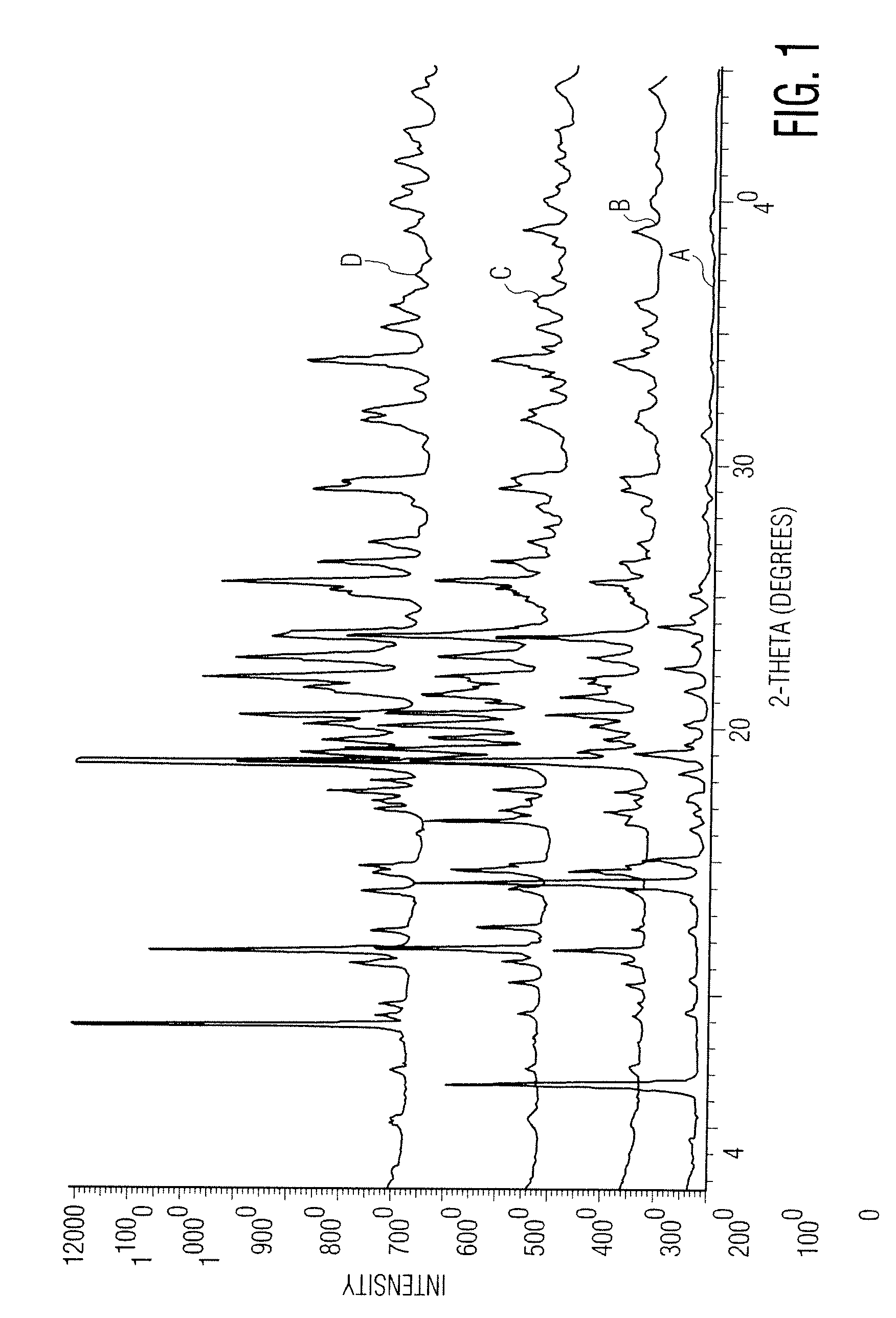
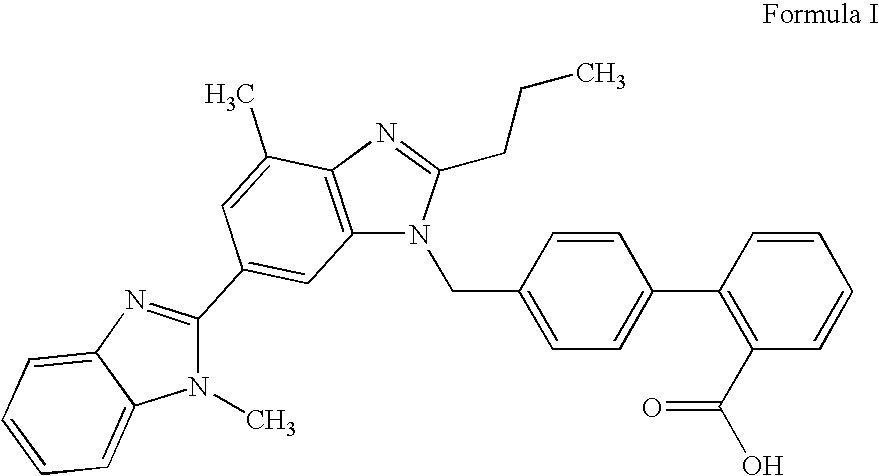
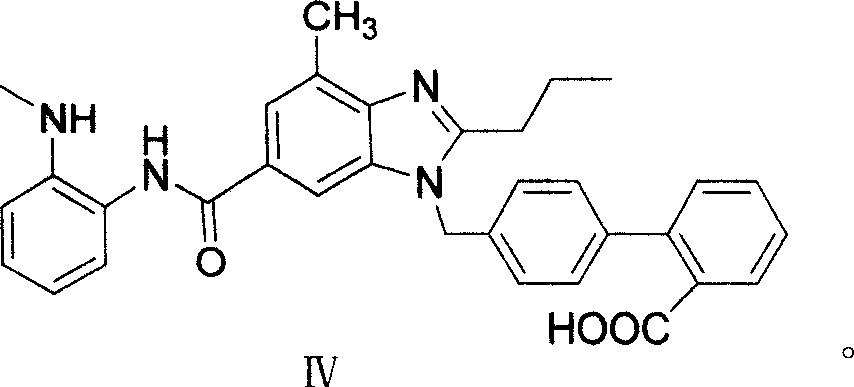
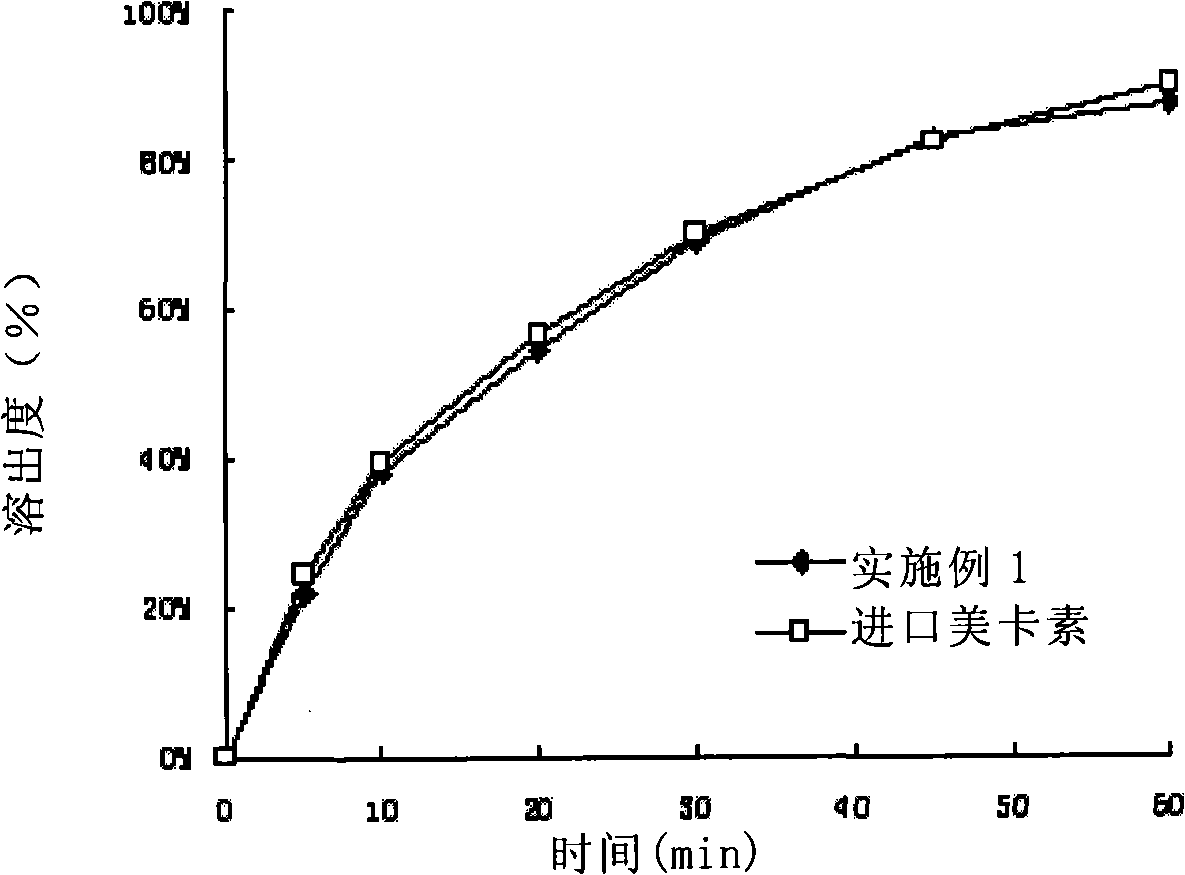
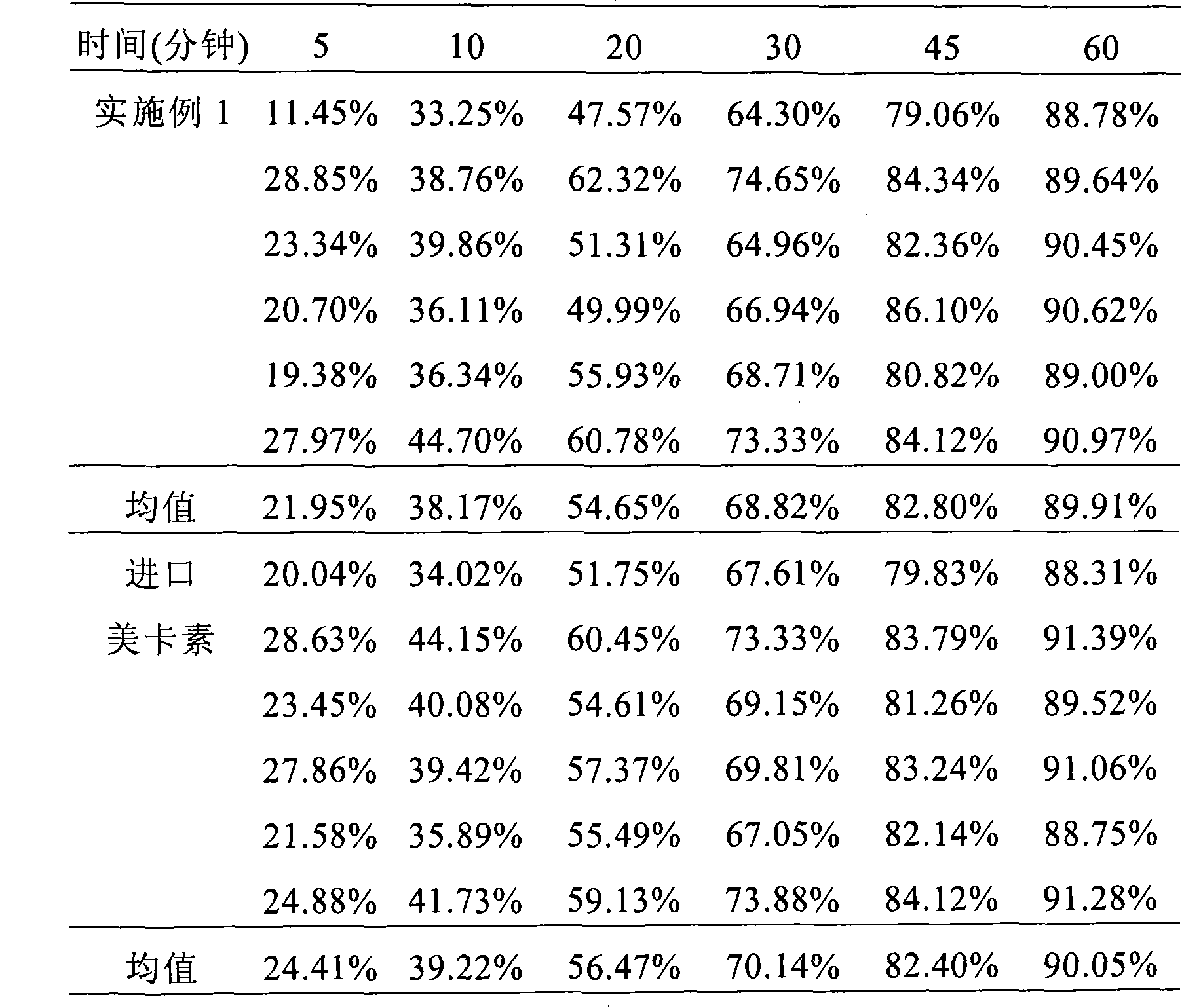
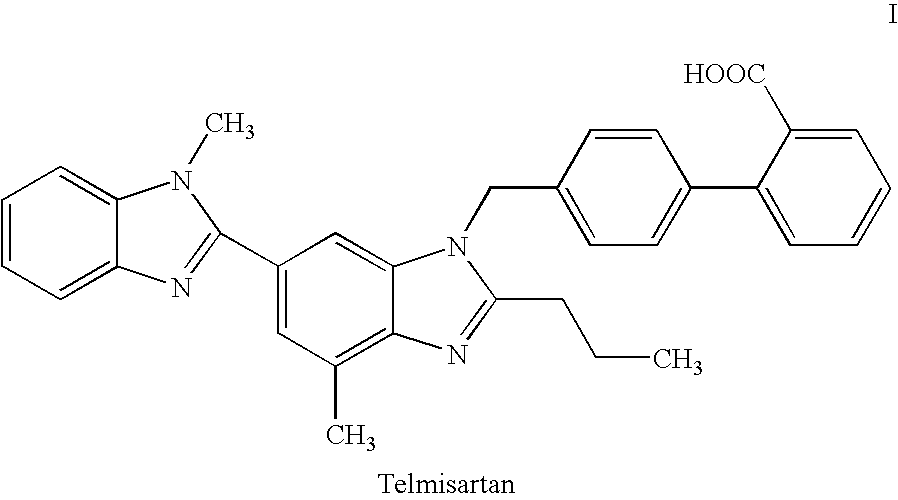
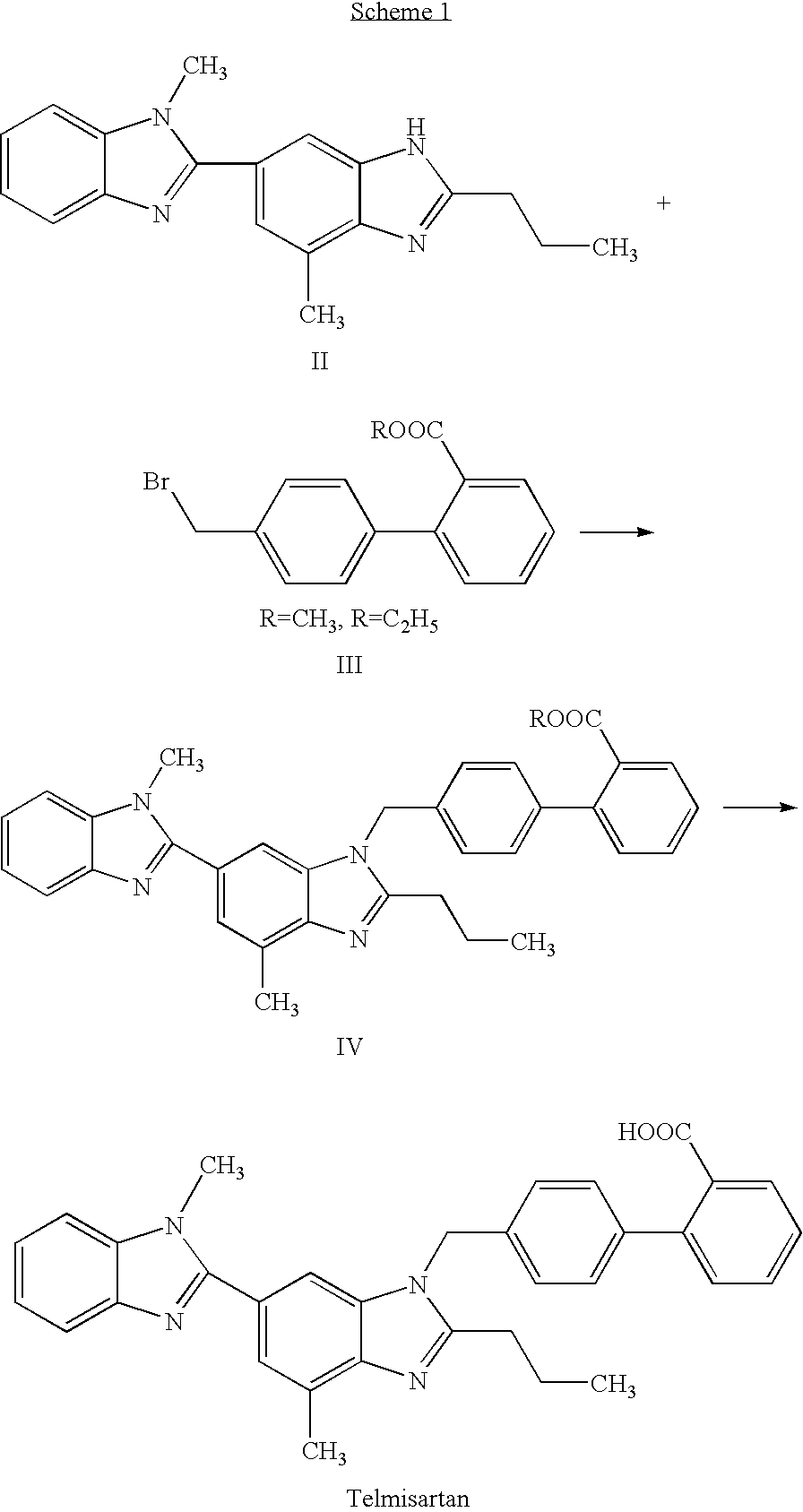
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
247 results about "Telmisartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat high blood pressure (hypertension).
Bilayer pharmaceutical tablet comprising telmisartan and a diuretic and preparation thereof
InactiveUS20050089575A1Low dissolution rateOvercome problemsMaterial analysis by electric/magnetic meansPharmaceutical non-active ingredientsHydrochlorothiazideImmediate release
The present invention relates to a bilayer pharmaceutical tablet comprising a first layer formulated for immediate release of the angiotensin II receptor antagonist telmisartan from a dissolving tablet matrix which contains telmisartan in substantially amorphous form, and a second layer formulated for immediate release of a diuretic like hydrochlorothiazide from a fast disintegrating tablet matrix. A method of producing the bilayer tablet is also disclosed.
Owner:BOEHRINGER INGELHEIM PHARM KG
Telmisartan and hydrochlorothiazide combination therapy
InactiveUS20060159747A1Increased riskGood blood pressureBiocidePill deliveryHydrochlorothiazideCombination therapy
A pharmaceutical composition comprising about 80 mg of telmisartan or a salt thereof and about 25 mg of hydrochlorothiazide or about 160 mg of telmisartan or a salt thereof and about 50 mg of hydrochlorothiazide, and methods of treating hypertension in patients with such combination.
