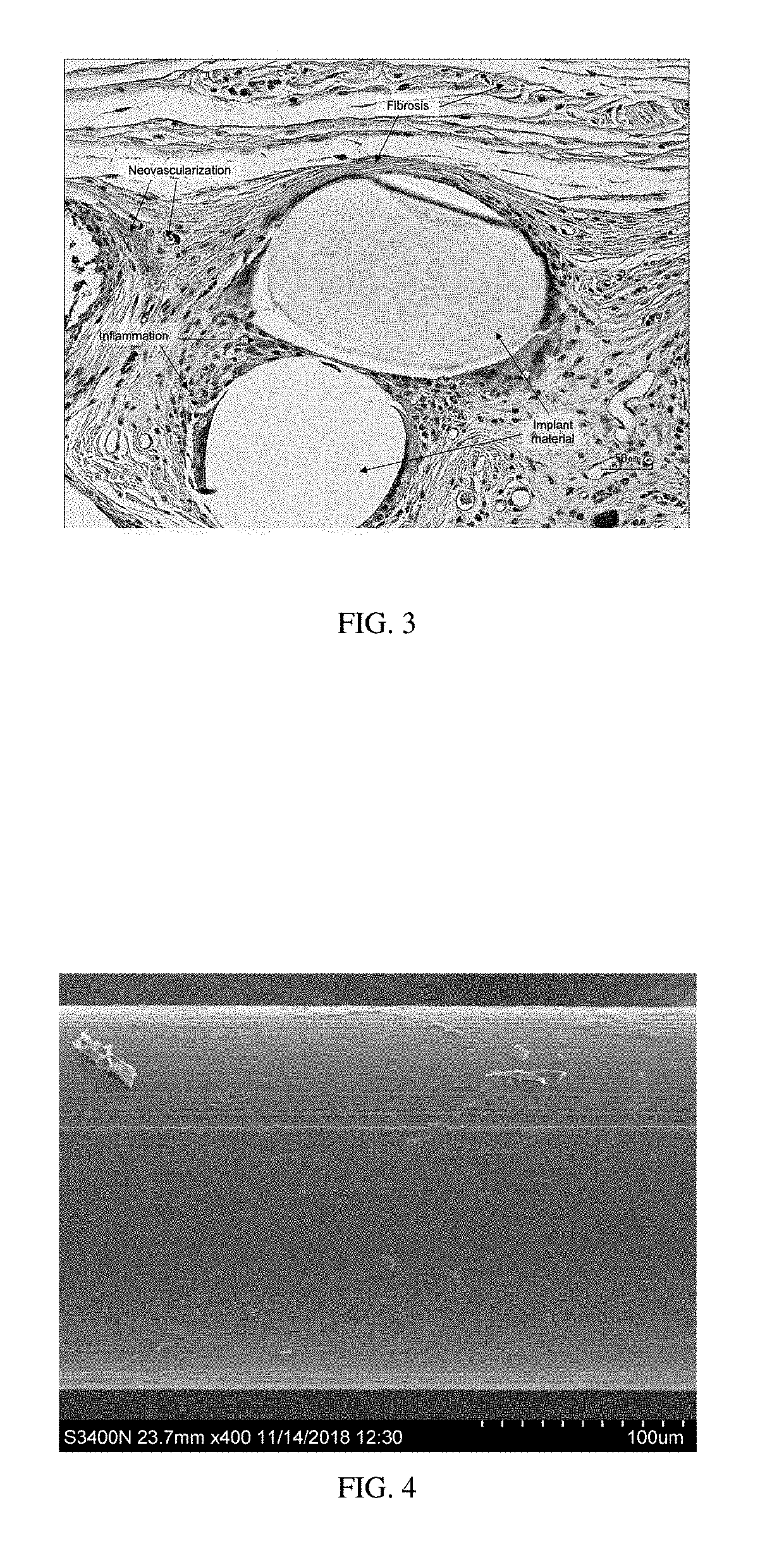
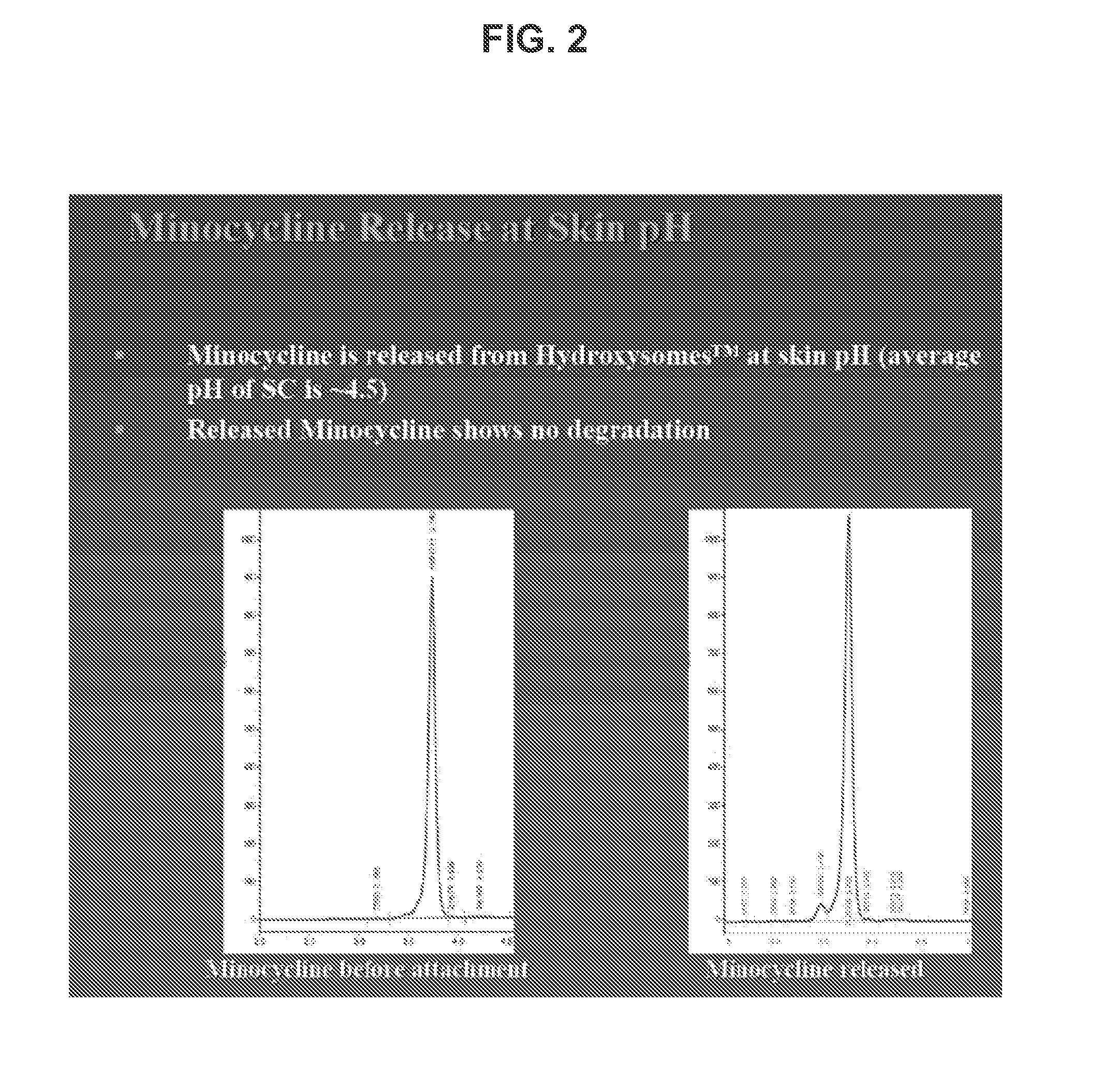
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
162 results about "Minocycline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
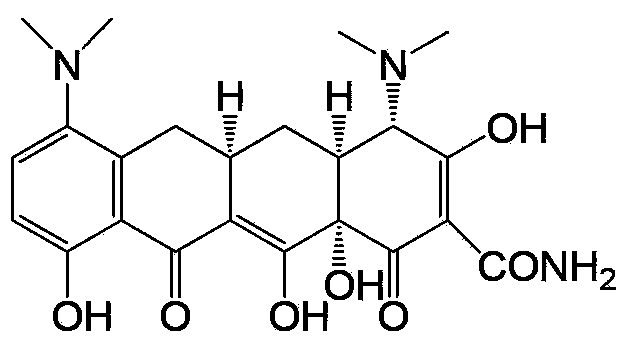
Minocycline is used to treat a wide variety of infections. It may also be used along with other medications to treat severe acne.
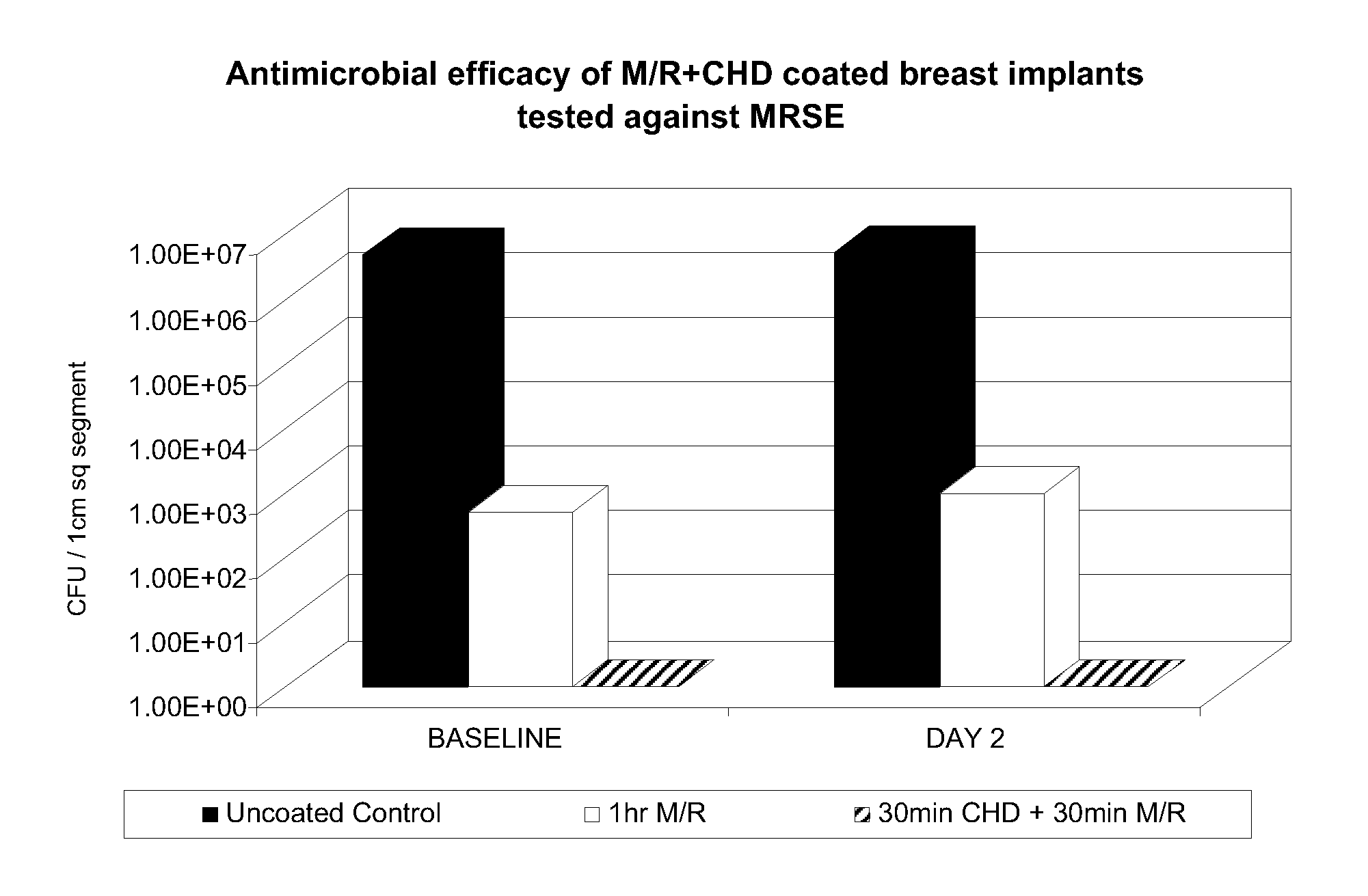
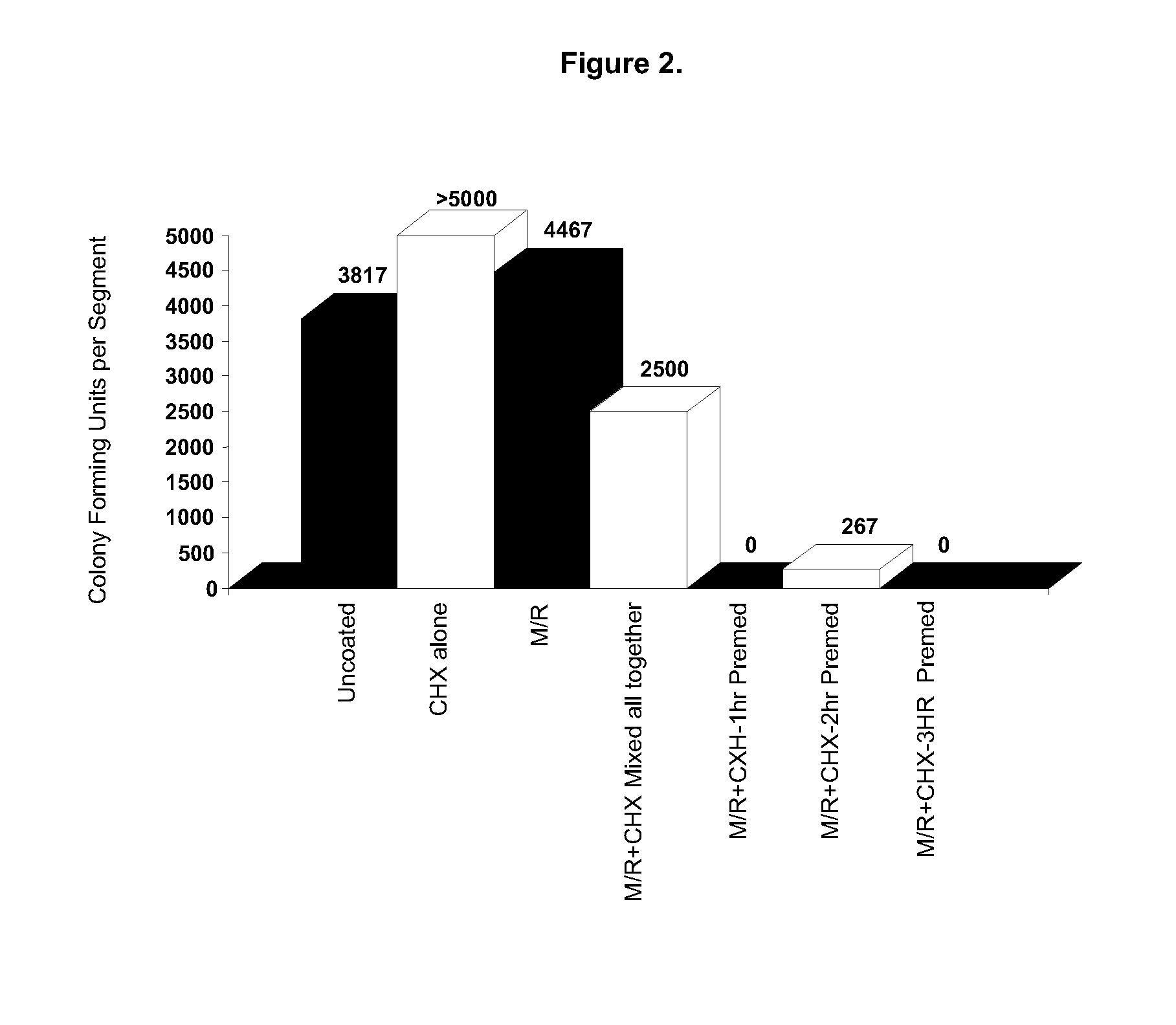
Combinations of antiseptic and antibiotic agents that inhibit the development of resistant microorganisms
InactiveUS6582719B2Toxic reductionLong-term efficacySuture equipmentsHeavy metal active ingredientsTriclosanChlorhexidine
The present invention relates to compositions comprising a combination of one or more antiseptic and an antibiotic. It is based, at least in part, on the discovery that such combinations tend to deter the formation of antibiotic-resistant organisms. In preferred, nonlimiting embodiments of the invention, the antibiotic is minocycline and the antiseptic is a chlorhexidine compound, triclosan, or benzalkonium chloride, and in particular embodiments, a silver salt or a bismuth salt is added. Examples of specific, nonlimiting embodiments of the invention include combinations of (i) minocycline, triclosan, and a bismuth salt; (ii) minocycline, a chlorhexidine compound, and a bismuth salt; and (iii) minocycline, benzalkonium chloride, and a bismuth salt. The present invention further provides for articles, such as, but not limited to, medical articles, which have been treated with or which otherwise comprise a combination of antiseptic and antibiotic.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Topical minocycline ointment for suppression of allergic skin responses
The method of the present application is directed towards a method for suppressing an allergic response in response to an allergic trigger. This method comprises the following steps; applying, topically, to an affected area an effective amount of a minocycline composition so that the minocycline composition contacts the affected area for an effective amount of time and removing the minocycline composition from the affected area.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Pharmaceutical tetracycline composition for dermatological use
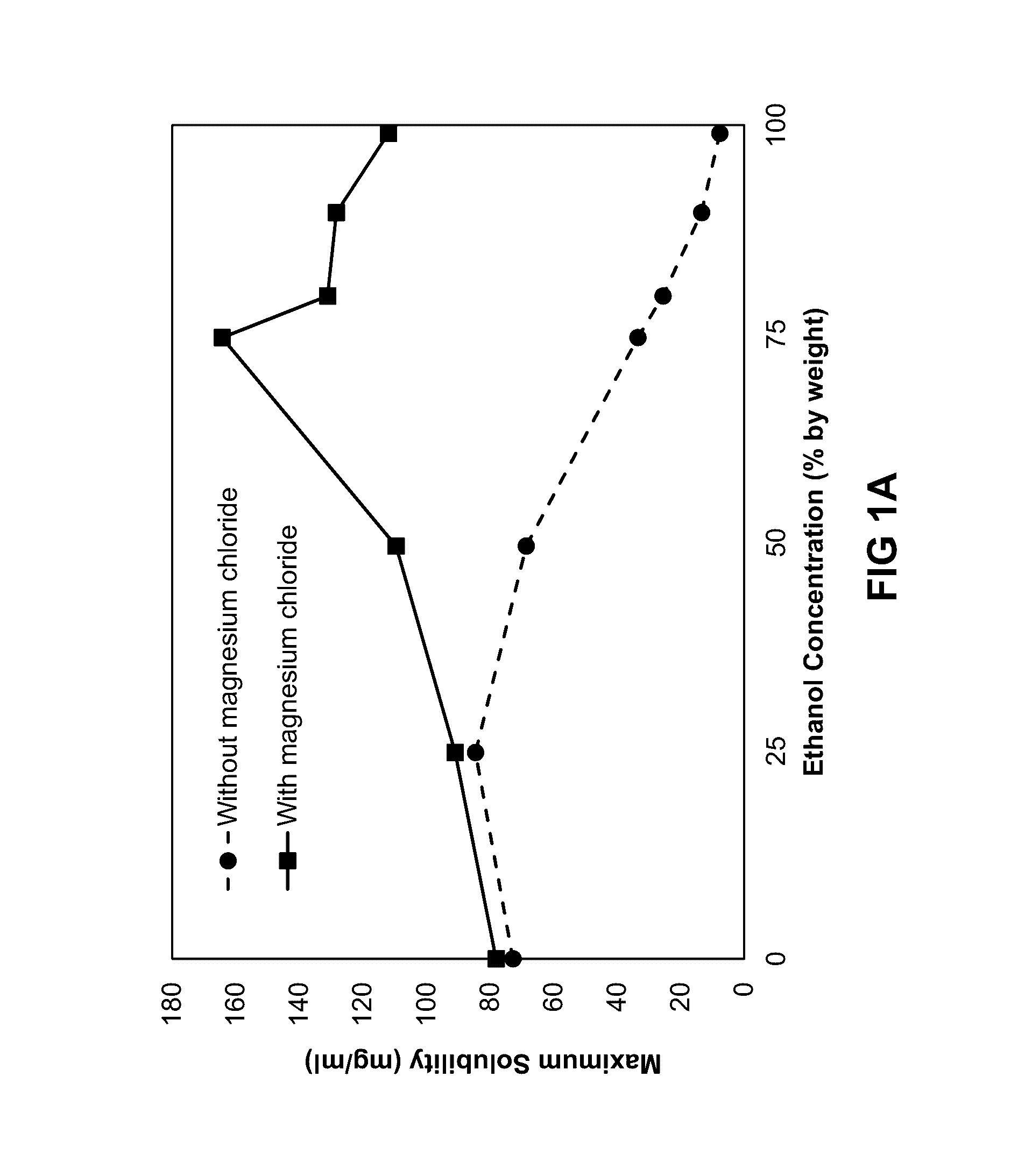
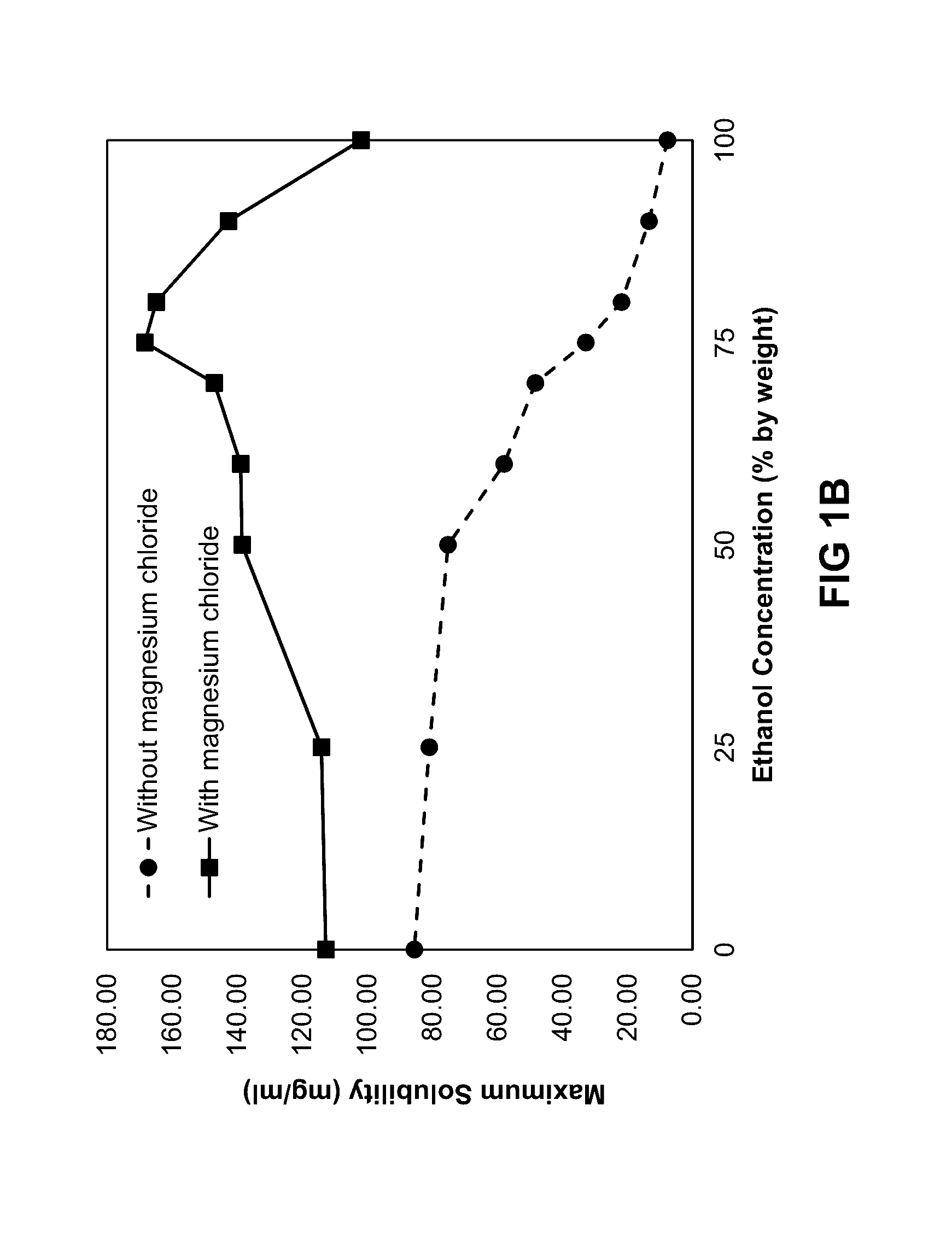
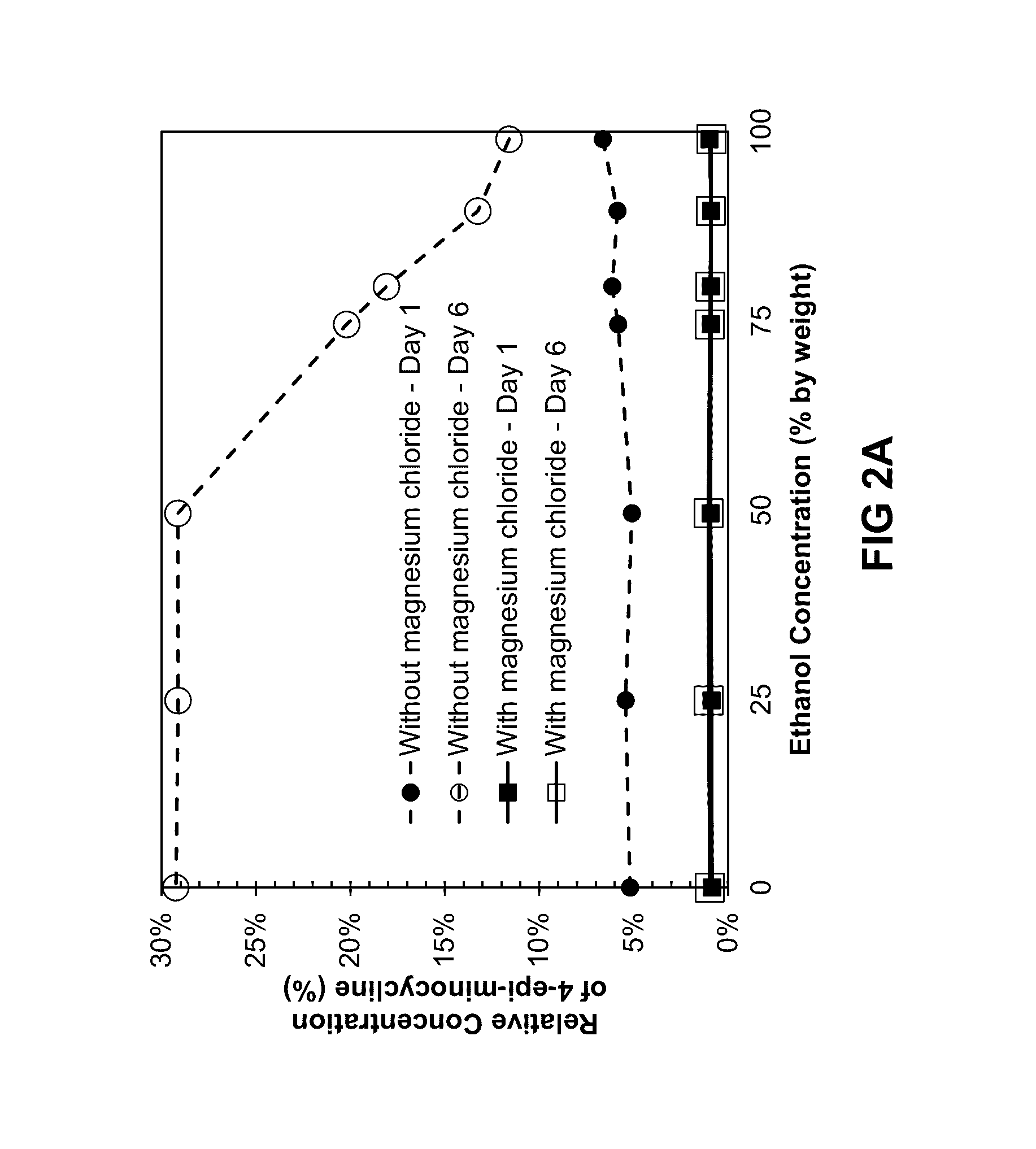
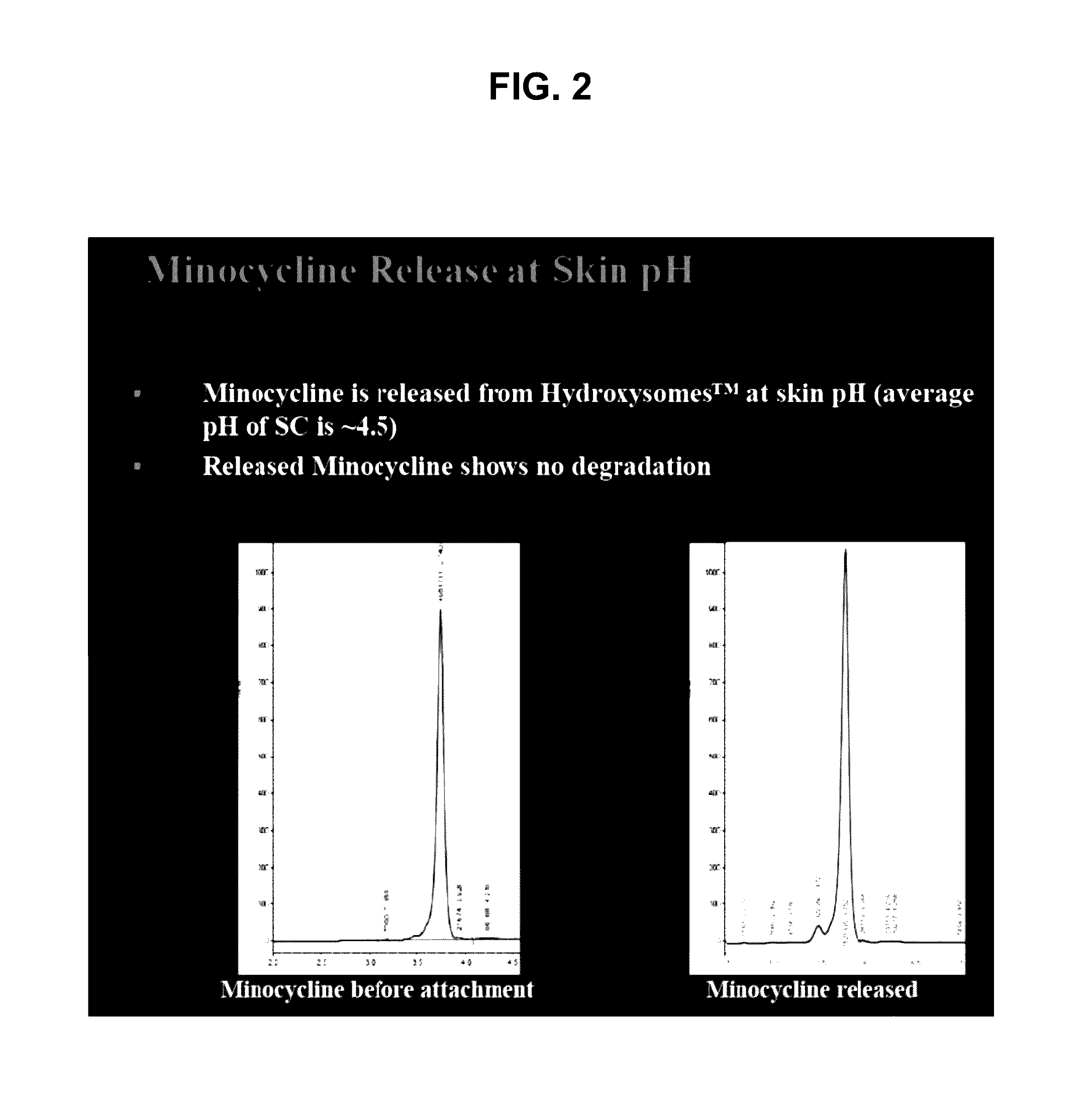
