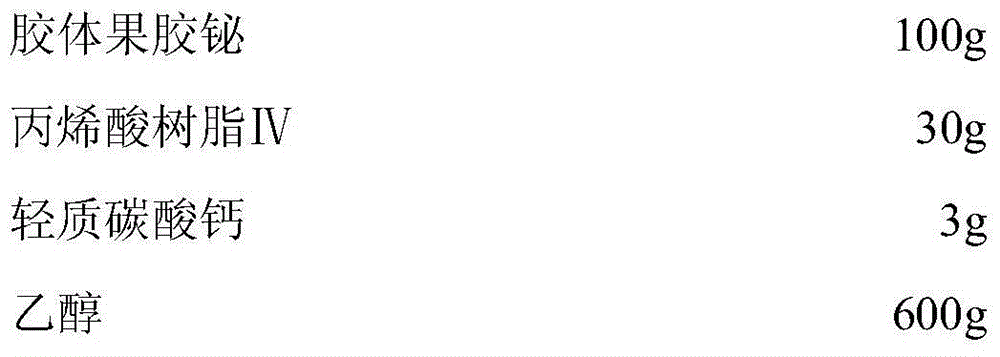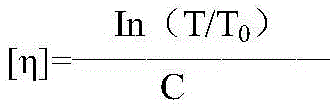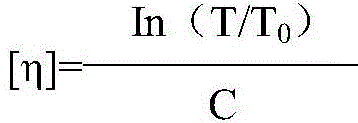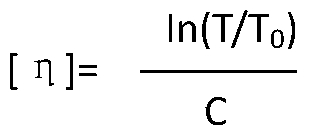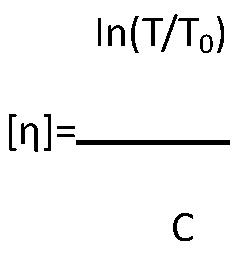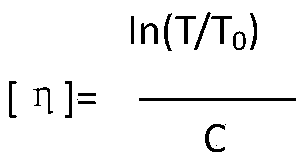Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Bismuth / pectin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colloidal Bismuth Pectin is a medicine available in a number of countries worldwide. A list of US medications equivalent to Colloidal Bismuth Pectin is available on the Drugs.com website. Skip to Content
Colloid bismuth pectin compound and quality control method of pharmaceutical compositions thereof
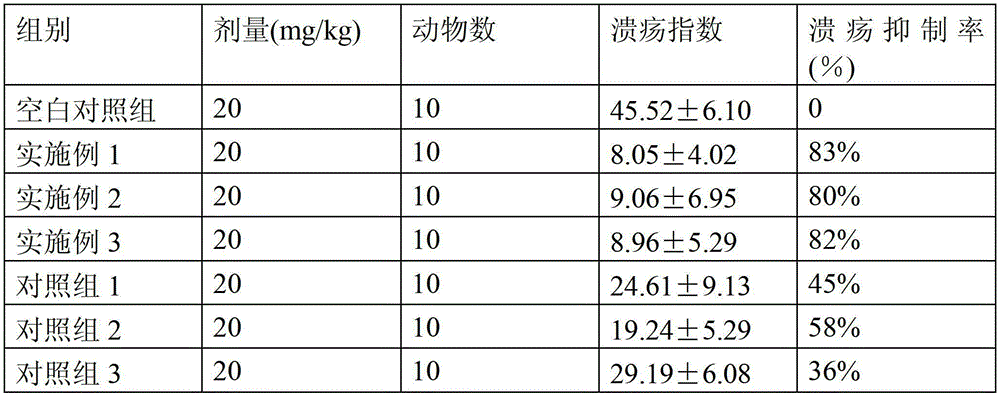
InactiveCN102507381AStrong selective adhesionGood selective adhesionMaterial analysis by observing effect on chemical indicatorDirect flow property measurementClinical efficacyBismuth / pectin
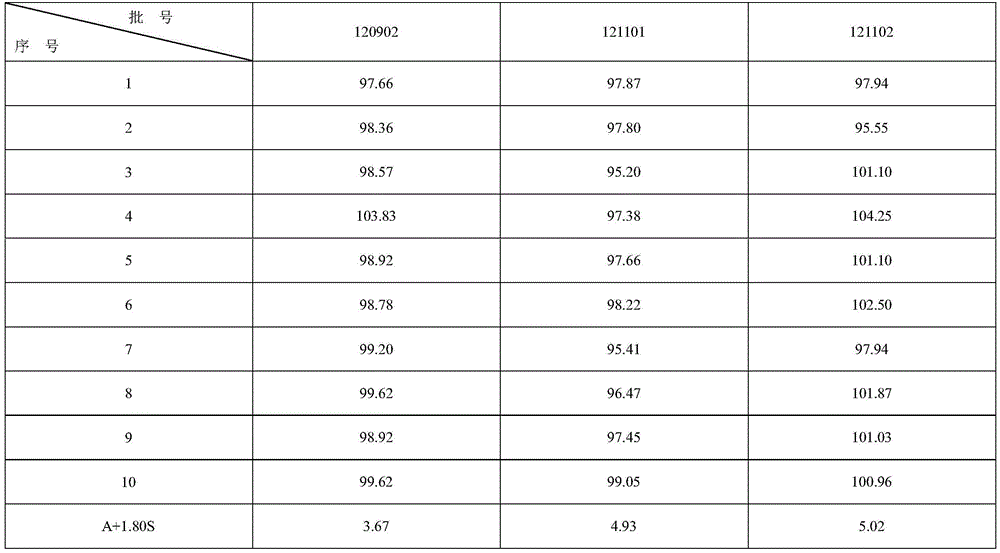
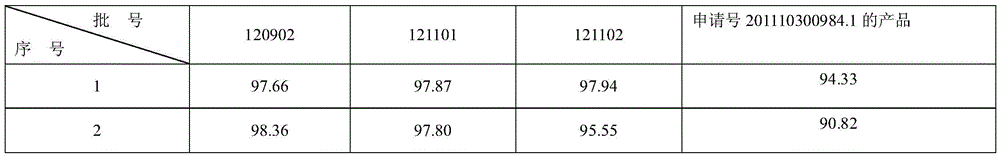
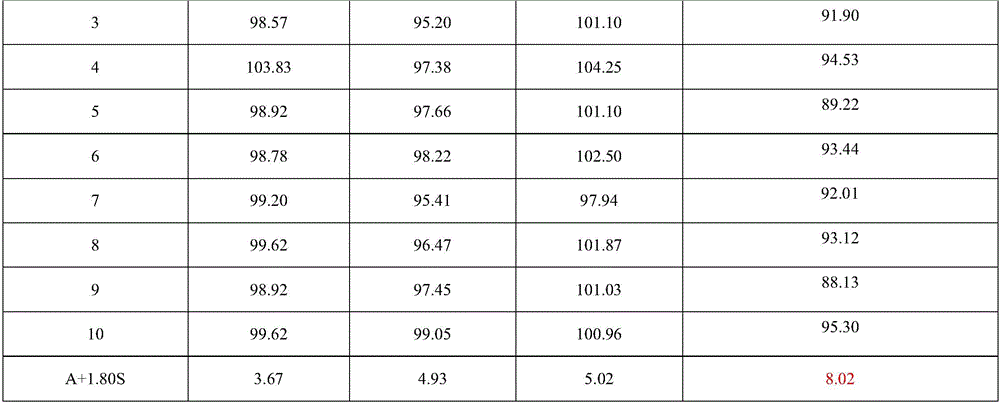
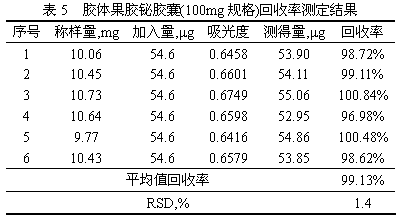
The invention relates to a colloid bismuth pectin compound and a quality control method of pharmaceutical compositions of the colloid bismuth pectin compound, and specifically provides a colloid bismuth pectin compound, new quality control indexes of pharmaceutical compositions of the colloid bismuth pectin compound and a detection method of the colloid bismuth pectin compound and the pharmaceutical compositions of the colloid bismuth. In the prior art, the quality of products having poor efficacy can not be well controlled because the efficacy-related indexes are not controlled strictly in the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof. Based on the existing quality control methods of colloid bismuth pectin and pharmaceuticals thereof, the following detection items and indexes are added: intrinsic viscosity, gel property, uniformity and galacturonic acid content. The invention can effectively ensure clinical efficacy of products, make the product quality standards more scientific, reasonable and controllable, and has great significance in quality control of the colloid bismuth pectin and the pharmaceutical preparation of colloid bismuth pectin.
Owner:于学敏
Method for determining content of bismuth in colloidal bismuth pectin or preparation containing colloidal bismuth pectin