Owner:BOEHRINGER INGELHEIM INT GMBH
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
Use of telmisartan for the prevention and treatment of vascular headache
The present invention relates to a method for the prophylaxis of vascular headaches which do not originate from hypertension, especially migraine, the method comprising administration of telmisartan to a subject in need of such a treatment. The present invention relates also to a method for the prophylaxis of vascular headaches, comprising the co-administration of telmisartan in combination with other drugs suitable for migraine prophylaxis and / or acute treatment of migraine.
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical formulations comprising telmisartan and hydrochlorothiazide
InactiveUS20100247649A1Lower blood pressureReduce liquid volumePowder deliverySolution deliveryHydrochlorothiazideImmediate release
Pharmaceutical tablets comprising a first layer formulated for immediate release of telmisartan from a dissolving matrix and a second layer formulated for immediate release of hydrochlorothiazide from a dissolving matrix, methods for producing tablets and methods of use for treating hypertension.
Owner:DR REDDYS LAB LTD
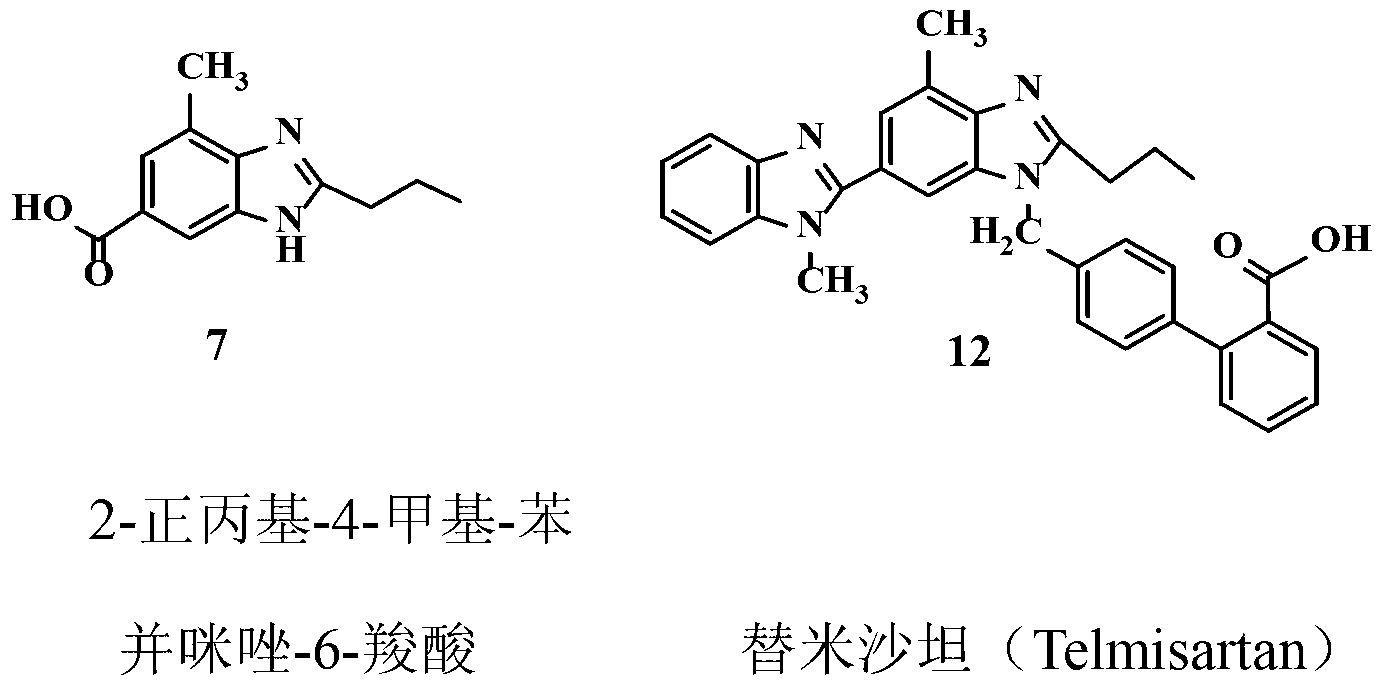
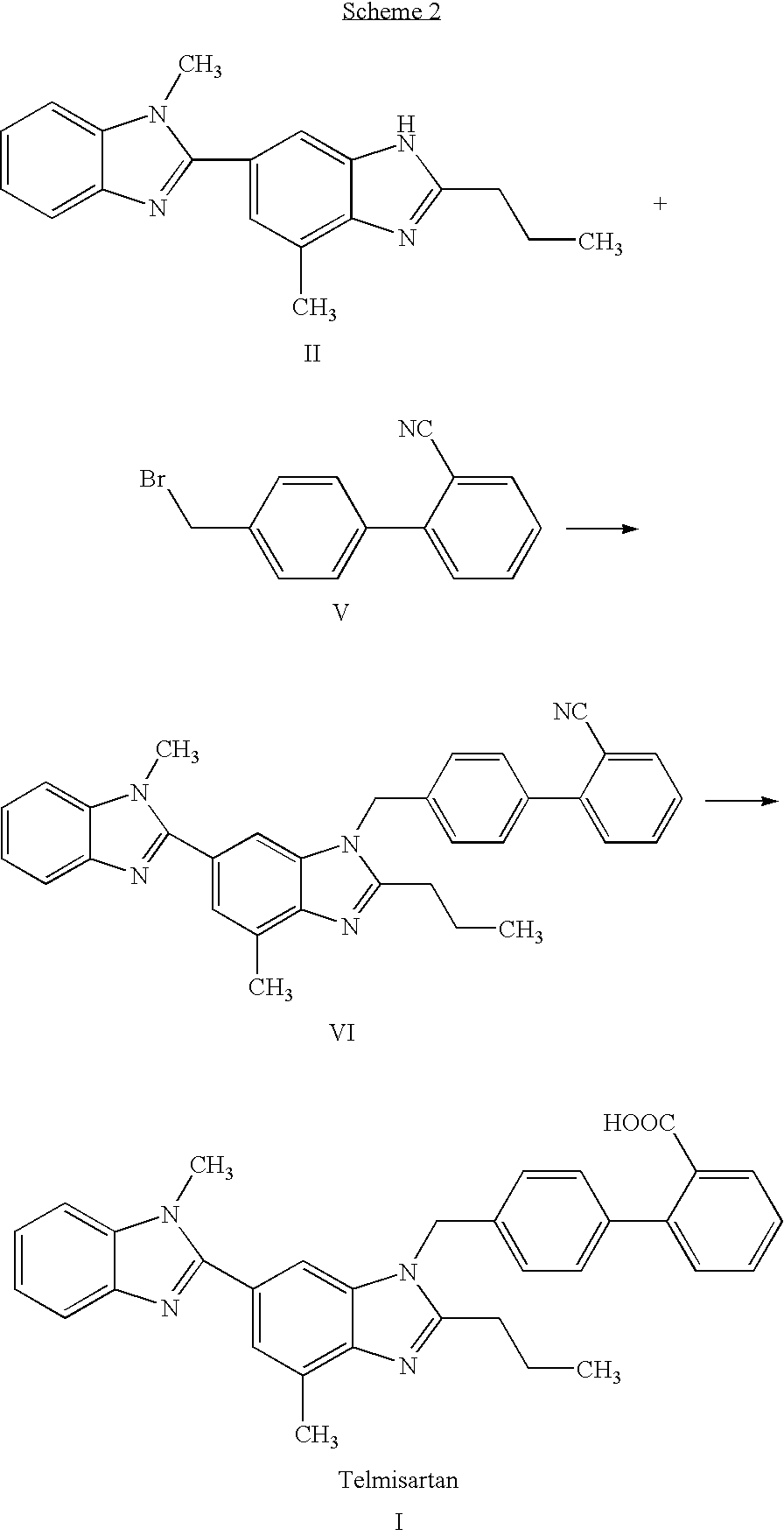
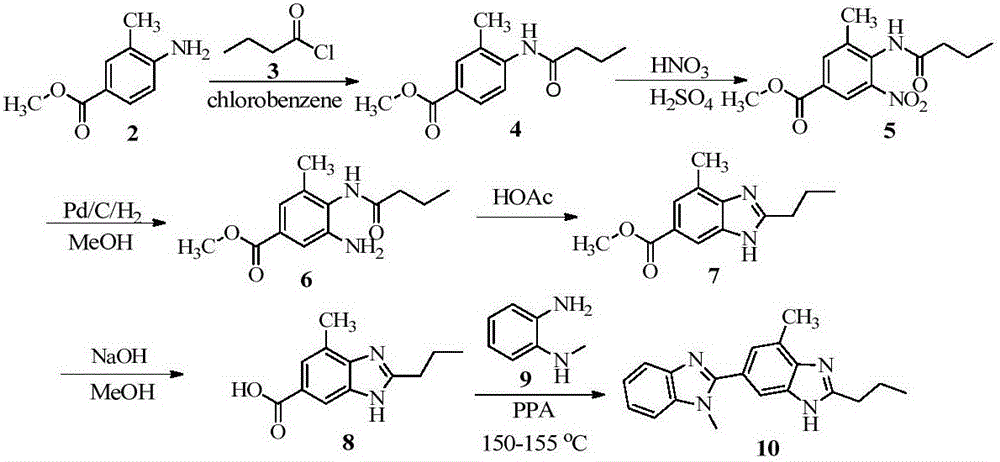