ActiveUS20160279152A1Prevent scalingAntibacterial agentsTetracycline active ingredientsMagnesium saltSolvent
Provided herein is a topical composition and related methods for making and using the composition. In a first aspect, the topical composition comprises minocycline, a magnesium salt, and a sulfite compound in a non-aqueous solvent. In yet another aspect, the topical composition comprises a tetracycline-class drug, a source of magnesium, a monohydric aliphatic alcohol, and a polyol, wherein (i) the ratio between the monohydric aliphatic alcohol and the propylene glycol is in the range of 1:1 to 99:1 by weight and (ii) the tetracycline-class drug is dissolved in the topical composition.
Owner:BIOPHARMX
Topical minocycline compositions and methods of using the same
Owner:LAB SKIN CARE INC
Tetracycline derivatives with reduced antibiotic activity and neuroprotective benefits
ActiveUS8338477B2Prevent death and loss functionBiocideNervous disorderTetracyclineBlood–brain barrier
The present disclosure is directed to compositions and methods which utilize the tetracycline scaffold, preferably the scaffold of tetracycline or minocycline, and which significantly lack antibiotic activity. The compounds have neuroprotective attributes without interfering with the drugs capacity to pass through the blood brain barrier. These compounds have neuroprotective activity because of their inhibition of neuronal cell cycle progression. The compounds are characterized in part by a fifth ring joining positions 9 and 10.
Owner:NEUMEDICS
Combinations of antiseptic and antibiotic agents containing medical devices
InactiveUS20050192547A1Toxic reductionLong efficacyBiocideDead animal preservationTriclosanChlorhexidine
The present invention relates to compositions comprising a combination of one or more antiseptic and an antibiotic. It is based, at least in part, on the discovery that such combinations tend to deter the formation of antibiotic-resistant organisms. In preferred, nonlimiting embodiments of the invention, the antibiotic is minocycline and the antiseptic is a chlorhexidine compound, triclosan, or benzalkonium chloride, and in particular embodiments, a silver salt or a bismuth salt is added. Examples of specific, nonlimiting embodiments of the invention include combinations of (i) minocycline, triclosan, and a bismuth salt; (ii) minocycline, a chlorhexidine compound, and a bismuth salt; and (iii) minocycline, benzalkonium chloride, and a bismuth salt. The present invention further provides for articles, such as, but not limited to, medical articles, which have been treated with or which otherwise comprise a combination of antiseptic and antibiotic.