ActiveCN104880428AEliminate distractionsAccurate determination of bismuth contentAntibacterial agentsMaterial analysis by observing effect on chemical indicatorBismuth / pectinColloid
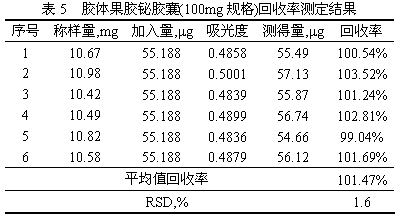
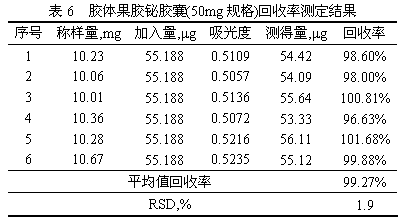
The invention provides a method for determining the content of bismuth in colloidal bismuth pectin or a preparation containing the colloidal bismuth pectin. According to the method, the bismuth is dissociated from the colloidal bismuth pectin with protonic acid, the characteristic reaction principle that bismuth reacts with potassium iodide to generate yellow bismuth potassium iodide is used, ultraviolet-visible spectrophotometry is adopted for determining, and the content is calculated through an external standard method. The established method for determining the content of the bismuth in the colloidal bismuth pectin or the preparation containing the colloidal bismuth pectin is high in specificity, good in linearity, high in accuracy, repeatability and sensitivity and capable of serving as a quality control method for the bismuth in the colloidal bismuth pectin or the preparation containing the colloidal bismuth pectin to effectively control product quality.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Dispersion preparation containing colloidal bismuth pectin and preparation method thereof
ActiveCN104147041ASatisfy colloidal stability characteristicsConforms to dispersion uniformity regulationsOrganic active ingredientsInorganic active ingredientsBismuth / pectinColloid
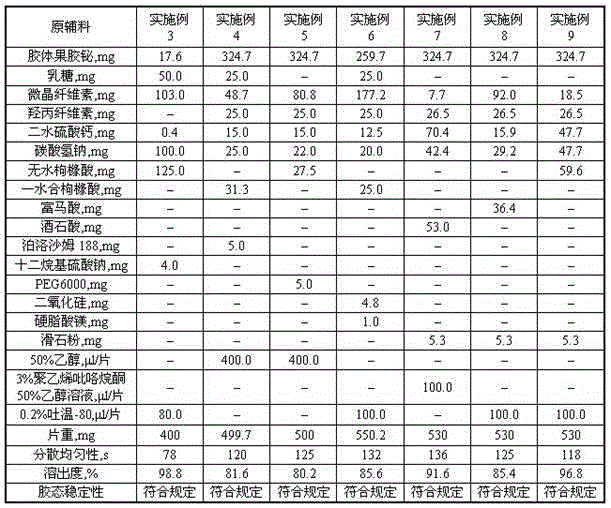
The invention discloses a dispersion preparation containing colloidal bismuth pectin and a preparation method thereof. 1 g is used as a unit for the dispersion preparation; in each unit of the preparation, colloidal bismuth pectin or a drug containing colloidal bismuth pectin, in terms of colloidal bismuth pectin, is 44.0 to 900.0 mg, and each unit further contains 1.0 to 500 mg of a penetration enhancer, 2.0 to 312.5 mg of an acid-source pore forming agent, 2.0 to 250.0 mg of an alkali-source pore forming agent and 1.0 to 400.0 mg of a disintegrating agent. The dispersion preparation containing colloidal bismuth pectin has a dissolution rate of more than 80%, satisfactory colloidal stability and dispersion uniformity of less than 2.5 min, so regulations for dispersion uniformity are met, effective dissolution is realized, and the characteristic of colloidal stability of colloidal bismuth pectin is realized.