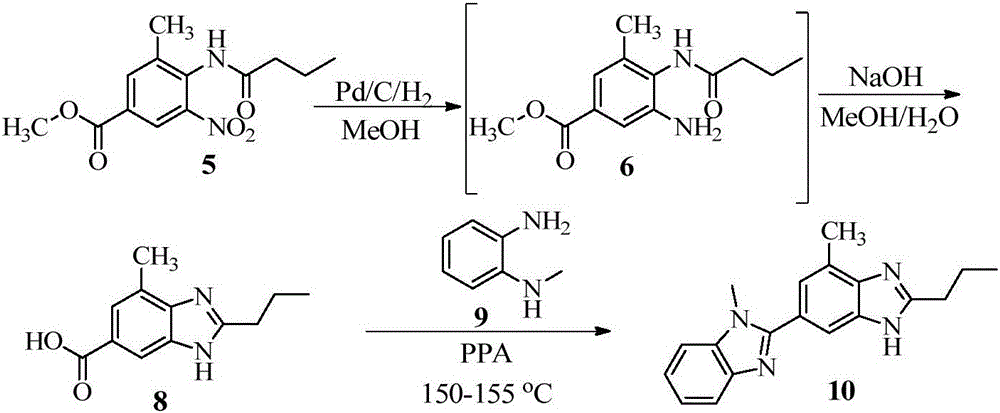
Intermediate of telmisartan, its preparation and use
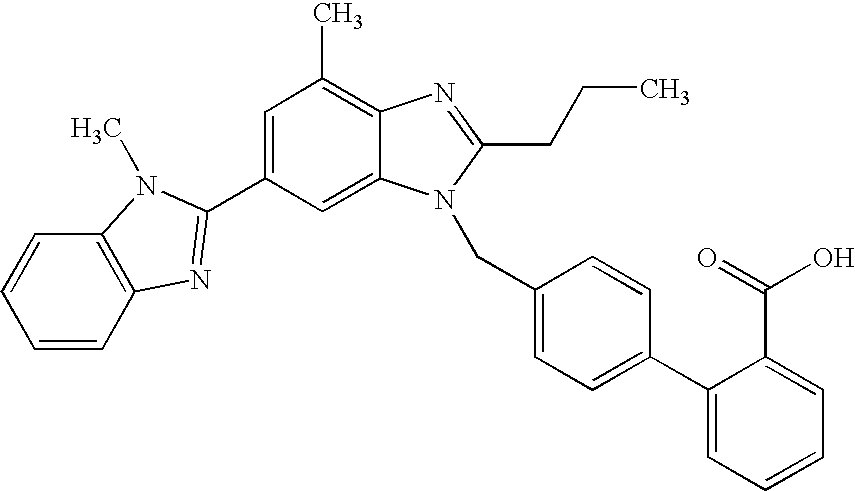
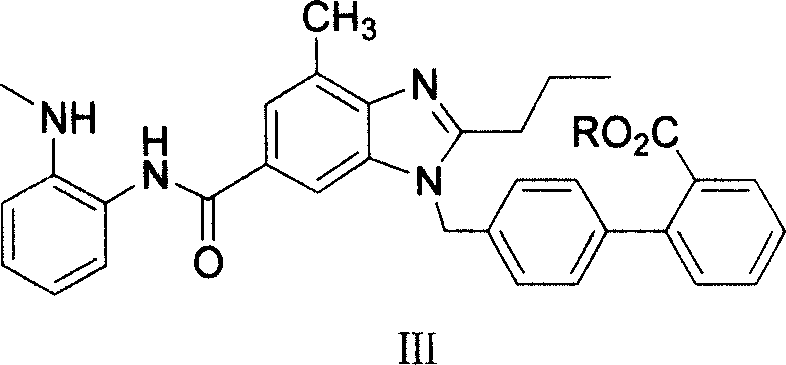
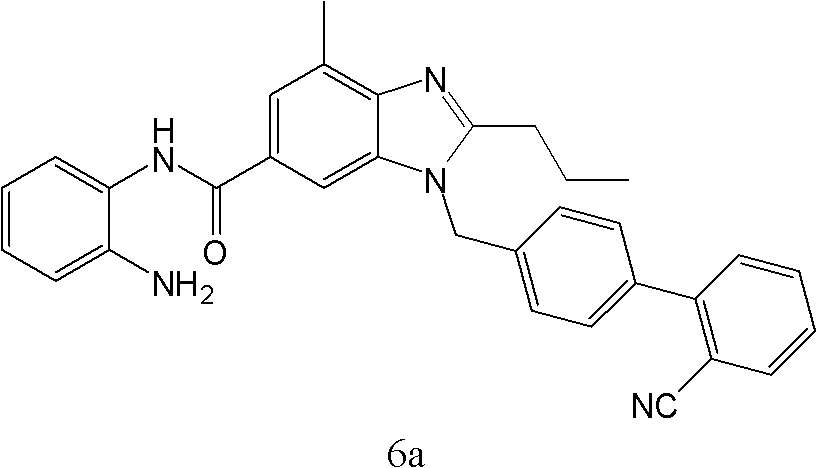
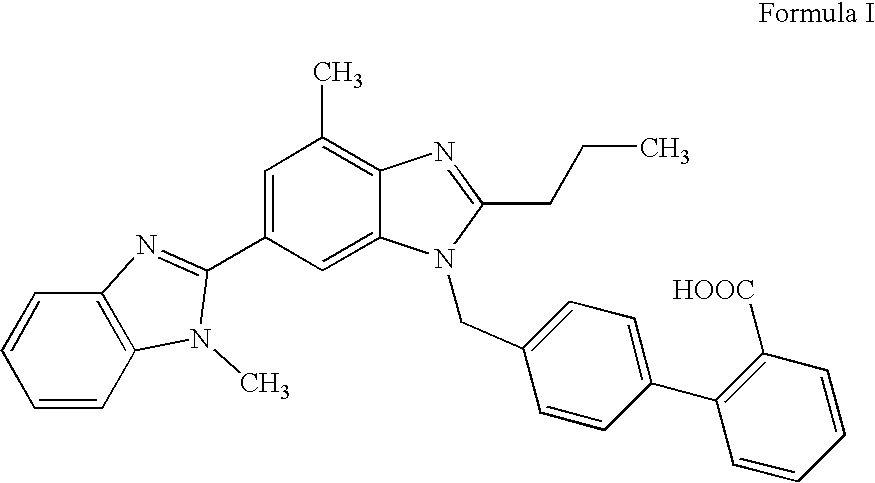
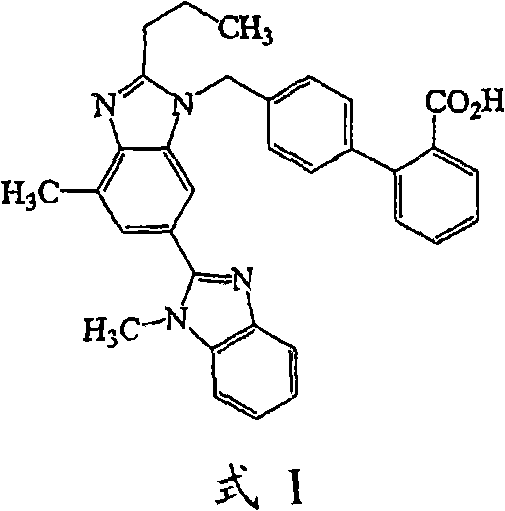
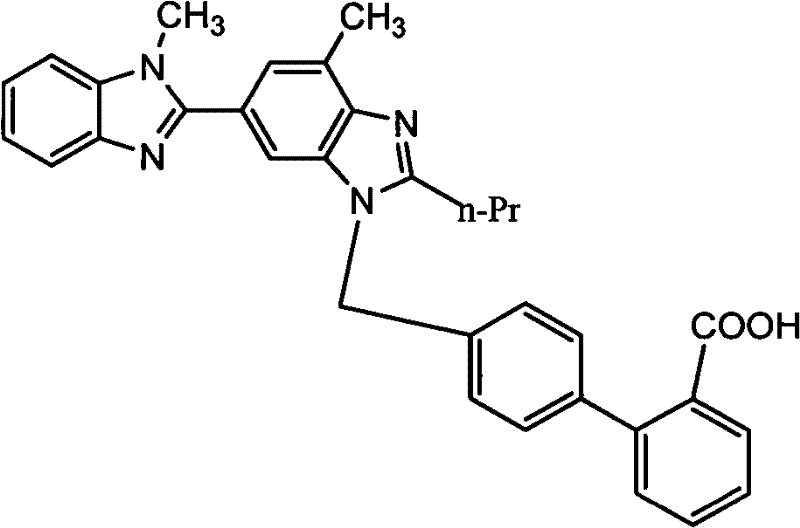
The invention supplies an intermediate to make intermediate of Telmisartan that has the structure of 4'-((4- methyl-6-(2- methylamino) carbanilino)-2-propyl-1H- benzimidazole-1-radicle) methyl) biphenyl-2-carboxylic acid. It has the advantages of high yield, save operation, low environment pollution, etc.