Owner:MODAK SHANTA M +2
Surgial mesh implants containing poly(butylene succinate) and copolymers thereof
PendingUS20190269817A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Topical Minocycline Compositions and Methods of Using the Same
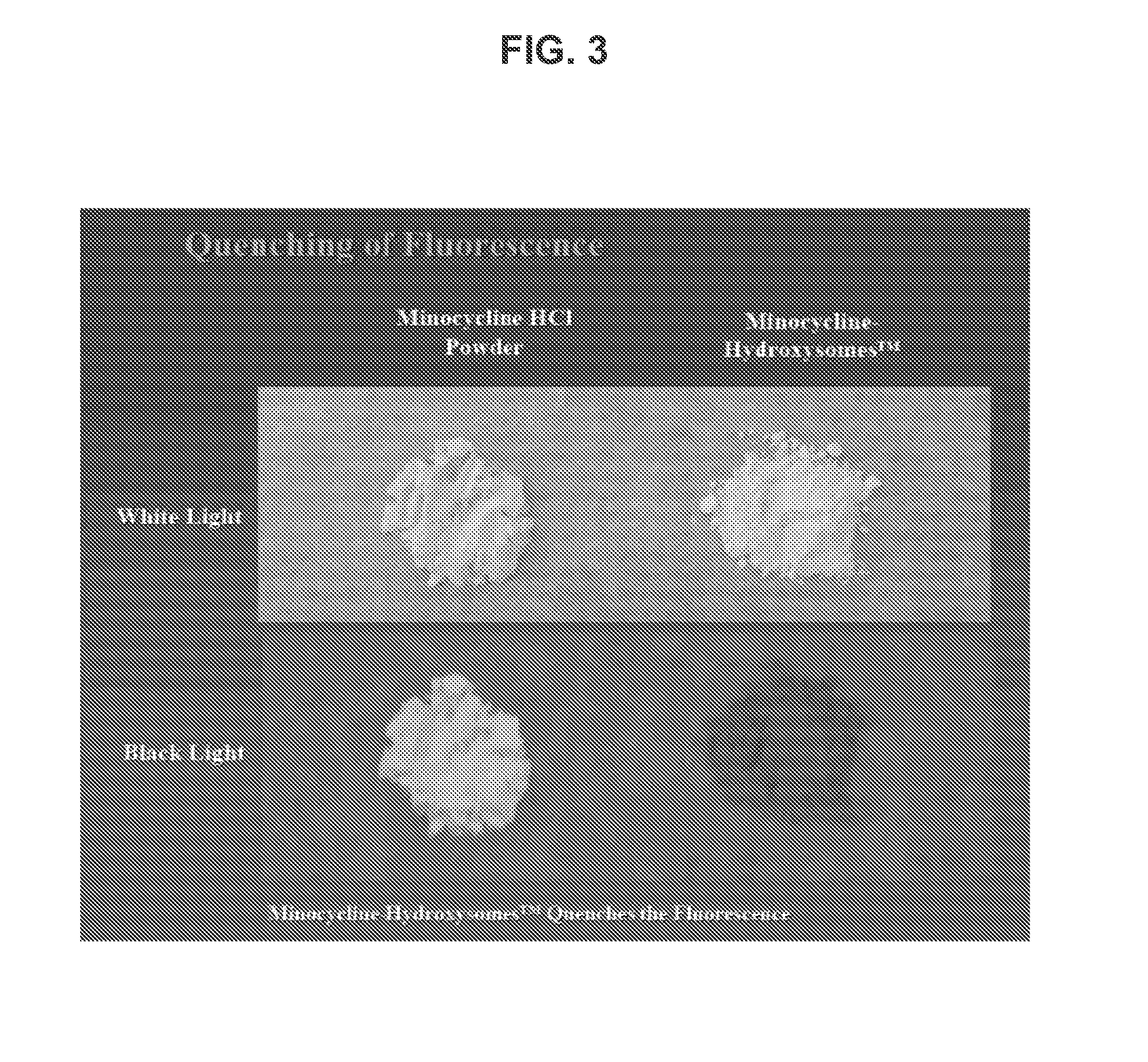
Topical minocycline compositions with reduced fluorescence are provided. In some instances, the compositions include an amount of a minocycline active agent associated with porous calcium particles. Also provided are methods of using the compositions, e.g., in the treatment of acne.
Owner:LAB SKIN CARE INC
Sterilized minocycline and rifampin-containing medical device
InactiveUS20080075628A1Maximize recoveryGood curative effectSurgeryLavatory sanitoryMedical deviceBiomedical engineering
A sterilized medical device comprising minocycline and rifampin is described. The device is setrerilized by e-beam raditation such that degradation of minocycline and rifampin is minimized. Methods for sterilizing minocycline and rifampin-containing devices by e-beam radiation and methods of manufacturing devices, which methods include e-beam sterilization, are also described.
Owner:MEDTRONIC INC
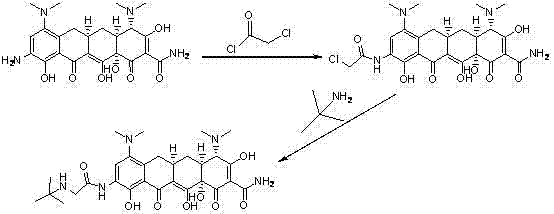
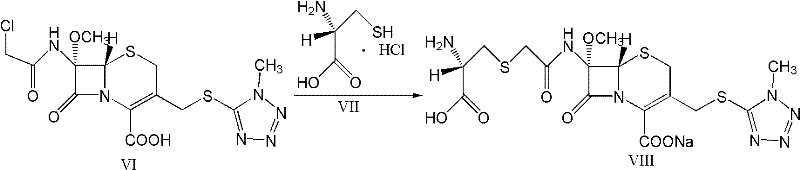
Preparation method and intermediate of minocycline
ActiveCN103387512ALow priceEasy to operateOrganic compound preparationCarboxylic acid amides preparationAlcoholPalladium
The invention discloses a preparation method and an intermediate of minocycline. A preparation method for the intermediate M-M is to subject demethylated aureomycin and dimethylamine to reactions as described in the specification in an amine solvent or amide solvent under the catalysis of a palladium complex. The preparation method for minocycline comprises the following steps: (1) subjecting demethylated aureomycin and dimethylamine to the reactions in the amine solvent or amide solvent under the catalysis of the palladium complex so as to prepare the compound M-M; and (2) subjecting the compound M-M prepared in step (1) to hydrogenation and dehydroxylation in an alcohol solvent including an acid under the catalysis of a catalyst. The preparation method provided by the invention has the advantages of easily available raw materials, low cost, simple two-step reaction operation, high product yield, good product quality, recoverability and reusability of the solvents and easy industrial production.