Owner:SHANXI ZHENDONG ANXIN BIOLOGICAL PHARM CO LTD
Method for detecting dissolution rate of colloidal bismuth pectin preparation
ActiveCN104897668AGuaranteed uniformityImprove effectivenessMaterial analysis by observing effect on chemical indicatorSurface/boundary effectBismuth / pectinHydrogen-Ion Concentrations
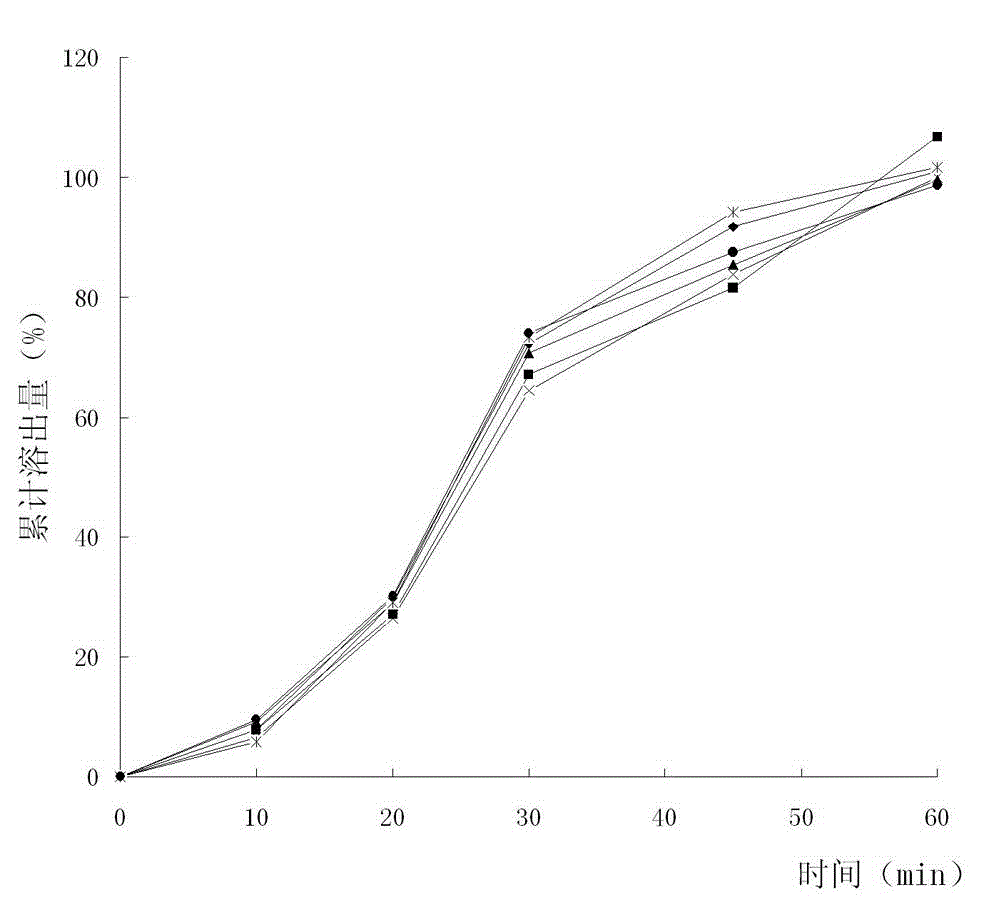
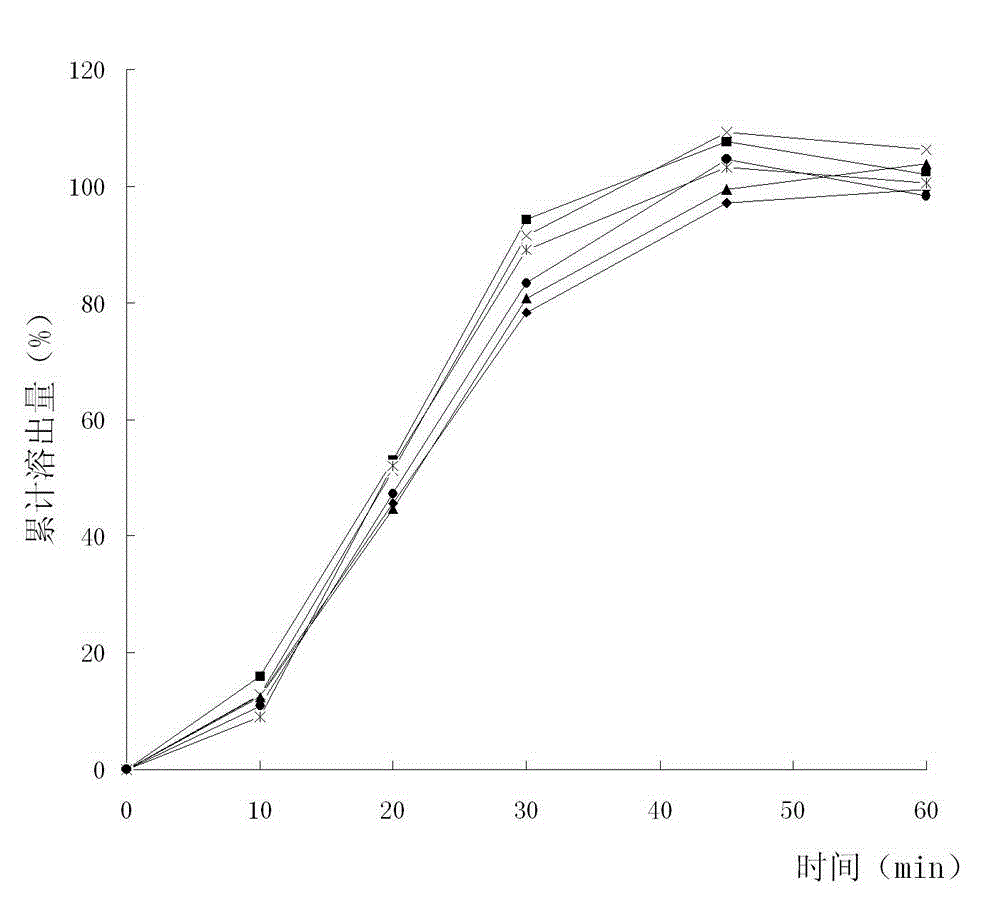
The invention discloses a method for detecting dissolution rate of a colloidal bismuth pectin preparation. The method comprises the following steps of dissolving the colloidal bismuth pectin preparation in dissolution media by using the Ch. p 2010(2) Appendix XC of a Chinese Pharmacopoeia dissolution rate measuring method; directly taking digested liquor; adding proton acid dissociation agents in the digested liquor until the hydrogen ion concentration in the digested liquor is 0.8-1.2 mol / L; performing centrifugal separation to obtain liquid supernatant; adding citric acid or ascorbic acid and chromophoric solution of potassium iodide in the liquid supernatant to perform developing; and measuring and calculating the digested amount of colloidal bismuth pectin in bismuth by using a spectrophotometric method. By the method for detecting the dissolution rate of the colloidal bismuth pectin preparation, the dissolution curve and the dissolution rate of colloidal bismuth pectin in the colloidal bismuth pectin preparation can be measured well, and requirements on quality control are met.