Owner:ZHEJIANG MENOVO PHARMA
Key intermediate of telmisartan, synthesis method thereof and method for synthesizing telmisartan by intermediate
ActiveCN102050791ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistrySynthesis methodsCombinatorial chemistry
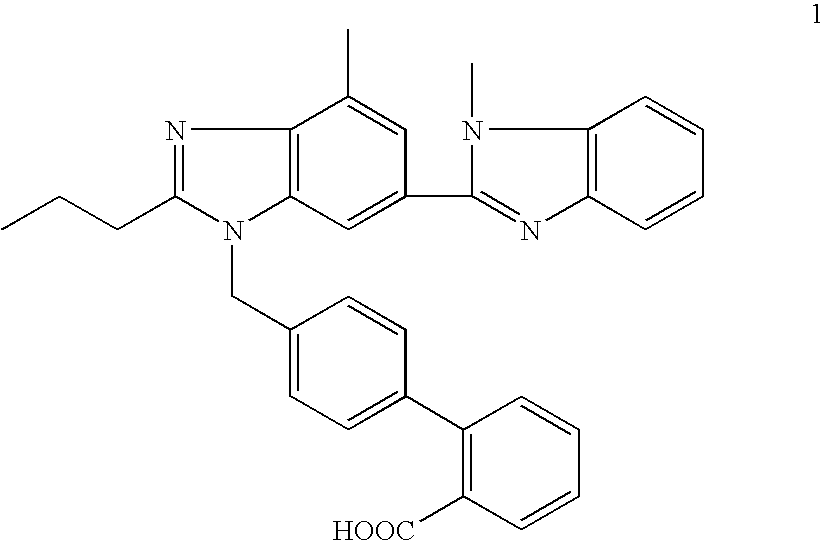
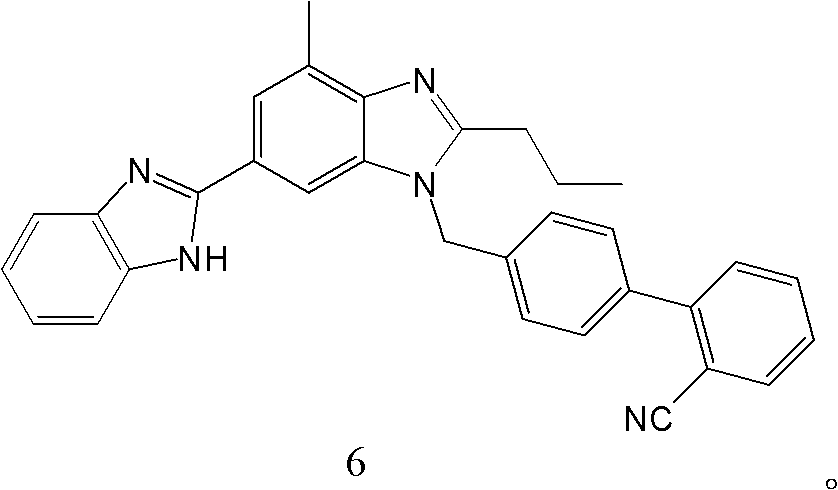
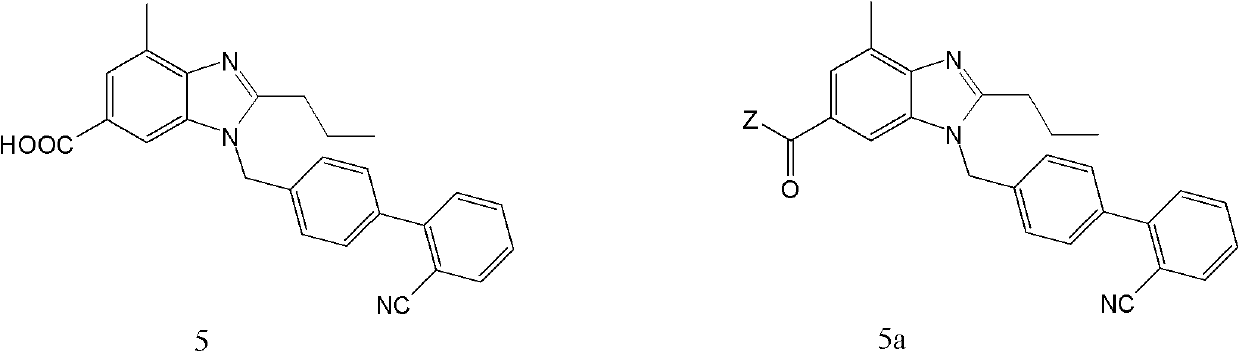
The invention discloses a key intermediate of telmisartan. A chemical name of the key intermediate is 4'-[(4'-methyl-2'propyl[2,6'-bi-1H-benzimidazole]-1'-yl) methyl], and the key intermediate has a structural formula (6) shown below. The invention also discloses a synthetic method for the key intermediate of the telmisartan and a method for synthesizing the telmisartan by the intermediate. A newprocess for synthesizing the telmisartan by the intermediate thereof has the advantages of low-cost and readily available materials, mild reaction condition, low production cost, high yield and quality and the like.