Owner:CHENGDU CHEMPARTNER
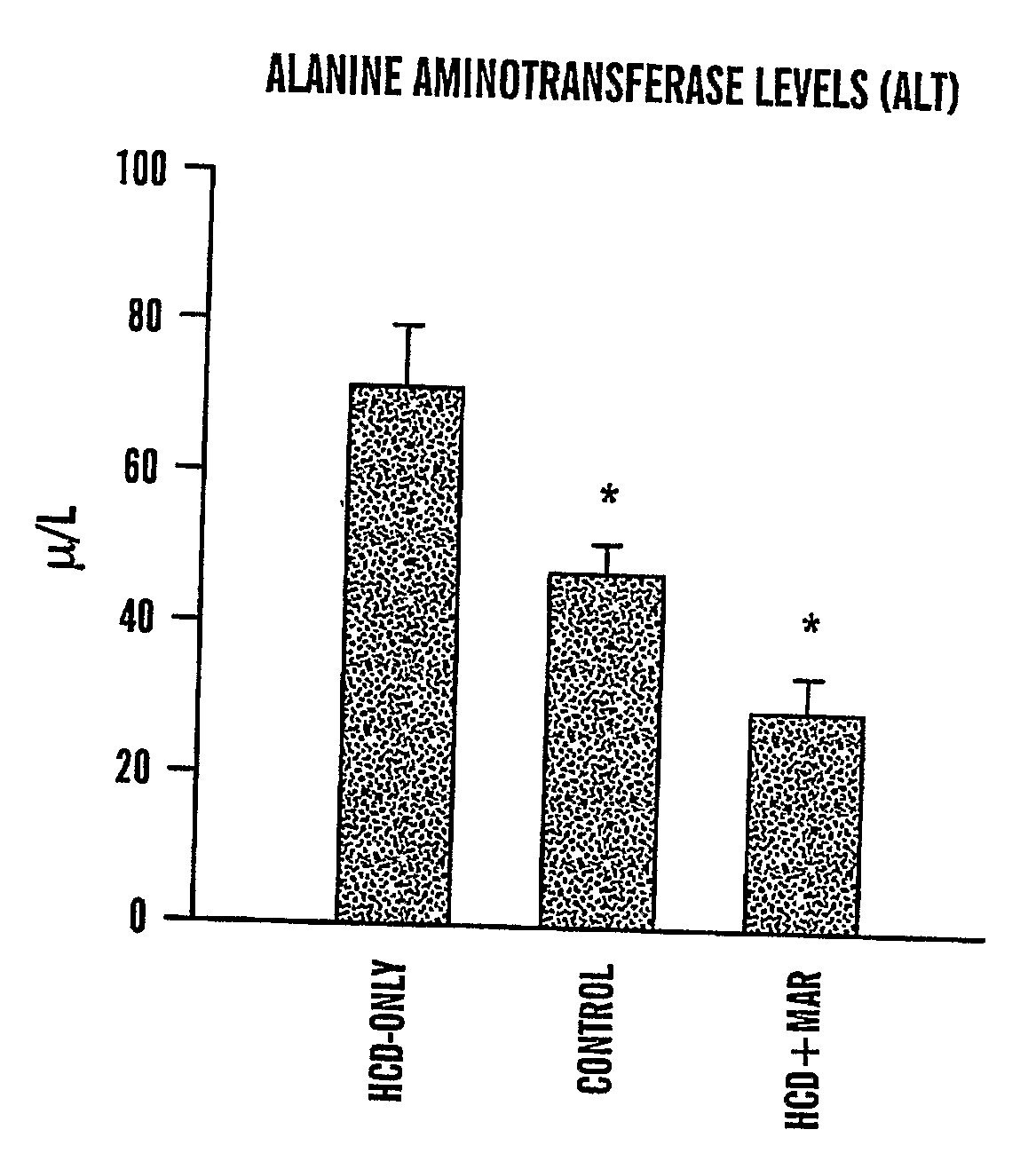
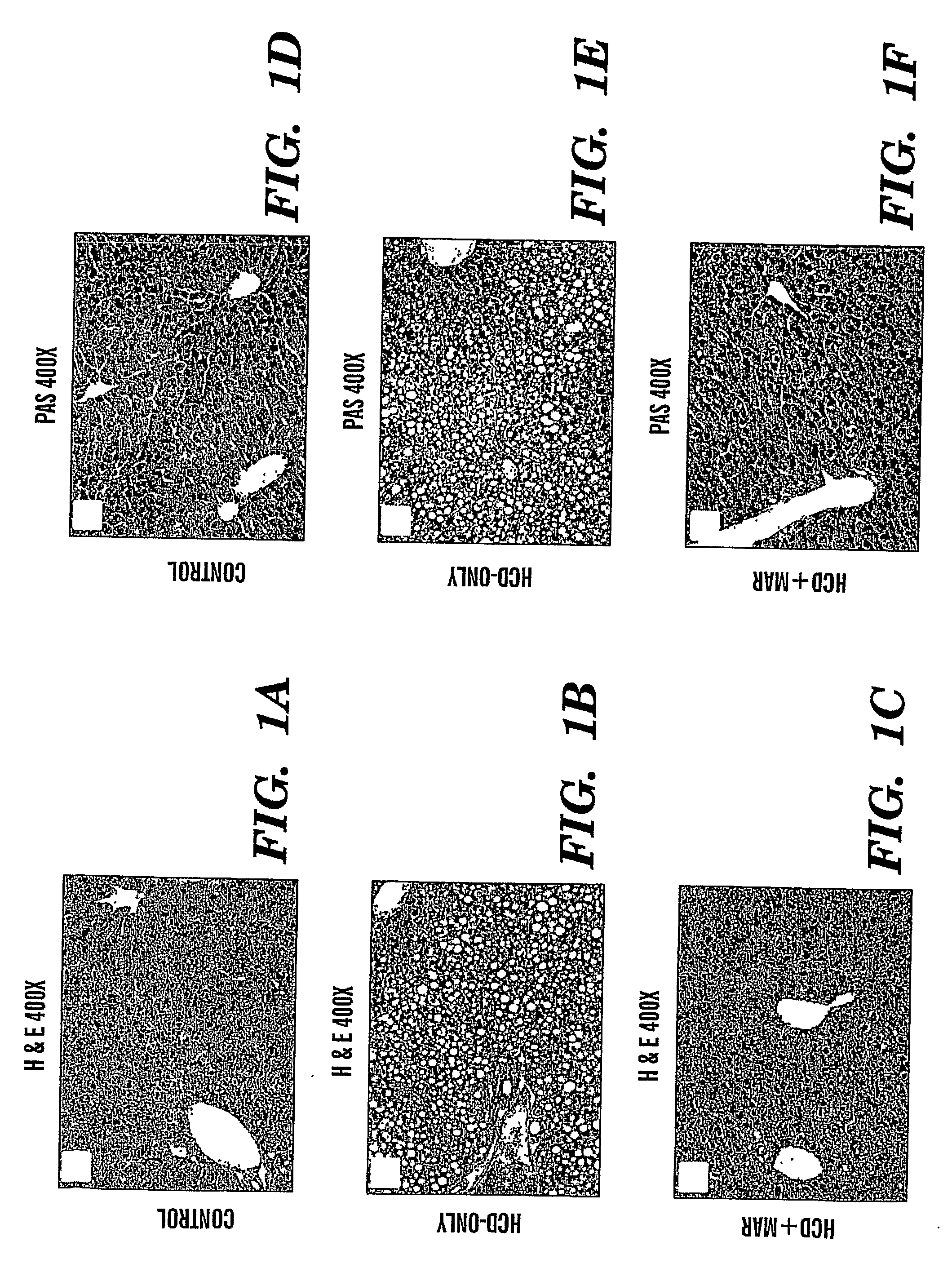
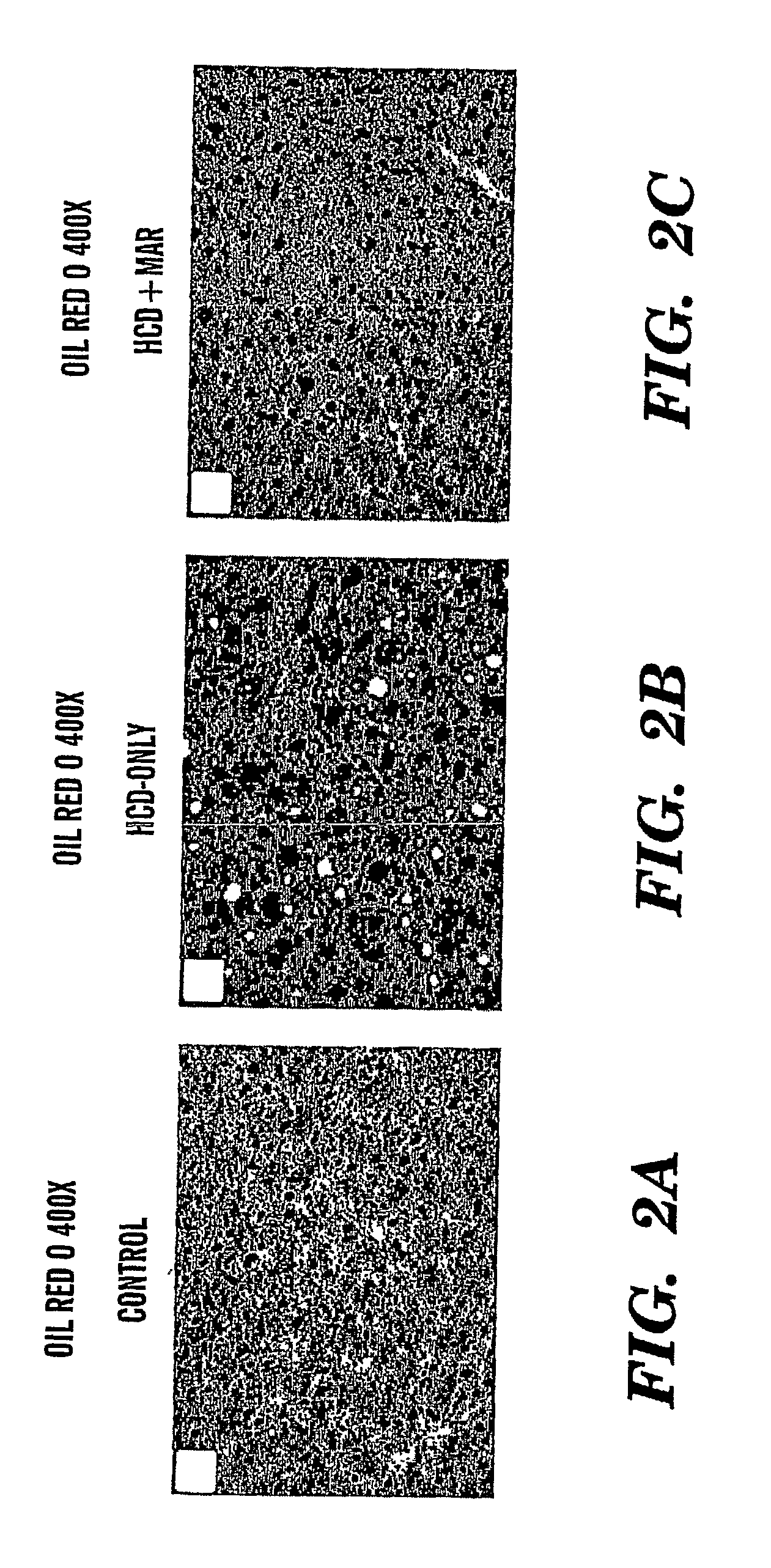
Method of treating fatty liver disease
The present invention relates to a method for treating a fatty liver disease or disorder in a patient in need thereof. The method comprises administering at least one matrix metalloproteinase (“MMP”) inhibitor to the patient. Fatty liver disease or disorders include, for example, NAFLD, NASH, ALD, fatty liver associated with chronic hepatitis infection, TPN, steroid treatment, tamoxifen treatment, gastrointestional operations, diabetes and Reye's Syndrome. The method is particularly useful when the fatty liver disease is associated with TPN and the patient is an infant or when the patient is obese. MMP inhibitors useful in the present invention include, for example, Marimastat, tetracyclines, Prinomastat, Batimastat, BAY 12-9566, AG3340, BMS-275291, Neovastat, BB-3644, KB-R7785, TIMP1, TIMP2, doxycycline, minocycline, RS-130,830; CGS 27023A, Solimastat, Ro 32-3555, BMS-272591, and D2163. Marimastat is a preferred MMP inhibitor.