Owner:SHANXI ZHENDONG ANXIN BIOLOGICAL PHARM CO LTD
Preparation method of medical micromolecular pectin
ActiveCN109879985APromote dissolutionImprove extraction efficiencyOrganic active ingredientsAlcoholBismuth / pectin
The invention discloses a preparation method of medical micromolecular pectin. The method comprises the following steps of pretreatment, extraction, separation, purification and drying. The cellulaseand nitric acid are used for treatment; the extraction time is about 2 hours. Compared with a conventional acidolysis method, the preparation method has the advantages that the extraction efficiency can be greatly improved; the pectin molecules can be more dispersed through cellulase; the content of small molecule pectin in the solution is further improved; vacuum concentration and alcohol precipitation are used for purifying small molecule pectin; the purification purity is higher; the impurity content is small; the quality of the small molecule pectin is very good. The process design is reasonable; the obtained small molecule pectin has very good use effects in aspects of capsules of colloidal bismuth pectin capsules and the like.
Owner:广州市莱檬生物科技有限公司 +3
Low-moisture-absorption colloidal bismuth pectin capsule and preparation technology thereof
InactiveCN104825419AAct as a porogenFast releaseAntibacterial agentsOrganic active ingredientsAcrylic resinBismuth / pectin
The invention discloses a low-moisture-absorption colloidal bismuth pectin capsule and a preparation technology thereof. In a preparation of the low-moisture-absorption colloidal bismuth pectin capsule, acrylic resin IV is taken as a coating material and light calcium carbonate is taken as an anti-sticking agent to coat colloidal bismuth pectin to obtain a coated pellet, and the obtained coated pellet is used to fill a capsule shell. The low-moisture-absorption colloidal bismuth pectin capsule and the preparation technology have the advantages that the problems that the colloidal bismuth pectin preparation is prone to absorbing moisture and clustering and not easy to disperse uniformly are solved, therapeutic efficacy of the colloidal bismuth pectin preparation is improved, less auxiliary materials are used, the preparation technology is simple, and the low-moisture-absorption colloidal bismuth pectin capsule is easy to produce industrially in large scale.
Owner:浙江得恩德制药股份有限公司
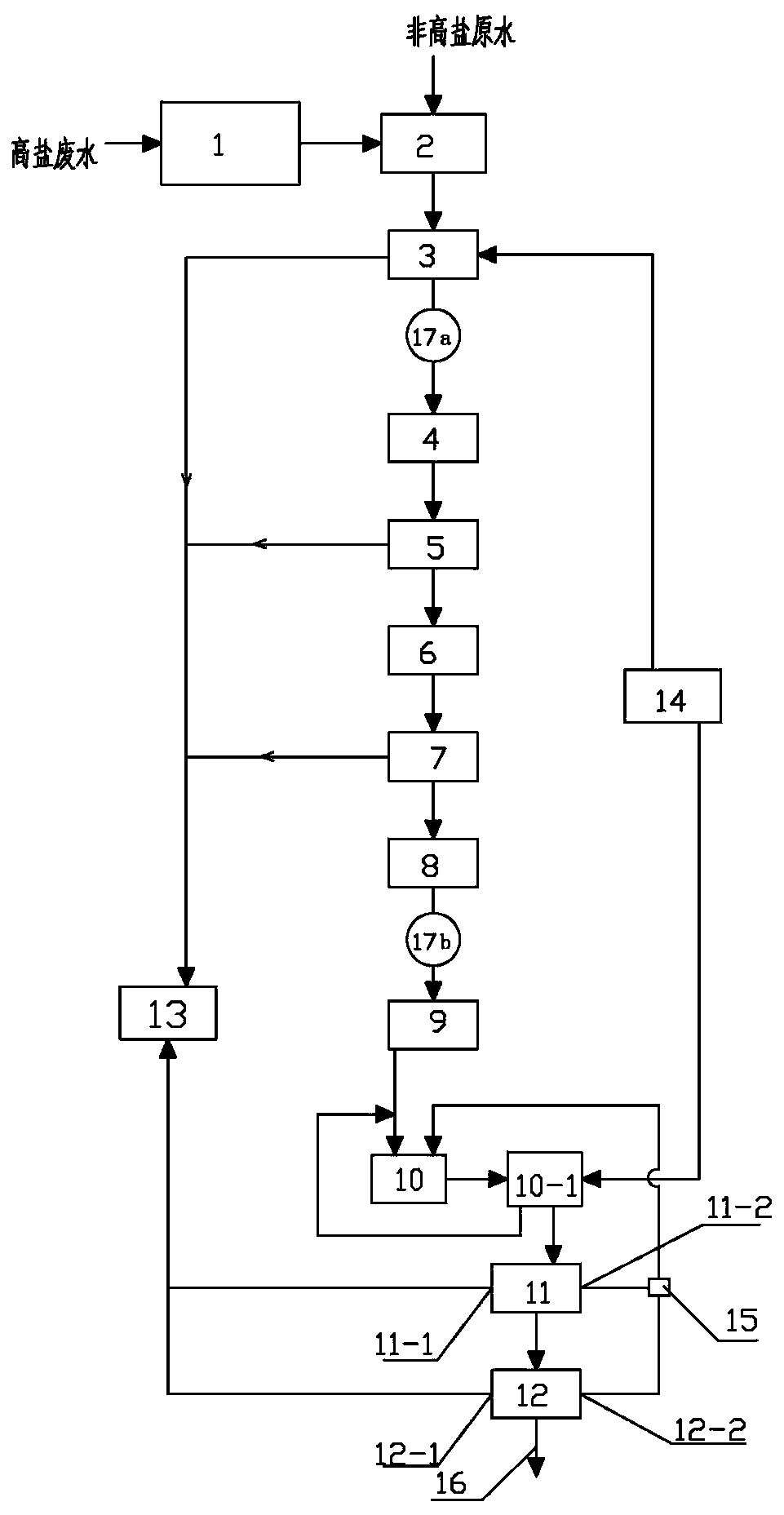
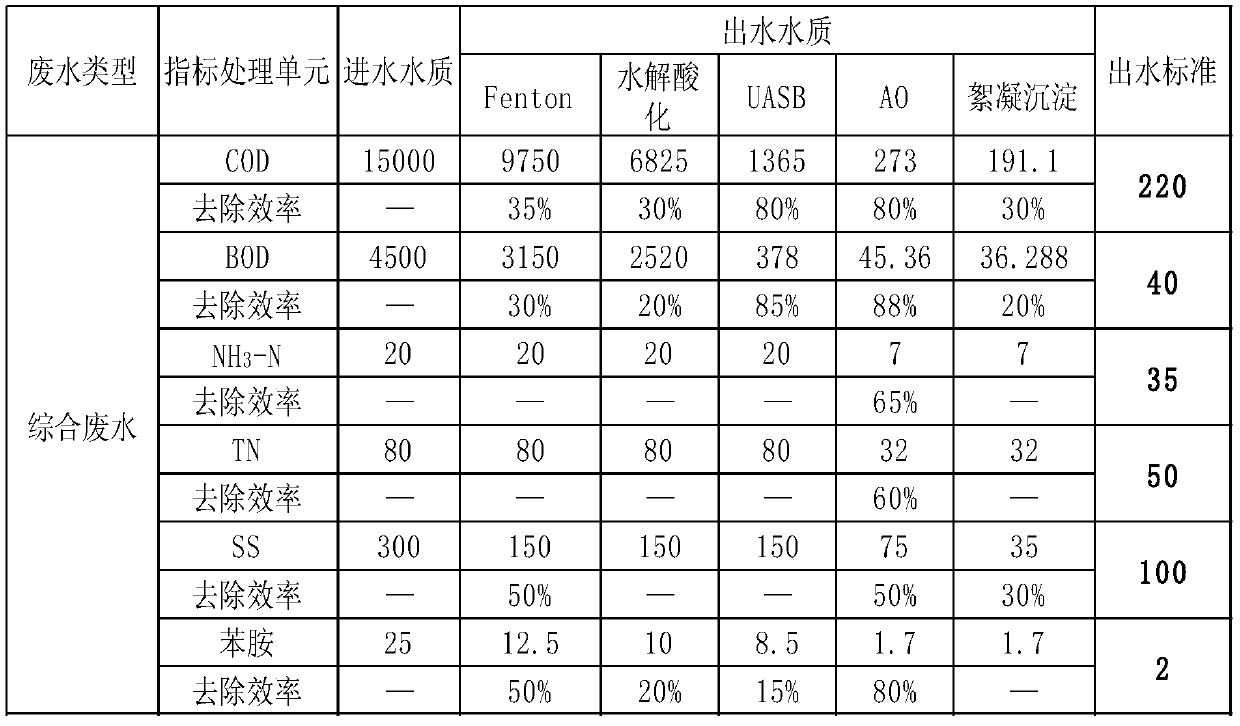
Comprehensive wastewater treatment method for bismuth pectin bulk drug, acridone acetic acid, acyclovir and thiodipropionate medical intermediate
InactiveCN111470718AReduce concentrationEfficient removalWater treatment parameter controlWater treatment compoundsPropanoic acidBismuth / pectin
The invention relates to a comprehensive wastewater treatment method for bismuth pectin bulk drugs, acridone acetic acid, acyclovir and thiodipropionate medical intermediates. The method comprises thefollowing steps: 1) performing wastewater desalination; 2) adjusting the pH of the wastewater; 3) carrying out pH acidification treatment on the wastewater; (4) carrying out Fenton reaction; 5) performing wastewater pH back-regulation treatment; 6) performing hydrolytic acidification of the wastewater; 7) performing