientific, reasonable, high in accuracy and high in repeatability, can completely and effectively control the quality of the capsules for treating gynecological inflammation and hysteromyoma to assure the clinic effectiveness of the preparation.
Owner:贵阳春科药业技术研发有限公司
Method for detecting vitamin D content in vitamin D drop
ActiveCN105372337AGood stability and repeatabilityImprove analysis efficiencyComponent separationChromatography columnChemistry
The present invention discloses a method for detecting the vitamin D content in a vitamin D drop. According to the present invention, through a large number of optimizations, the optimal mobile phase composition, the optimal flow rate, the optimal detection wavelength, the optimal chromatography column and other analysis conditions are obtained, and the multiple experiment results show that the method has characteristics of good stability, good repeatability, high analysis efficiency and good separation, and can sensitively and accurately perform qualitative and quantitative detection on the vitamin D3, such that the quality of the vitamin D drop can be objectively, completely and accurately evaluated, and the important significance can be provided for the vitamin D drop quality control and the clinical treatment effect ensuring.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Radix astragali medicinal materials, intermediate body and method for testing fingerprint of formulation thereof as well as standard fingerprint
ActiveCN101327246AImprove controllabilityThe pretreatment method is simpleComponent separationMetabolism disorderDrugChemistry
The invention discloses a fingerprint map detecting method of radix astragali drug, intermediate compound and a preparation thereof, the method is as follows: taking onocol as reference substance, obtaining each sample solution through the processes of extracting / dissolving, determining constant volume and the like, performing HPLC detection under the condition of the same chromatogram, performing gradient elution, recording a chromatogram map, and obtaining the product. In the method, the preliminary treatment method for each sample is simple, the reservation of the characteristic components is complete, the sample solution is stable; the precession is high, reproduction quality is good, and the solution has a certain specificity; the separation effect of each characteristic peak in the obtained fingerprint map is good, the fingerprint map of the radix astragali drug, intermediate compound and the preparation has better correlation; the method can be used for identifying the fake of the radix astragali drug, increases the controllability of the production process of the radix astragali preparation, and is in favour of ensuring the quality stability and the clinical healing efficacy of the radix astragali preparation. The invention also discloses a standard fingerprint map of radix astragali drug, intermediate compound and preparation thereof obtained by the fingerprint map detecting method.
Owner:SICHUAN BAILI PHARM CO LTD
Antibacterial anti-inflammatory capsule quality detection method
InactiveCN105606756AQuality improvementAdd TLC DiscriminationComponent separationTest sampleMedicine
The invention discloses an antibacterial anti-inflammatory capsule quality detection method. Antibacterial anti-inflammatory capsules are prepared from honeysuckle flowers, radix stemonae, Chinese rhubarb, folium isatidis, radix scutellariae, rhizoma anemarrhenae and a christina loosestrife herb. The antibacterial anti-inflammatory capsule quality detection method comprises the four steps of suitability test on chromatographic conditions and a system, preparation of a reference substance solution, preparation of a test sample solution and determination. In addition, the radix stemonae, the Chinese rhubarb, the radix scutellariae, the rhizoma anemarrhenae and the honeysuckle flowers are subjected to thin-layer identification. The antibacterial anti-inflammatory capsule quality detection method has the advantages of being simple and easy to operate and capable of effectively controlling the product quality.
Owner:贵州省科晖制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com