Owner:CHILDRENS MEDICAL CENT CORP
Method for imparting antimicrobial activity to a medical device
A method for imparting broad spectrum antimicrobial activity to a medical device. The medical device is sequentially contacted with a first antimicrobial component, such as an antiseptic, and thereafter with a second antimicrobial component, such as a mixture of antibiotics. The first component may be a guanidium compound, such as chlorhexidine. The second component may be a mixture of a tetracycline, such as minocycline, and a rifamycin, such as rifampin.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Composition comprising minocycline as an effective component for prevention and treatment of dementia, and learning and memory impairments
InactiveUS20060148766A1Avoid cell deathAvoid cytotoxicityBiocideTetracycline active ingredientsMemory disorderBULK ACTIVE INGREDIENT
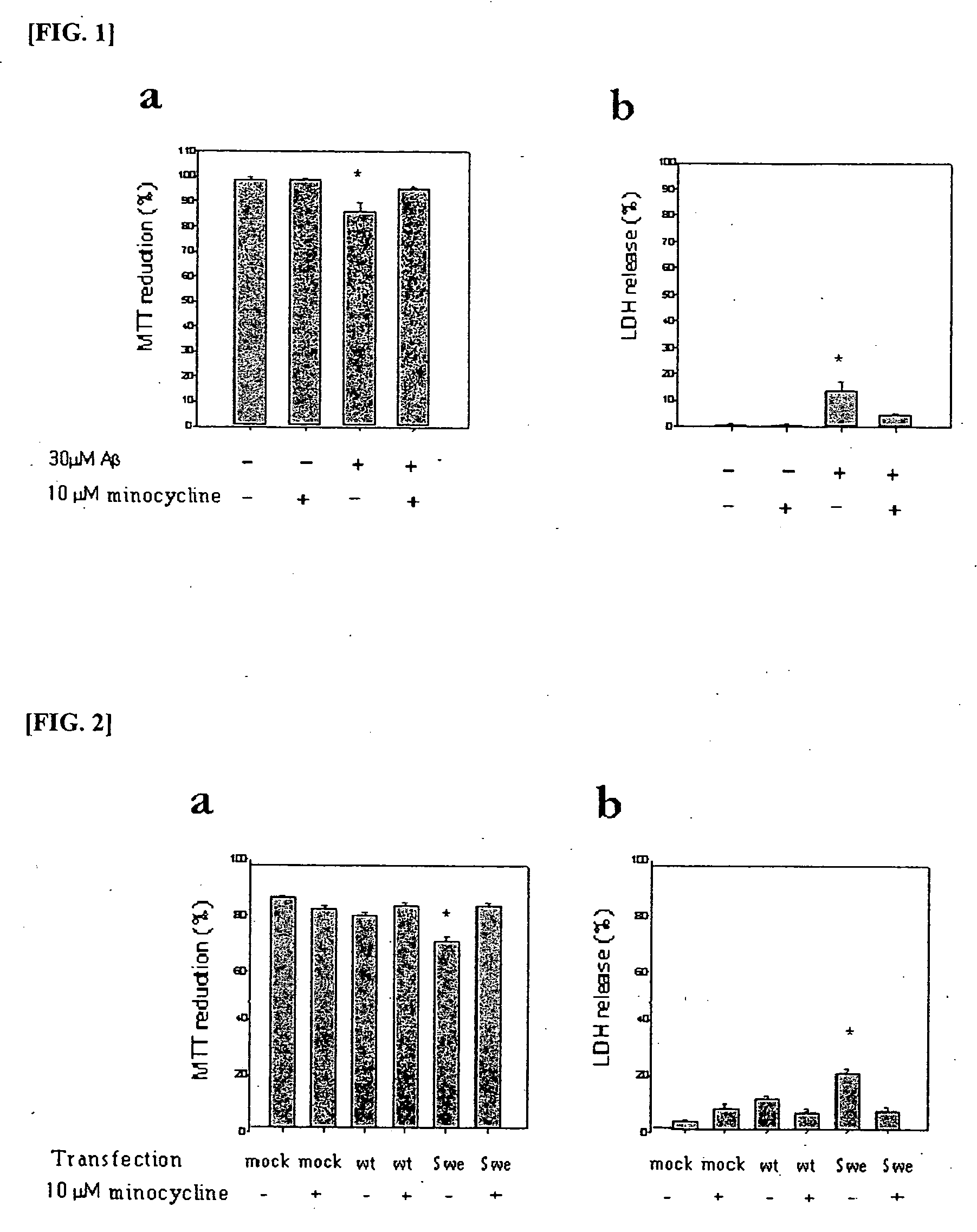
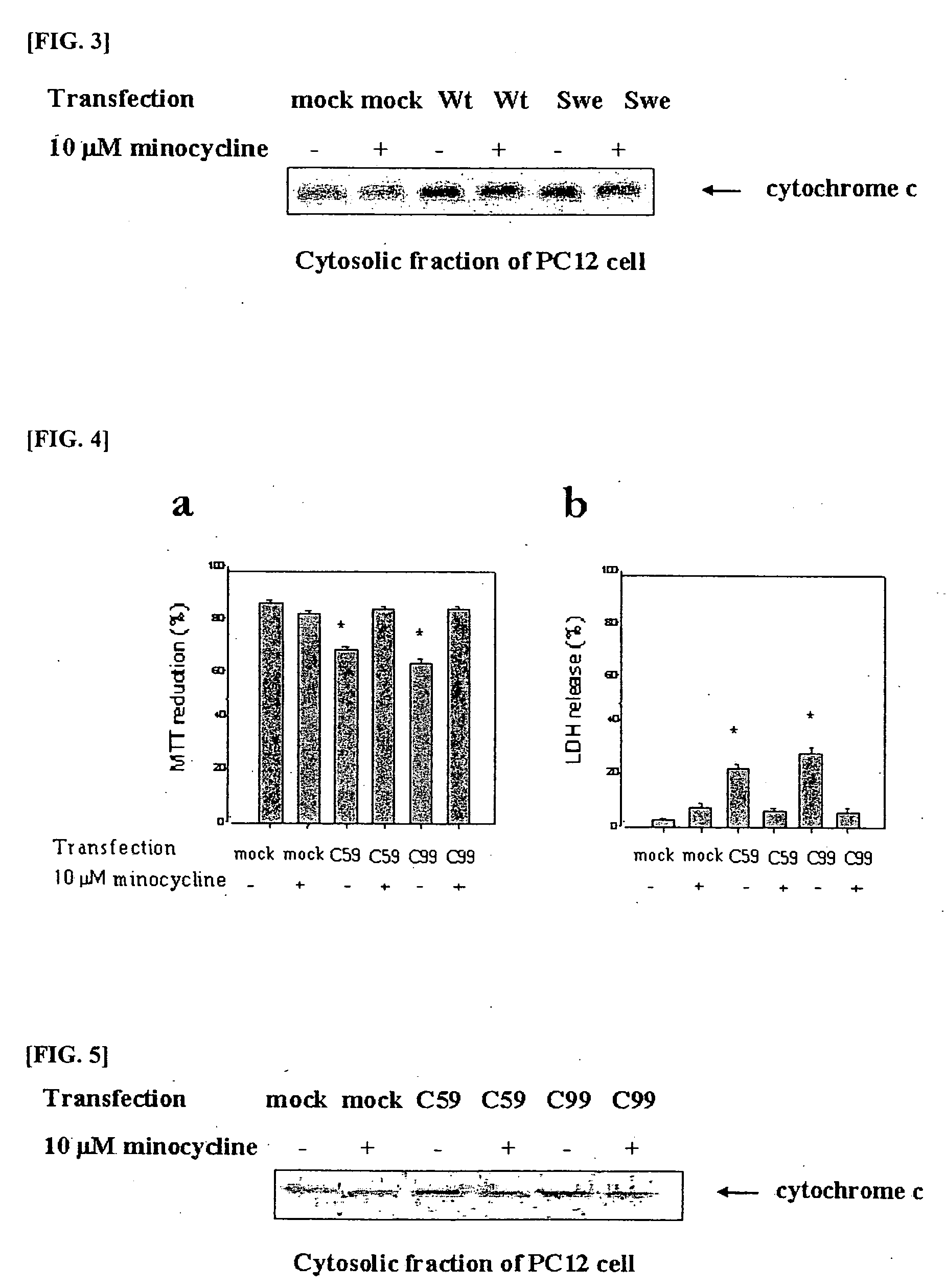
The present invention relates to a composition for preventing and treating dementia and memory impairment, which contains minocycline as active ingredient. The composition of the present invention has an effect of inhibiting brain cell death and memory impairment, which are induced by amyloid beta-protein and C-terminal protein. Thus, the composition of the present invention is useful for the prevention and treatment of various dementias, including Alzheimer's disease, and the impairment of learning and memory and cognitive function.
Owner:SUH YOO HUN +4
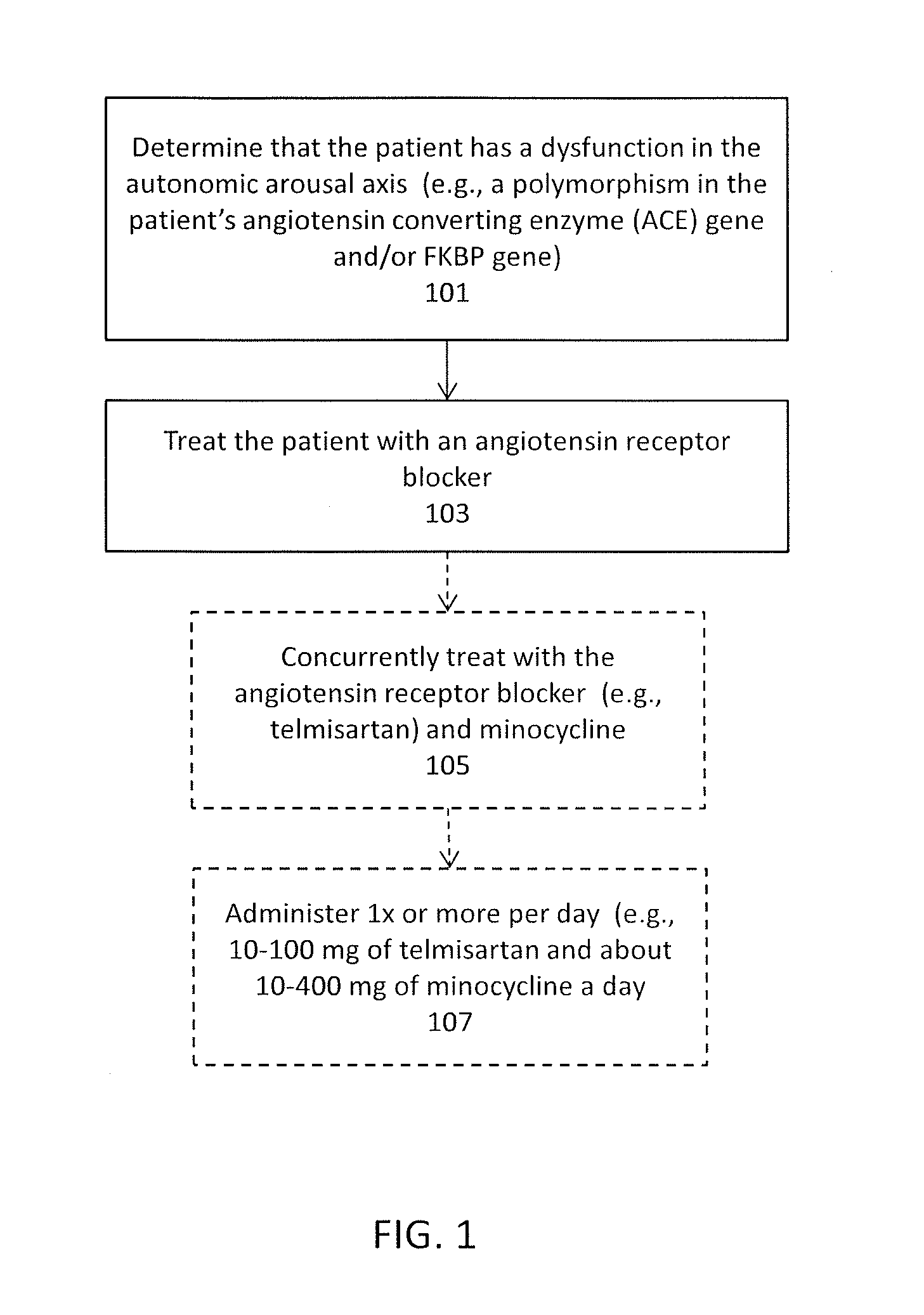
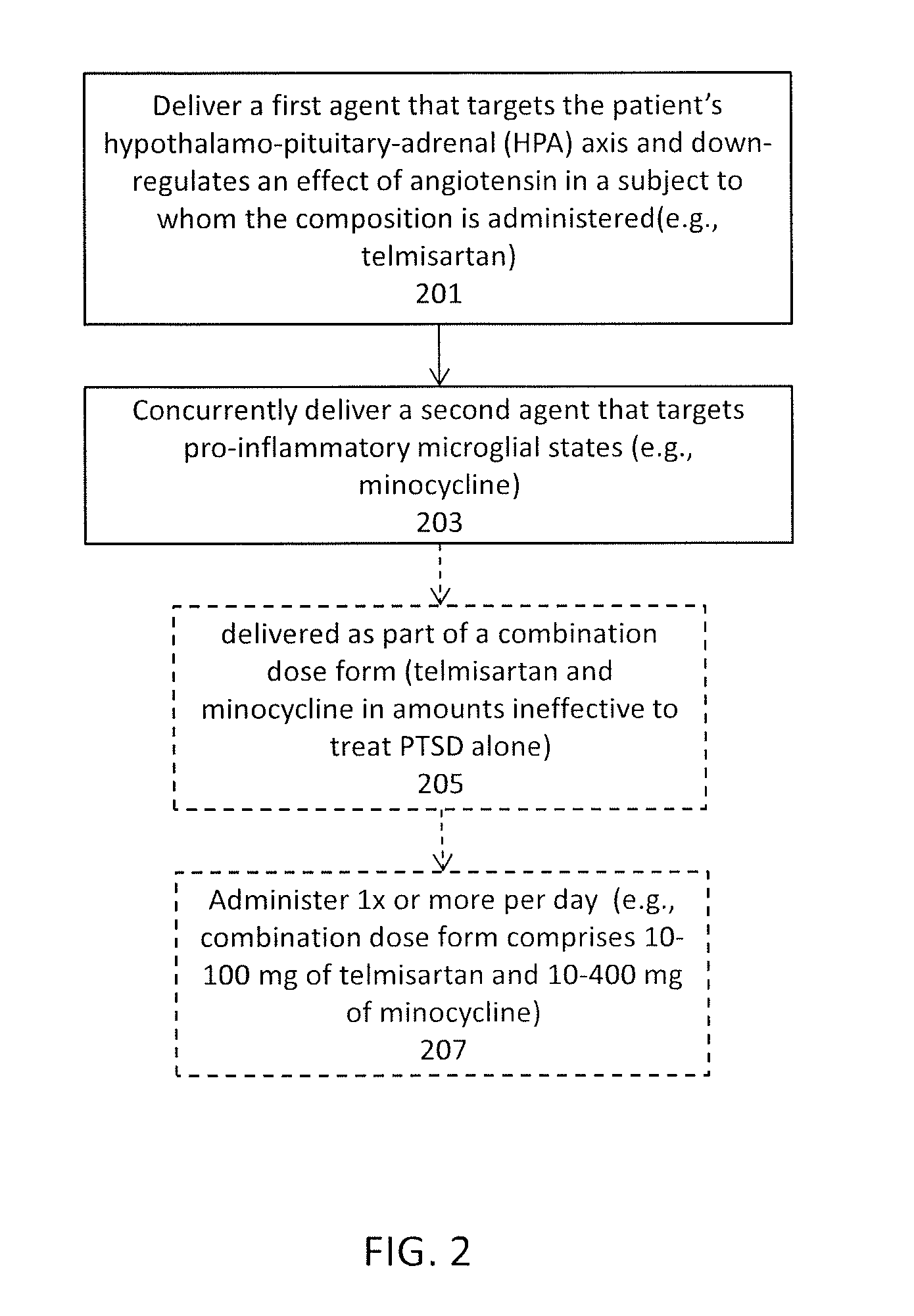
Methods and compositions for the treatment of post-traumatic stress disorder
InactiveUS20170029892A1Improve blood-brain barrier permeabilityReduce hyperactivityTetracycline active ingredientsMicrobiological testing/measurementAngiotensin receptorAngiotensin Receptor Blockers
Methods and compositions are disclosed to treat neuropsychiatric disorders post-traumatic stress disorder (PTSD). In particular, described herein are angiotensin receptor blockers (ARBs), and in particular the combination of one or more ARB (such as telmisartan) and an agent that enhances the delivery of the ARB across the blood-brain barrier (such as minocycline). PTSD may be treated using a combination of telmisartan and minocycline at levels of each that are, by themselves, infective to treat PTSD. Also described herein are methods for treating PTSD by first identifying patents for whom the use of an ARB treatment would be effective, by determining that patient has a dysfunction in their angiotensin converting enzyme and / or other genes in the autonomic arousal axis.
Owner:GENOMIND
Anti-infection venous catheter and preparation method thereof
ActiveCN102500033ALost fastImprove efficacyAntibacterial agentsTetracycline active ingredientsVeinIntravenous catheter
The invention relates to an anti-infection venous catheter and a preparation method thereof. The venous catheter comprises a conduit main body tube, a tip end and a tube seat, and preferably also comprises a connecting seat and a plurality of epitaxial tubes, wherein the parts retained in a human body are the conduit main body tube and the tip end; and an anti-infection medicament rifampicin, an anti-infection medicament minocycline or combination of the two are uniformly loaded on the conduit main body tube. The preparation method of the venous catheter comprises the following steps of: dissolving the anti-infection medicament or the medicament combination to form soak solution; soaking the conduit into the soak solution to fully soak medicaments into the conduit; and drying to remove solvent to prepare the anti-infection venous catheter. During use, the medicaments are slowly released to fulfill the anti-infection aim of the conduit and avoid infection of the conduit during retention in the human body in surgery.