anaerobic treatment on the wastewater; 8) performing AO reaction; 9) performing secondary precipitation; 10) flocculating and precipitating: adding 10% by mass of PAC and 0.1% by mass of PAM into a flocculation sedimentation tank; enabling the wastewater to react for 0.5 to 1h ina flocculation sedimentation tank; allowing part of sludge generated in the reaction process to flow back to the anoxic tank, enabling residual sludge to enter a sludge tank, and discharging supernatethrough a wastewater up-to-standard discharge pipeline; according to the present invention, the method has characteristics of low cost, convenient operation management, reduced wastewater pollutant concentration, and thus further reaching treatment standard, effective removing COD, BOD and NH3-N, achieving discharge standard, and providing huge social and economic benefits.
Owner:HENAN HENGAN ENVIRONMENTAL PROTECTION TECHCO LTD
Method for preparing colloidal bismuth pectin and method for controlling adhesiveness of medicine composition of colloidal bismuth pectin
ActiveCN105461823AIncrease the uniformity of bismuth ion contentObvious potential jump pointAntibacterial agentsOrganic active ingredientsBismuth / pectinColloid
The invention relates to a method for preparing colloidal bismuth pectin and a method for controlling the adhesiveness of a medicine composition of colloidal bismuth pectin. The method for preparing colloidal bismuth pectin includes the steps that firstly, a bismuth salt solution is prepared; secondly, colloidal bismuth pectin is prepared; thirdly, colloidal bismuth pectin is purified. The method for controlling the adhesiveness of a dry suspension of the medicine composition of colloidal bismuth pectin includes the step of measuring and controlling one or more indexes including the intrinsic viscosity, bismuth ion content uniformity and galacturonic acid content. Colloidal bismuth pectin prepared through the method is reasonable in galacturonic acid content, bismuth ion content, bismuth ion content uniformity and other index contents, high in intrinsic viscosity, and good in clinical effect. The treatment effect of colloidal bismuth pectin is improved, the gastritis cure rate and the Hp eradication rate of colloidal bismuth pectin are greatly increased, and the gastric mucosa protection function of colloidal bismuth pectin is greatly improved.
Owner:HUNAN WARRANT PHARMA +1
A kind of low hygroscopic colloid bismuth pectin capsule and its preparation process
InactiveCN104825419BAct as a porogenFast releaseAntibacterial agentsOrganic active ingredientsAcrylic resinBismuth / pectin
The invention discloses a colloidal bismuth pectin capsule with low hygroscopicity and a preparation process thereof. The preparation uses acrylic resin IV as a coating material and light calcium carbonate as an anti-sticking agent to coat the colloidal bismuth pectin. The obtained Coated pellets are filled with capsule shells, which greatly improves the problem of easy moisture absorption and difficult dispersion of colloidal pectin bismuth preparations in the storage process, and improves the curative effect of the drug; at the same time, there are fewer types of excipients used, and the preparation process is simple and easy for industrialization Big production.