Owner:BEIJING DEMAX MEDICAL TECH
Oriented implants containing poly(butylene succinate) and copolymer, and methods of use thereof
PendingUS20190269816A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Method for preparing absorbable and antibacterial guided tissue regeneration membrane for bone-like structure
ActiveCN104436318AFunction as a biological barrierImprove biological activitySurgerySodium phosphatesPhosphorylation
The invention provides a method for preparing an absorbable and antibacterial guided tissue regeneration membrane for a bone-like structure, and belongs to the field of biological medical materials. The method comprises the following steps: firstly, preparing a cell free bovine pericardial collagen fiber membrane by adopting a repeated freeze-thaw method and a surface active agent TritonX-100, or preparing a demineralized cell free collagen fiber membrane of a cattle lamellar bone by using a method of EDTA decalcification by using the surfactant TritonX-100; treating by using sodium trimetaphosphate to obtain a phosphorylated collagen fiber membrane; with the assistance of a direct-current electric field, mineralizing in agar hydrogel containing calcium and phosphate, thereby obtaining a mineralized collagen membrane with a bone-like structure with certain hardness; and finally soaking the mineralized collagen membrane into a minocyline solution, and preparing an antibacterial bone-like structured composite membrane structure by virtue of the characteristics of combined minocyline and hydroxyapatite. The guided tissue regeneration membrane provided by the invention has a bone-like structure, is capable of maintaining a bone defect space, is good in operation forming property and antibacterial property, and can be applied to clinical practice for bone defect remediation and reconstruction in periodontal departments, dental implant mediation and occlusalf surfaces.
Owner:李柏霖 +1
Synthetic method for high-purity tigecycline
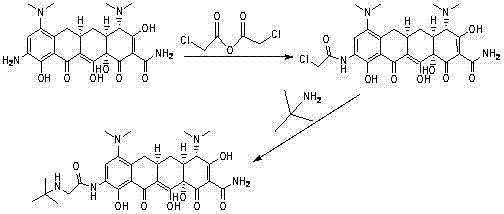
ActiveCN102391148AAvoid degradationShort synthetic routeOrganic compound preparationCarboxylic acid amides preparationGlycineTigecycline
The invention relates to a novel preparation method for antibioticdrug tigecycline. 9-amino minocycline and N-tert-butyl group glycine are taken as starting materials. The novel preparation method is characterized in that the N-tert-butyl group glycine is dissolved in indifferent solvent, under the existence of an acidic acceptor and an amino acid condensating agent, reaction with 9-amino minocycline is carried out along the route of amino acid condensation for 3 hours to 10 hours, and then an indifferent solvent is cooled to a room temperature. Tigecycline is obtained through acidification, neutralization, extraction, drying, concentration and refining. In the preparation method disclosed by the invention, the operation is simplified, the purity of the obtained product reaches more than 99.5 percent, individual impurities are controlled to be lower than 0.1 percent, and an epimer is controlled to be lower than 0.5 percent; and the yield is high, the stability of the product is good, and the preparation method is suitable for industrial production.
Owner:NANJING HAIRUN PHARM CO LTD
Minocycline hydroehloride sustained- release tablets and preparation method thereof
InactiveCN101658501AGood curative effectImprove securityAntibacterial agentsTetracycline active ingredientsCurative effectPharmacology
The invention aims to provide minocycline hydroehloride sustained-release tablets with higher stability of medicament release and administrative safety, which are characterized by consisting of minocycline hydroehloride, a slow release material, a filler, a binder, a lubricating agent and a coating material. The minocycline hydroehloride sustained-release tablets have the characteristics of havingconvenient administration, lasting effect and stable curative effect, reducing untoward effect, improving the compliance of patients and the like.
Owner:COSCI MED TECH CO LTD
Sustained-releasing oral mucosa medicinal film
InactiveCN1883461ABroaden applicationLong release timeOrganic active ingredientsDigestive systemDiseasePharmaceutical formulation
Disclosed is a medicinal preparation for treating oral mucosa diseases comprising active medicinal constituents and auxiliary materials, which can be in the form of paster obtained through sheet pressing or thin films obtained through liquid phase film forming, the effective medicinal constituents can be antibiotic medicaments including Minocycline, hibitane, fortimicin, achromycin or metronidazole, the auxiliary materials include binding agent, disintegrating agent and flavoring agent.
Owner:何元
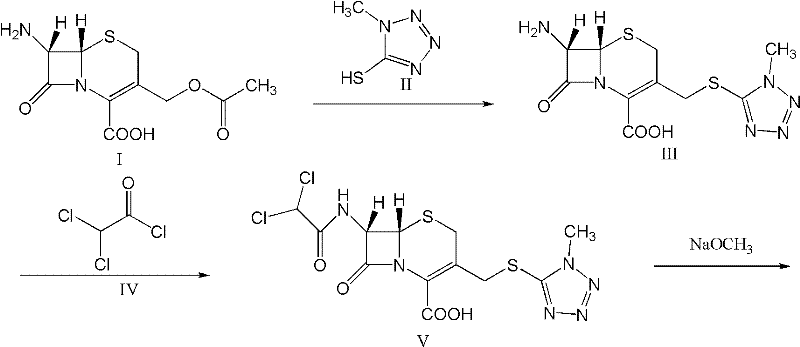
A kind of preparation method of cefminox sodium
The present invention relates to a kind of preparation of medicinal compound, particularly a kind of preparation method of cefminox sodium. The present invention adopts a synthesis route using 7-ACA as a starting material, and the cost of raw materials is lower than that of a synthesis route using 7-MAC as a starting material. The new synthetic route uses dichloroacetyl chloride instead of bromoacetyl bromide, which reduces the toxicity and cost of raw materials. The new synthetic route increases the intermediate treatment process, reduces the amount of impurities brought by the intermediate into the next reaction, and improves the quality and stability of the finished product. The present invention overcomes the shortcomings of the synthetic route using 7-MAC as the starting material through the above improvements.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Salts and polymorphs of 9-(2,2-dimethylpropyl-aminomethyl) minocycline
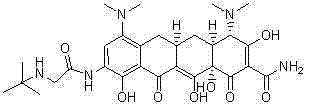
Crystalline forms, including salts and polymorphs, of a compound useful in the treatment of tetracycline compound-responsive states are provided herein. The crystalline compounds are useful for the treatment or prevention of conditions and disorders such as bacterial infections and neoplasms, as well as other known applications for tetracycline compounds in general.
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
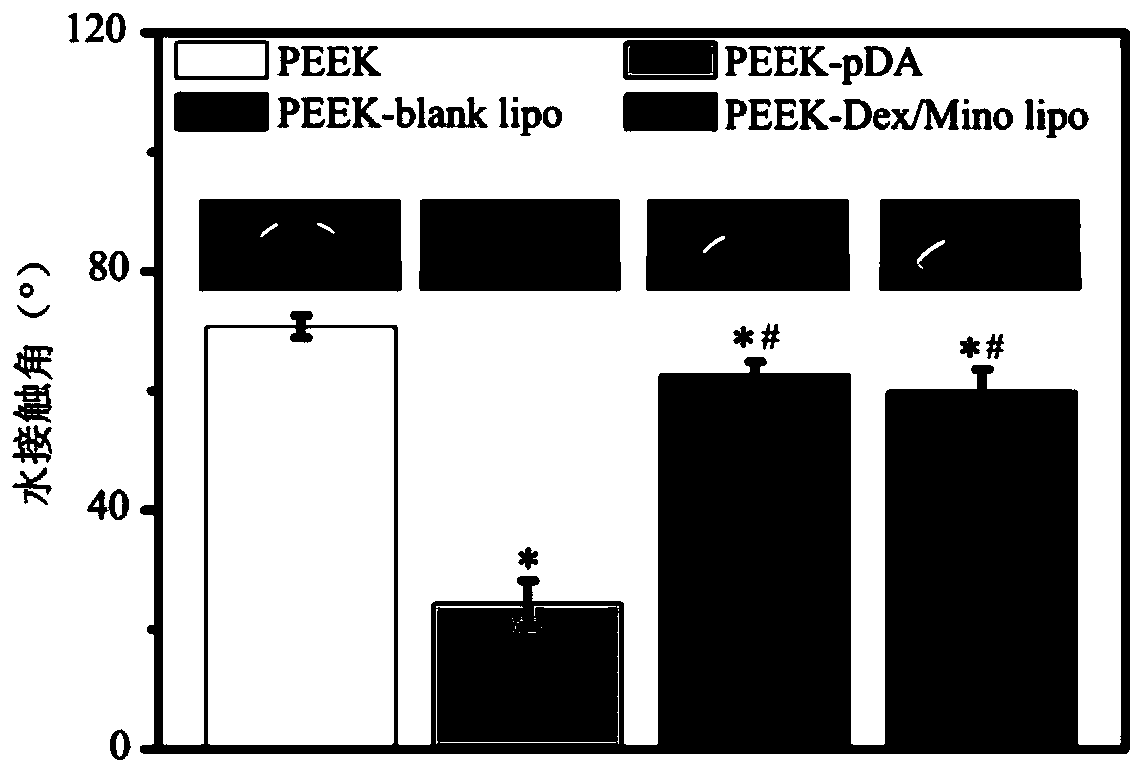
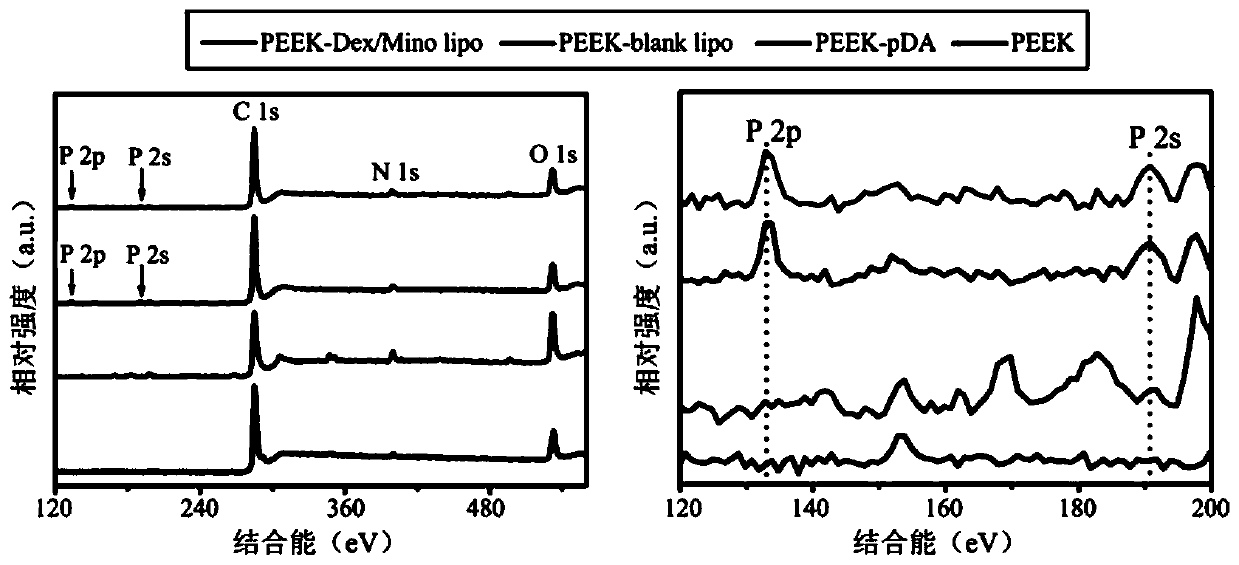
Lipidosome-based method for modifying PEEK (polyetheretherketone) surface with hexadecadrol/minocycline and application
InactiveCN110279890AImprove anti-infective activityGood anti-inflammatory activityTetracycline active ingredientsSkeletal disorderWater bathsPolycarbonate membranes
The invention discloses a lipidosome-based method for modifying a PEEK (polyetheretherketone) surface with hexadecadrol / minocycline and an application of the method. Lipidosome loaded with hexadecadrol and minocycline is prepared with a thin film dispersion method and then modifies the PEEK surface. The method comprises following steps: (1), hexadecadrol and lipids are dissolved in a methanol and chloroform mixture (1:1, v / v); (2), methanol and chloroform are removed by a rotary vacuum evaporator, and then a lipid membrane is hydrated by a hydration solution; lipidosome loaded with hexadecadrol is obtained by water bath ultrasonic treatment, and is extruded by a porous polycarbonate membrane; dialysis is performed overnight; (3), lipidosome is mixed with a minocycline storage solution, the mixture is shaken, and lipidosome loaded with hexadecadrol / minocycline is obtained, and is stored for standby application at 4 DEG C after being dialyzed overnight; (4), PEEK implant is put in a dopamine solution for reaction, and ultrasonic cleaning is performed; (5), the PEEK implant is added to a hexadecadrol / minocycline lipidosome solution for soaking and is slightly cleaned, and a product is obtained. The obtained product can promote bone repair, regeneration and integration.
Owner:BEIJING SHIJITAN HOSPITAL CAPITAL MEDICAL UNIVERSTY +1
Oriented p4hb implants containing antimicrobial agents
ActiveUS20160082160A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsPharmaceutical containersFiberMedicine
Oriented resorbable implants made from poly-4-hydroxybutyrate (P4HB) and copolymers thereof, have been developed that contain one or more antimicrobial agents to prevent colonization of the implants, and reduce or prevent the occurrence of infection following implantation in a patient. These oriented implants are particularly suitable for use in procedures where prolonged strength retention is necessary and there is a risk of infection. Coverings and receptacles made from poly-4-hydroxybutyrate and copolymers thereof, containing antimicrobial agents, have also been developed for use with implantable devices to prevent colonization of these devices, and to reduce or prevent the occurrence of infection following implantation of these devices in a patient. These coverings and receptacles may be used to hold, or partially or fully cover, devices such as pacemakers and neurostimulators. Preferably, the coverings and receptacles are made from meshes, non-wovens, films, fibers, and foams, and contain rifampin and minocycline.