Owner:浙江得恩德制药股份有限公司
Compound preparation for treating broiler proventriculitis
InactiveCN104771757AImprove stabilityEasy to transportAntibacterial agentsHeavy metal active ingredientsRegimenBismuth / pectin
The invention discloses a compound preparation for treating broiler proventriculitis. The compound preparation comprises 1-5wt% of a proton pump inhibitor, 5-30wt% of vitamin U, 3-15wt% of colloidal bismuth pectin and 50-91wt% of a filler. The composition of the compound preparation is screened and optimized, medicines have very good synergistic effects, and the compound preparation has the advantages of low medicine cost, simple production technology and controllable quality. The clinic effective rate of the compound preparation to proventriculitis initiated by feed replacement, moldiness and environment stress reaches above 94%, the clinic cure rate of one treatment course reaches 90%, the compound preparation has an obviously better clinic effect than the single proton pump inhibitor, the treatment course of the compound preparation is shortened to 3d from that of the proton pump inhibitor of 5-7d, the clinic effective rate increases by 6%, and the cure rate increases by 18%. The compound preparation can be used as a combined medicine for treating broiler proventriculitis initiated by virus or bacterium infection, helps broiler flocks recover stomach and intestine digestion functions, and rapidly recovers the feed intake.
Owner:SHENYANG VICA ANIMAL HUSBANDRY TECH
A kind of colloidal bismuth pectin capsule and its preparation process
ActiveCN104116721BWell mixedSmall particle sizeAntibacterial agentsOrganic active ingredientsBismuth / pectinChemistry
The invention discloses a colloidal bismuth pectin capsule and a preparation process thereof. The capsule is formed by filling content into a capsule shell, and the content contains kieselguhr of adsorbing colloidal bismuth pectin. By adopting the preparation process, the colloidal bismuth pectin and the kieselguhr are added to a ball grinder so as to be crushed, and the colloidal bismuth pectin can be evenly adsorbed on the surface of the kieselguhr or into holes after the colloidal bismuth pectin and the kieselguhr are crushed, so that the medicine is prevented from easily agglomerating in water.
Owner:桂林华信制药有限公司
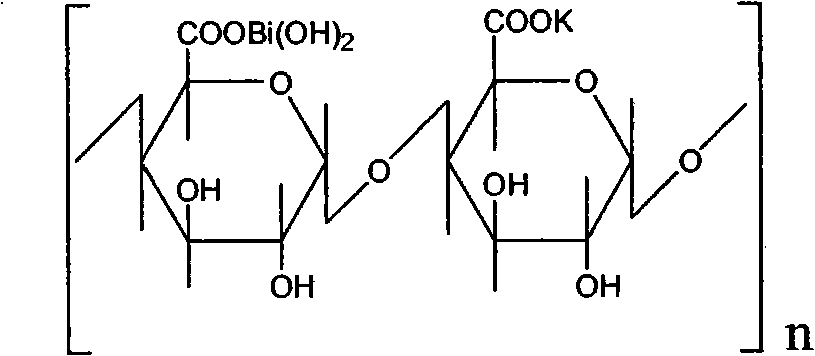
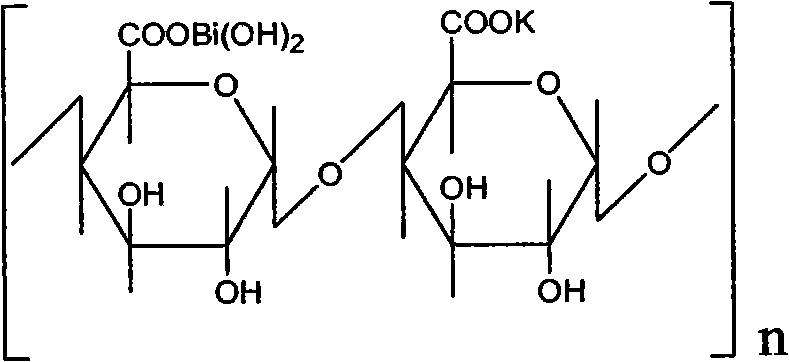
High-purity colloidal bismuth pectin compound and confirmation of structural formula, molecular formula and molecular weight thereof
InactiveCN107118285AOrganic active ingredientsInorganic active ingredientsBismuth / pectinStructural formula
The invention discloses a preparation method of a high-purity colloidal bismuth pectin compound, confirmation of a chemical structural formula, a molecular formula and the molecular weight thereof, and an application technology. The compound is different from a compound prepared by a colloidal bismuth pectin preparation method (patent) disclosed and implemented previously. According to the original patent process, due to reasons of raw materials in an intermediate liquid pectin and a bismuth salt solution for the production of colloidal bismuth pectin and the preparation method thereof, the product contains lots of organic impurities (sorbitol, pectin, etc.) and inorganic impurities (nitrate and bismuth hydroxide), purity of the product is low (only 80%), quality standard level is low, and curative effect is poor. The invention provides several preparation methods for preparation of a high-purity (98%) colloidal bismuth pectin compound, including five optimization methods. Through spectrometry and analysis, the structural formula, the molecular formula and the molecular weight of the compound are determined. A novel medicinal preparation prepared from the high-purity compound has obvious clinical advantages.
Owner:于学敏
Dispersion preparation containing colloidal bismuth pectin and preparing method therefor
InactiveUS20170095507A1Delayed disintegration timeEffective dispersionOrganic active ingredientsHeavy metal active ingredientsBismuth / pectinColloid
A dispersion preparation containing colloidal bismuth pectin is provided. 1 g is adopted as a unit for the preparation. Each unit of the preparation includes: 44.0 to 900.0 mg of colloidal bismuth pectin, 1.0-500 mg of a penetration enhancer, 2.0-312.5 mg of acid-source pore forming agent, 2.0-250.0 mg of alkali-source pore forming agent and 1.0-400.0 mg of a disintegrating agent.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Pharmaceutical composition for treating helicobacter pylori infection and application of pharmaceutical composition
ActiveCN106729719AReduce usageReduced responseAntibacterial agentsDigestive systemBismuth / pectinColloidal bismuth subcitrate
The invention discloses a pharmaceutical composition for treating helicobacter pylori infection and application of the pharmaceutical composition. The pharmaceutical composition is prepared from, by weight, 7.5-40 parts of proton pump inhibitor, 150-1000 parts of antibiotic, 220-300 parts of bismuth agent and 60-140 parts of volatile extracts, wherein the proton pump inhibitor is selected from one of omeprazole, lansoprazole, esomeprazole, pantoprazole and rabeprazole; the antibiotic is selected from one of amoxicillin, clarithromycin, levofloxacin, metronidazole, furazolidone, tetracycline and minocycline; the bismuth agent is selected from one of bismuth potassium citrate, colloidal bismuth subcitrate and colloidal bismuth pectin; the volatile extracts are volatile components extracted from chenopodium ambrosioides and twigs and leaves of Pilular Adina. By means of the pharmaceutical composition, the eradication rate of helicobacter pylori can be improved, the effective rate is increased, and the drug resistance rate and the rate of adverse reactions are reduced.
Owner:DONGZHIMEN HOSPITAL OF BEIJING UNIV OF CHINESE MEDICINE
Colloidal bismuth pectin capsule preparation and preparation method
InactiveCN105616382AUniform concentrationImprove dispersion uniformityAntibacterial agentsOrganic active ingredientsBismuth / pectinColloid
The invention provides a colloidal bismuth pectin capsule preparation and a preparation process therefor. A capsule shell of the capsule is a hollow capsule shell containing hydroxypropyl starch. The capsule has good dispersion uniformity and uniform drug concentration in a gastric acid environment, and the preparation process is simple.
Owner:王萍
Traditional Chinese medicinal compound colloidal bismuth pectin capsule for treating peptic ulcer and preparation method thereof
InactiveCN105998050AGood dispersionLow hygroscopicityAntibacterial agentsInorganic active ingredientsDispersityBismuth / pectin
The invention discloses a traditional Chinese medicinal compound colloidal bismuth pectin capsule for treating a peptic ulcer and a preparation method thereof. The traditional Chinese medicinal compound colloidal bismuth pectin capsule for treating the peptic ulcer is prepared from the following raw materials in parts by weight: 35-45 parts of colloidal bismuth pectin (metered with the weight of bismuth), 15-25 parts of an acid inhibitor, 50-150 parts of an antibacterial agent, 20-40 parts of ganoderan extracts, 8-15 parts of hydroxypropyl methyl cellulose phthalate, 25-60 parts of a disintegrating agent and 20-60 parts of an emulsifying agent, wherein the acid inhibitor is clad with permethylated-[beta]-cyclodextrin. The obtained capsule is not only good in dispersity and obvious in sustained release effect, but also improves the immunity of an organism, and has an obvious restoring function for the hypoimmunity caused by western medicinal antibiotics.
Owner:ZHENGZHOU SIBIAN TECH CO LTD
Compound oral liquid for treating children diarrhea and preparation method thereof
InactiveCN105920606AImprove the environmentPromote digestion and absorptionHeavy metal active ingredientsDispersion deliverySodium lactateSide effect
The invention discloses a compound oral liquid for treating children diarrhea and a preparation method thereof. The compound oral liquid for treating the children diarrhea comprises the following components of Nutrilite Double powder, dideoxyinosine, gastrodia elata polypeptide, disaccharidase, ribavirin, racecadotril, bismuth pectin, hydrotalcite, dry yeast, probiotics, potassium chloride, sodium chloride, sodium lactate, calcium gluconate, corrigent, emulsifying agent, buffer liquid, and antioxidant. The compound oral liquid for treating the children diarrhea has the advantages that the environment of children gastrointestinal tracts is improved, the effective microbial community is increased, the digestion and adsorbing of gastrointestinal tract are promoted, the water-electrolyte metabolic disorder is prevented, and the body immunity is improved; the cost is low, the toxic or side effect is little, the compound oral liquid can be used for a long time, and the children diarrhea can be prevented and thoroughly cured after long-time administration.
Owner:钟志敏
Dropping pills containing colloidal bismuth pectin
Owner:CP PHARMA QINGDAO CO LTD
Pharmaceutical composition for treating gastric ulcer
The invention provides a pharmaceutical composition for treating gastric ulcer. The pharmaceutical composition comprises colloidal bismuth pectin and omeprazole, wherein colloidal bismuth pectin and omeprazole have synergistic treatment effects.
Owner:CP PHARMA QINGDAO CO LTD
Detection method of viscosity of colloidal bismuth pectin or colloidal bismuth pectin containing preparation
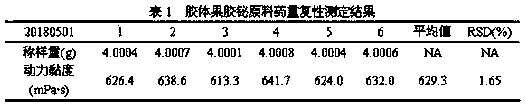
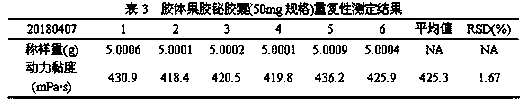
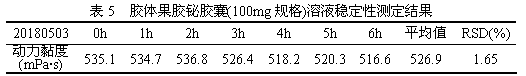
The invention discloses a detection method of viscosity of colloidal bismuth pectin or a colloidal bismuth pectin containing preparation. The colloidal bismuth pectin or the colloidal bismuth pectin containing preparation is dispersed with a proper amount of water and added with artificial gastric juice to form stable sol with high viscosity, and the dynamic viscosity of the sol is measured according to a third-method rotary viscosimeter measurement method of the 0633 viscosity measurement method in the four-part general rule of (the Chinese pharmacopoeia) 2015. The method in the invention issimple, convenient and rapid, and accurate in result, the dynamic viscosity is measured in the human body gastrointestinal environment, so that the viscosity situation of the colloidal bismuth pectinin a human body can be better embodied so as to reflect the colloid characteristics of the colloidal bismuth pectin or the colloidal bismuth pectin containing preparation.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Quality control method of colloidal bismuth pectin pharmaceutical composition
ActiveCN105572126AInhibit side effectsImprove securityPowder deliveryOrganic active ingredientsIon contentBismuth / pectin
The invention relates to a quality control method of a colloidal bismuth pectin pharmaceutical composition. The pharmaceutical composition is made into dry suspension and is made of the following components in parts by weight: 130 to 160 parts of bulk drug namely bismuth pectin (calculated by bismuth), 420 to 460 parts of filling agent namely mannitol, and 10 to 20 parts of flocculating agent namely disodium hydrogen phosphate and / or flavoring agent. The galacturonic acid transfer rate of the pharmaceutical composition is 96 to 102%. The content of galacturonic acid is not less than 0.4 gram for each 1.46 grams of dry suspension of colloidal bismuth pectin. The intrinsic viscosity number is not less than 1000; the bismuth ion content and uniformity of the pharmaceutical composition are controlled to improve the stability and treatment effect of dry suspension of colloidal bismuth pectin. The technology advancement of pharmaceutical industry is promoted, and the industry of quality control of colloidal bismuth pectin drugs is promoted.