Owner:TEPHA INC
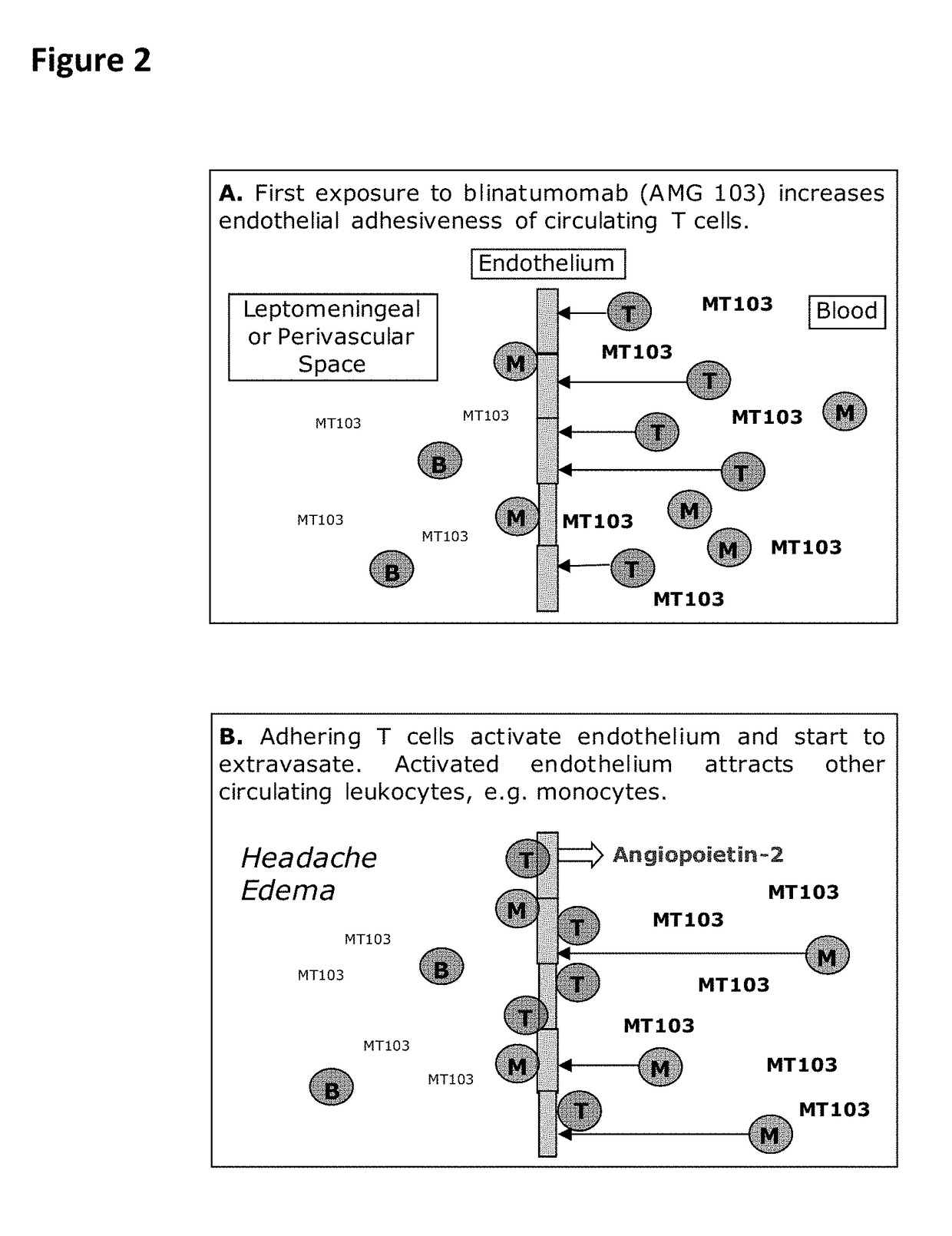
Anti-leukocyte adhesion for the mitigation of potential adverse events caused by CD3-specific binding domains
The present invention relates in essence to use of a compound, which decreases or inhibits the binding of mammalian T-cells to mammalian endothelial cells for use in a method of prophylaxis and / or amelioration and / or treatment of clinical adverse events caused by a therapy which comprises re-directing of T-cells against target cells in a patient. Such a therapy includes, but is not limited to, treatment with an antibody comprising a CD3 binding domain, such as a CD20×CD3 or a CD19×CD3 bispecific single chain antibody, e.g., blinatumomab (MT-103). Methods of treatment of patients having or being at risk of clinical adverse events caused by therapy which comprises re-directing of T-cells against target cells are also contemplated, as are methods of identifying a compound for administration in the methods of prophylaxis, amelioration and / or treatment. Such anti-adhesive type compounds include, but are not limited to, antibodies, like natalizumab, efalizumab, and etrolizumab; minocycline, (acetyl-)salicyclic acid, astilbin, and flavonoids; and thrombin and pentosanpolysulfate (PPS), or a pharmaceutically acceptable salt thereof.
Owner:AMGEN RES (MUNICH) GMBH
Camellia oleifera seed oil anti-acne ointment
InactiveCN102258632ARegulate metabolismRegulates metabolic turnoverTetracycline active ingredientsDermatological disorderBiotechnologyCamellia oleifera
The invention is "A Camellia Camellia Seed Oil Anti-Acne Ointment". field of biotechnology. Refined Camellia oleifera seed oil with strong affinity with sebum is used as the base, adding slug extract of heat-clearing, detoxifying, promoting blood circulation and removing stasis agent, reducing sebum secretion, dredging clogged pores agent vitaminate; under anaerobic environment, it is specially designed to kill Propionibacterium acnes Ointment made from minocycline. Preparation method: Weigh 2-5g of slug extract, 1-3g of vitaminate, 1-2g of minocycline, mix well, pass through a 100-mesh sieve for later use; weigh 50g-75g of glycerin, 0.5g-ethyl paraben Mix 1g with a certain amount of purified water, heat to 80°C for later use, this is the water phase; weigh 80-120g of tea oil, 5-8g of Tween 80, 10g of triethanolamine, heat to 80°C and stir to dissolve, this is the oil phase . Slowly add the oil phase to the water phase, add purified water to 1000 ml, and stir in the same direction to form a matrix. When the temperature of the matrix reaches 40°C, add the above-mentioned fine powder of the sieved drug, and continue to stir until the matrix is formed. paste. In this invention, the synergistic and additive effects of the combined use of various drugs can play the role of treating acne from multiple links such as sterilization, anti-inflammatory, reduction of sebum secretion, dredging of clogged pores, and improvement of skin microcirculation. Superior to all similar products in the current market.
Owner:DONGYUAN LVBAO BIOLOGICAL TECH DEV
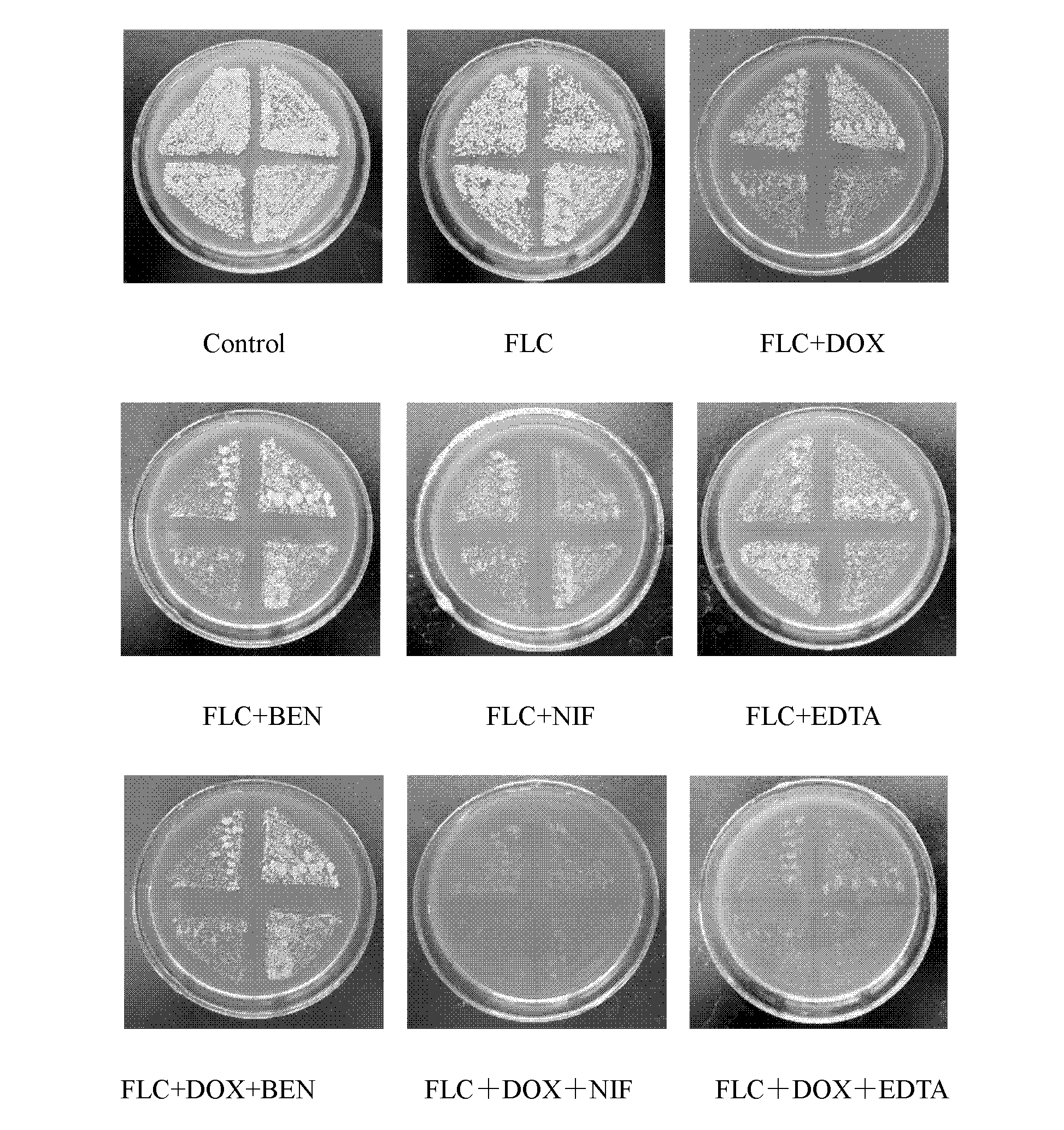
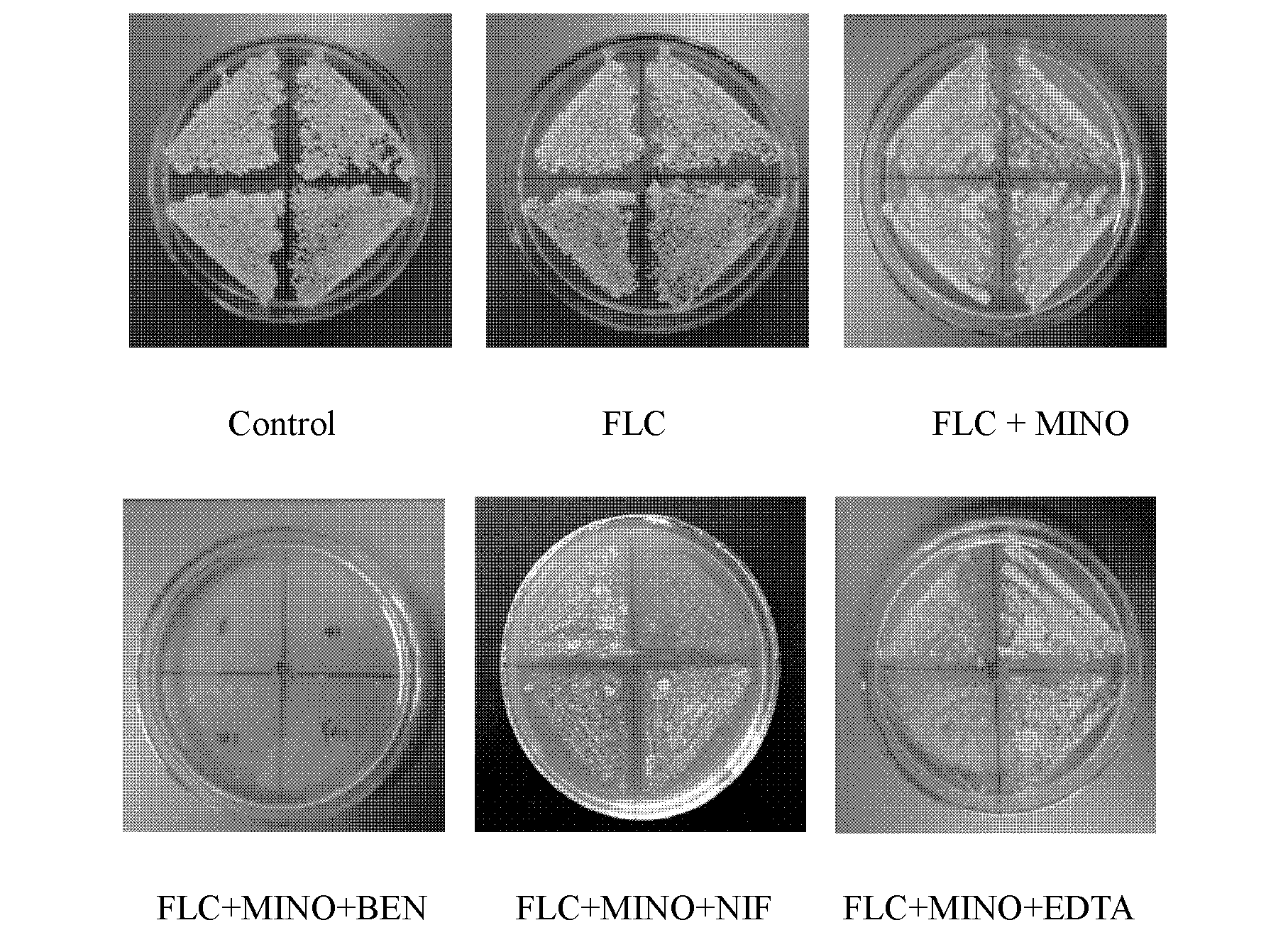
Application of combination of tetracycline medicine and fluconazole in preparation of antifungal product, and product thereof
InactiveCN102626415AMIC lowerIncrease concentrationAntimycoticsTetracycline active ingredientsCalcium modulationAntimicrobial drug
The invention discloses an application of a combination of a tetracycline medicine and fluconazole (FLC) in the preparation of an antifungal product, and the product thereof, and concretely discloses a synergistic effect of three cell calcium regulators of a non-selective calcium channel retarding agent benidipine (BEN), a selective L-type calcium channel retarding agent nifedipine (NIF) and a calcium ion chelating agent ethylene diamine tetraacetic acid (EDTA) to the FLC and tetracycline antifungal medicines of doxycycline (DOX) and minocycline (MINO), and an application of the three cell calcium regulators in Candida albicans resisting, wherein a synergistic antifungal effect can be generated by the combined application of the FLC, the DOX and the MINO. Results confirm that the combined application of the FLC and the DOX / MINO can generate the synergistic antifungal effect, and has a substantial killing effect on drug-resistant Candida albicans; the BEN, the NIF and the EDTA can obviously enhance the combined antifungal effect of the FLC and the DOX / MINO to the Candida albicans, and can obviously reduce the lowest effective concentration during the combined application of the FLC, the MINO and the DOX; and the combined application of the three calcium regulators and the FLC can enhance the antifungal effect.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
Treatment For Diseases Relying On Discovery That Thioredoxin Mediates Nitric Oxide
InactiveUS20110104308A1Increase pressureAntibacterial agentsOrganic active ingredientsDiseaseRestenosis
Patients having a disease associated with high level of thioredoxin system activity or a requirement for nitric oxide, e.g. large cell lymphoma or restenosis, are treated with a thioredoxin reductase inhibitor, e.g. auranofin or arsenic trioxide, and a nitric oxide donating compound, e.g. isosorbide mononitrite or isosorbide dinitrite or nitroglycerin or S-nittrosothiol. Patients having a disease associated with nitric oxide synthase overexpression or increased activity, e.g. Parkinson's disease or septic shock or pancreatic cancer, are treated with Trx / Trx reductase upregulator, e.g. aptamer that binds to thioredoxin reductase inhibitor, and agent causing depletion of nitric oxide (or adduct thereof), e.g. L-NMMA or L-NAME or minocycline or ascorbate or N-acetylcysteine.
Owner:DUKE UNIV
Multi-ingredient pharmaceutical composition for use in cancer therapy
ActiveUS20170232008A1Avoidance and reduction of side effectIncrease the likelihood of successTetracycline active ingredientsPhosphorous compound active ingredientsHMG-CoA reductaseValproic Acid
A pharmaceutical composition or kit of parts comprising at least three ingredients from a HMG-CoA reductase inhibitor, a leukotriene antagonist, a proton pump inhibitor, melatonin or a melatonin receptor agonist, a bioavailable preparation of a curcuminoid, a calciferol derivative, a compound from the group consisting of metformin and phenformin, valproate, minocycline and chloroquine and one or more pharmaceutically acceptable carriers or excipients.
Owner:TARGETED THERAPIES RES & CONSULTING CENT SPRL TTRCC
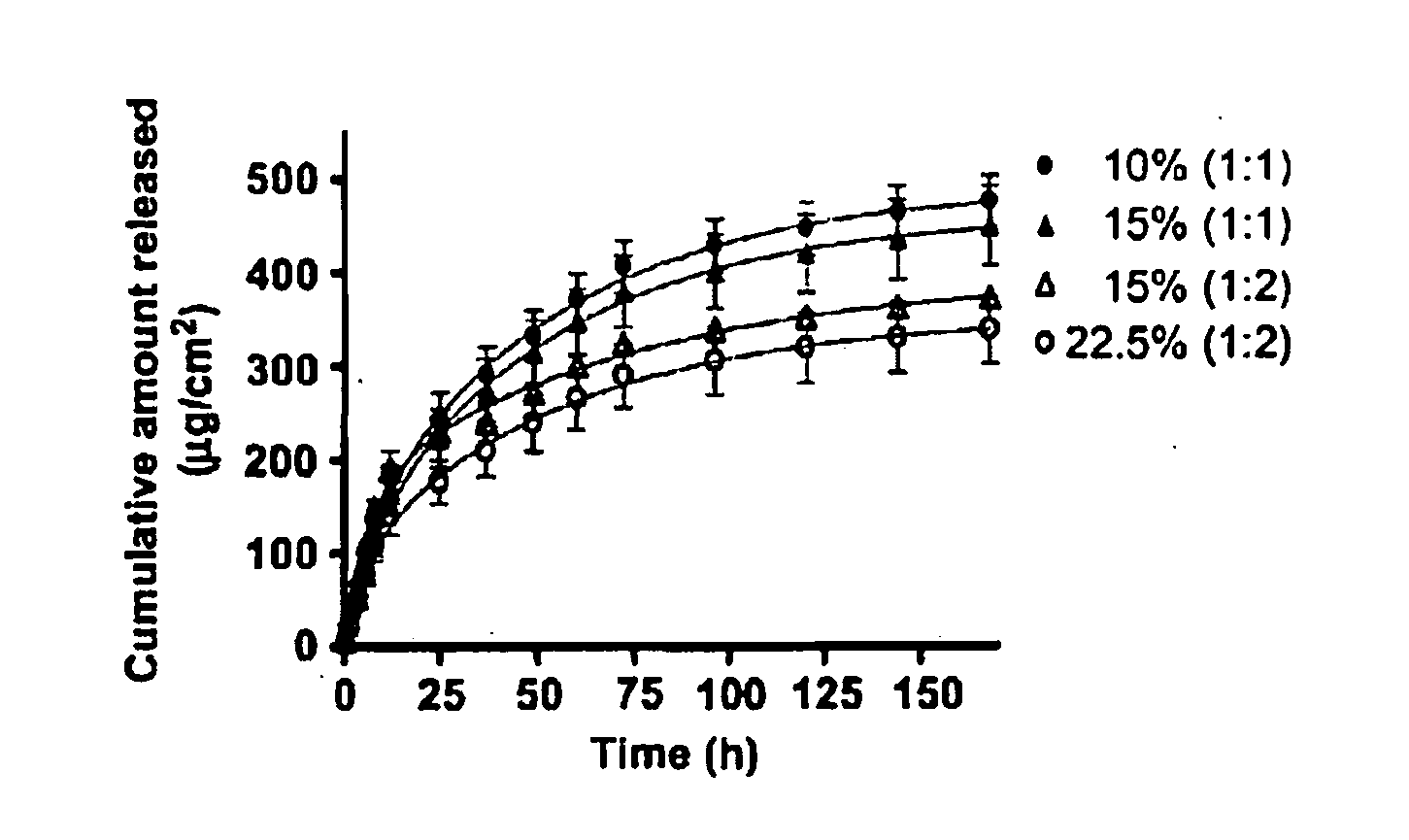
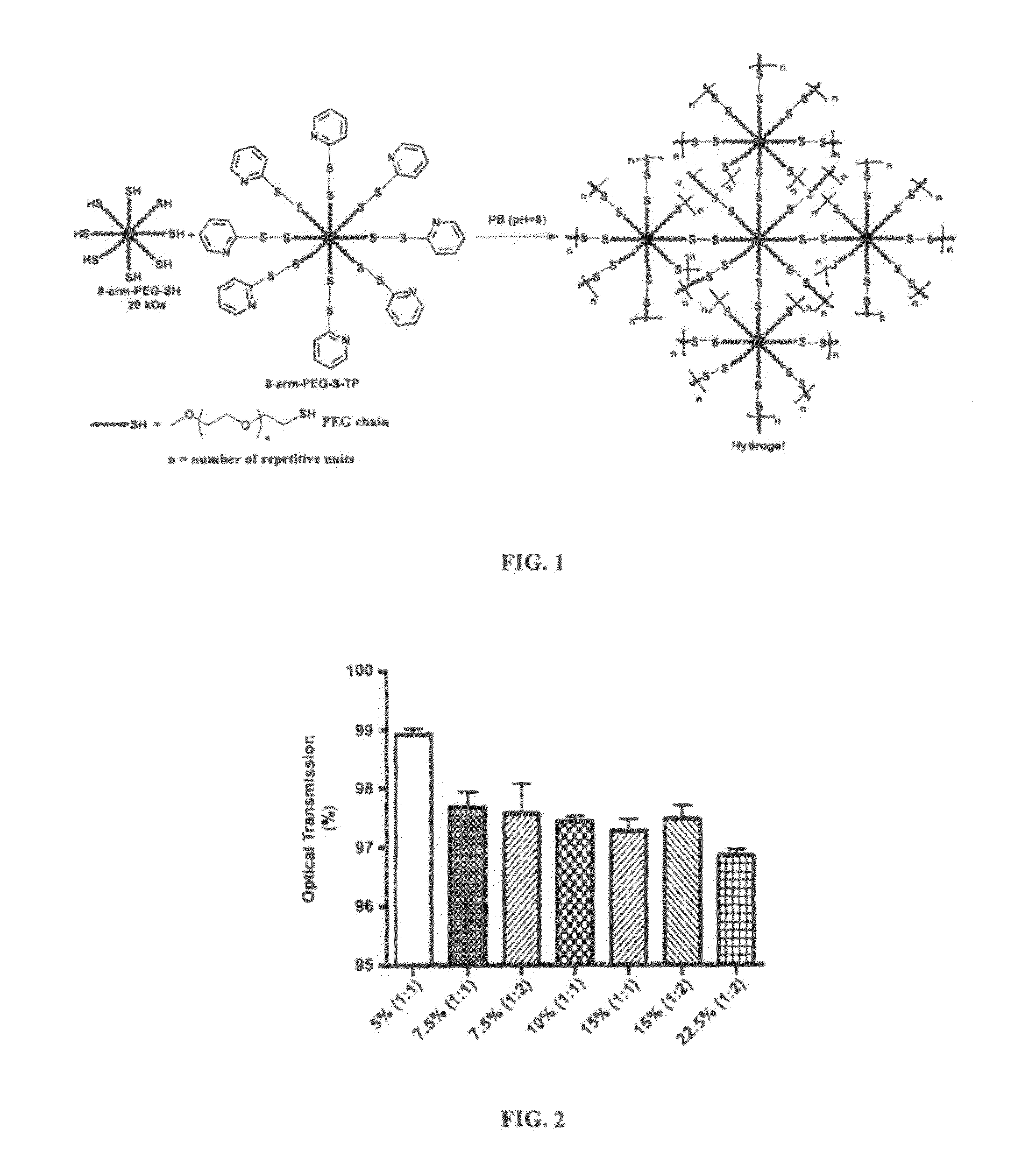
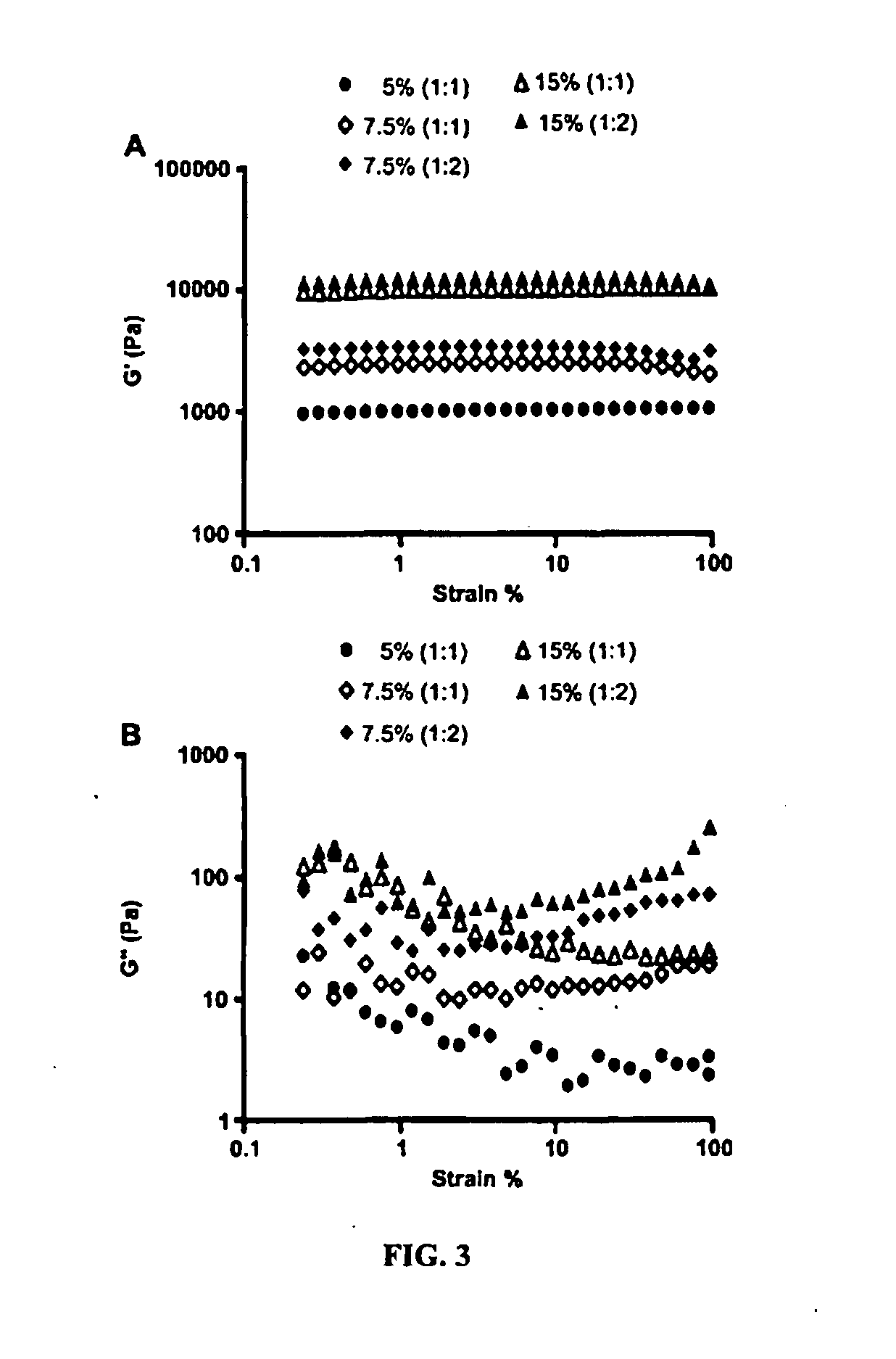
Hydrogel formulation for dermal and ocular delivery
Formulations of cross-linkable polymers, capable of forming non-toxic and biocompatible hydrogels in situ, containing at least one of doxycycline or minocycline. Methods of using the hydrogels for treating the skin or ocular tissues of mammals exposed to vesicant compounds such as sulfur mustard (SM), nitrogen mustard (NM) or half mustard (2-chloroethyl ethyl sulfide (CEES)) are also disclosed.
Owner:RUTGERS THE STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com