Owner:HUNAN WARRANT PHARMA +1
Control method for quality and safety of colloidal bismuth pectin pharmaceutical composition
ActiveCN105496968AGuaranteed clinical efficacyProduct quality standards are scientific, reasonable and controllableAntibacterial agentsOrganic active ingredientsFiller ExcipientBismuth / pectin
The invention relates to a control method for quality and safety of a colloidal bismuth pectin pharmaceutical composition. The pharmaceutical composition is in a dry suspension dosage form and is prepared from, by weight, 130-160 parts of colloidal bismuth pectin (according to bismuth) serving as the bulk pharmaceutical chemical, 420-460 parts of mannitol serving as filler and 10-20 parts of disodium hydrogen phosphate serving as flocculant and / or corrigent. The pharmaceutical composition is characterized in that the intrinsic viscosity of the pharmaceutical composition is controlled, the pharmaceutical stability and the treatment effect of the colloidal bismuth pectin dry suspension are improved, a significant impact will be imposed on improving technical progress of the product and the pharmaceutical industry, and the pharmaceutical composition will achieve obvious technical progress and promotion effects in the quality control field of the colloidal bismuth pectin.
Owner:HUNAN WARRANT PHARM TECH DEV CO LTD
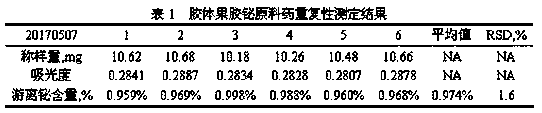
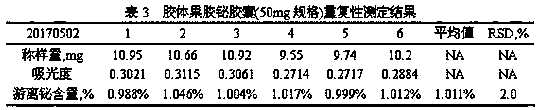
Method for detecting free bismuth in colloidal bismuth pectin or preparation containing colloidal bismuth pectin
ActiveCN109991184AAvoid product qualityStrong specificityChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorBismuth / pectinLinear relationship
The invention provides a method for detecting free bismuth in colloidal bismuth pectin or a preparation containing colloidal bismuth pectin. The method includes the following steps of: placing colloidal bismuth pectin or the preparation containing colloidal bismuth pectin in a plastic centrifuge tube, adding water to shake for no more than 1min, obtaining a colloidal solution with uniform dissolution, centrifuging the colloidal solution immediately, separating the supernatant, measuring the content of bismuth in the supernatant by complexometric titration or ultraviolet spectrophotometry, andcalculating the content of free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin. The method for detecting free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin, established by the invention, has the advantages of strong specificity, high accuracy, good repeatability, good linear relationship and high sensitivity,and can be used as a mass control method for free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin to effectively control the quality of products of colloidalbismuth pectin or the preparation thereof.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Colloidal bismuth pectin capsule containing galangal extract and preparation method of colloidal bismuth pectin capsule
InactiveCN105833153AUniform appearanceComplete appearanceAntibacterial agentsOrganic active ingredientsBismuth / pectinImmunocompetence
The invention discloses a colloidal bismuth pectin capsule containing a galangal extract and a preparation method of the colloidal bismuth pectin capsule. The colloidal bismuth pectin capsule containing the galangal extract is prepared from the following raw materials in parts by weight: 30 to 50 parts of colloidal bismuth pectin according to bismuth, 4 to 10 parts of the galangal extract, 80 to 160 parts of a filler, 7 to 11 parts of a disintegrating agent, 2 to 4 parts of talcum powder, 2 to 6 parts of an adhesive, 0.2 to 2.8 parts of a lubricating agent and 4 to 8 parts of gastric solubility opadry powder. The raw materials of the colloidal bismuth pectin capsule are reasonable in formula, and the prepared colloidal bismuth pectin capsule has a uniform and complete appearance; from protection of gastric mucosa, improvement of the immunity of a human body and improvement of the antibacterial ability of the human body, effects of quickly relieving gastric ulcer diseases and fundamentally curing gastric ulcer.
Owner:ZHENGZHOU SIBIAN TECH CO LTD
Streptococcus lactis enteric-coated tablets inhibiting helicobacter pylori (HP) and preparation method thereof
InactiveCN110075270AImprove immunityAntibacterial agentsOrganic active ingredientsPolythylene glycolSpirulina sp.
The invention provides streptococcus lactis enteric-coated tablets inhibiting helicobacter pylori (HP) and a preparation method thereof. The streptococcus lactis enteric-coated tablets are prepared from streptococcus lactis, colloidal bismuth pectin, furazolidone, spirulina, vitamin U, soybean lecithin, diethyl phthalate, polyacrylic acid resin No. 2, Tween-80, castor oil, polyethylene glycol-6000, xanthan gum, talc and starch. According to a formula, the streptococcus lactis is used as a main active ingredient for treating the helicobacter pylori; the spirulina is suitable for mucosa repair,regeneration and normal secretion of intestinal ulceration formed by helicobacter pylori pathogens; the soybean lecithin has the effects of nourishing the stomach, sterilizing and repairing gastric mucosae; the colloidal bismuth pectin has the effects of isolating gastric acid, protecting injured mucosae and killing the helicobacter pylori. The streptococcus lactis enteric-coated tablets providedby the invention achieves significant inhibiting and infiltrating effects for the treatment of the helicobacter pylori; meanwhile, the mucosal injury and ulceration of the gastrointestinal tract caused by the helicobacter pylori can be alleviated and repaired.
Owner:伟日(山东)生物科技有限公司
Method for measuring free bismuth in colloidal bismuth pectin or preparation containing colloidal bismuth pectin
ActiveCN110146500AGood reproducibilityStrong specificityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsSolubilityBismuth / pectin
The invention provides a method for measuring free bismuth in colloidal bismuth pectin or a preparation containing colloidal bismuth pectin. The method comprises the following steps of: adding ethanolwater solution with the volume concentration of 7-20% into the colloidal bismuth pectin or the preparation containing the colloidal bismuth pectin, and shaking to enable the dissolution to be uniform, or adding ethanol water solution with the volume concentration not more than 5% to shake and disperse, then adding ethanol until the volume concentration of the ethanol in the dispersion liquid is 30-50%, and shaking to enable the dissolution to be uniform to obtain colloidal solution; centrifuging to obtain supernatant; measuring by a complexometric titration method or an ultraviolet spectrophotometry method; and calculating the content of free bismuth in the colloidal bismuth pectin or the preparation containing the colloidal bismuth pectin. The ethanol aqueous solution not only has bettersolubility to free bismuth, but also can seal colloidal bismuth pectin molecules to a certain extent, and effectively prevent the dynamic bismuth salt release process of the colloidal bismuth pectin.According to the invention, the free bismuth is measured by dispersing the colloidal bismuth pectin with the ethanol aqueous solution, and the measuring result of the free bismuth is more accurate and reliable.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com