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
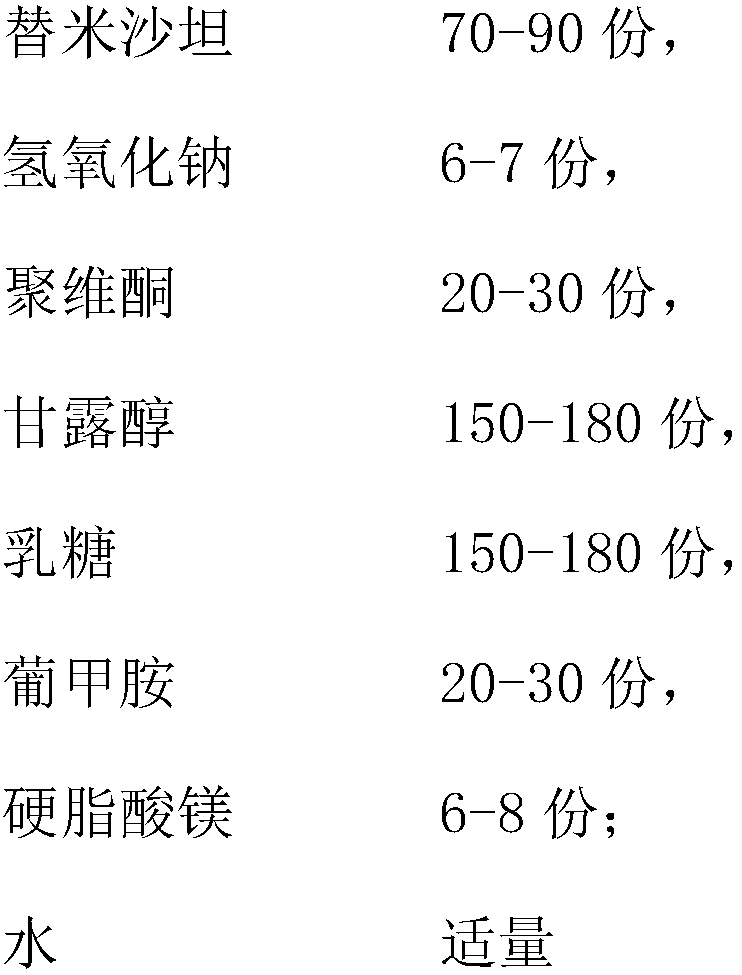
Telmisartan tablet composition
ActiveCN101897676ALow packaging requirementMeet stability requirementsOrganic active ingredientsPill deliveryMedicineMagnesium stearate
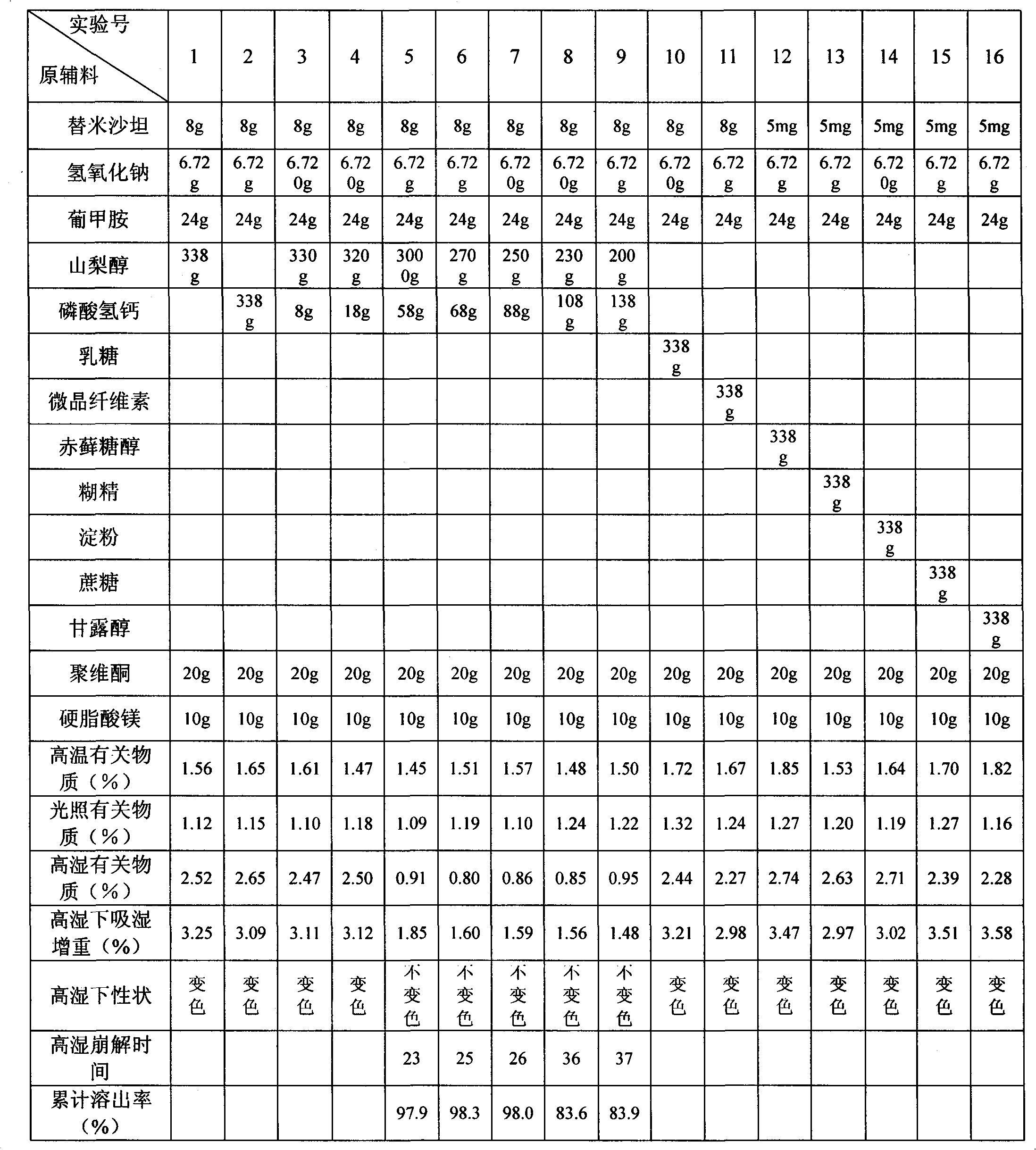
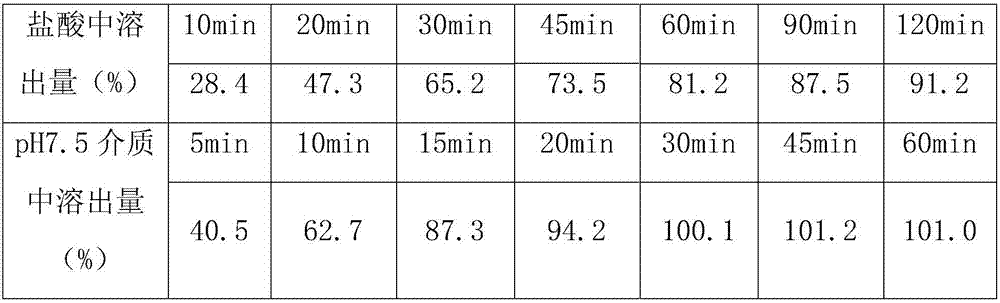
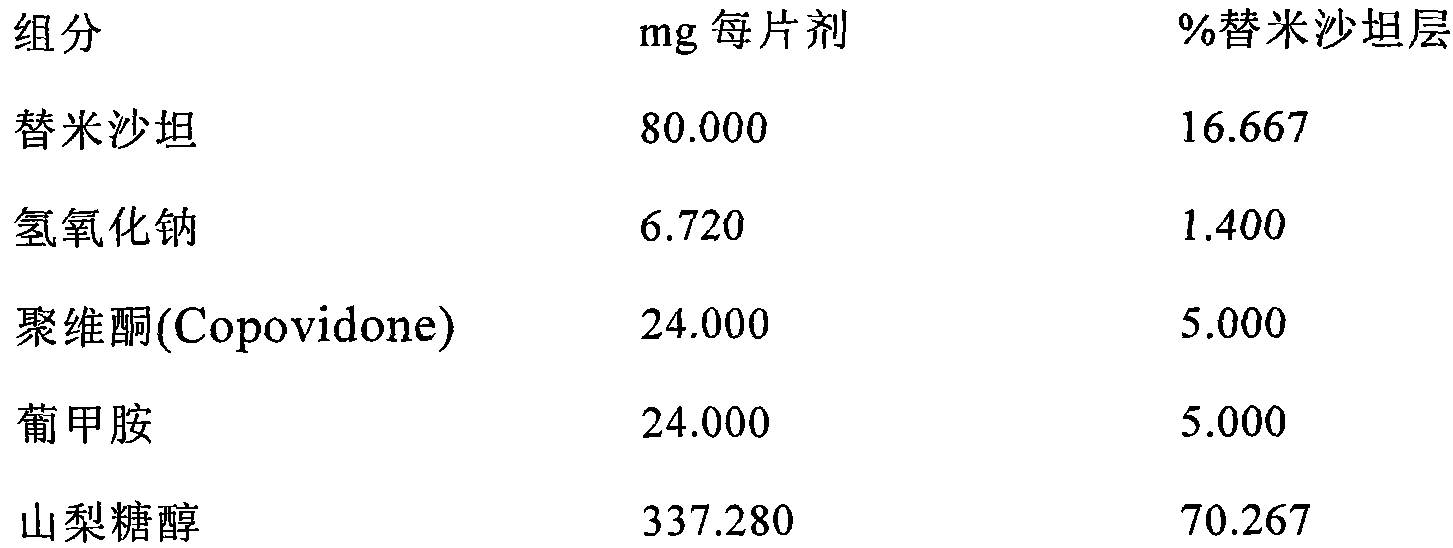
The invention belongs to the technical field of medicinal preparation, and in particular relates to a telmisartan tablet composition. The telmisartan tablet composition is characterized by being prepared from 80 parts of telmisartan, 6.72 parts of sodium hydroxide, 24 parts of meglumine, 250 to 280 parts of sorbierite, 58 to 88 parts of calcium hydrophosphate, 20 parts of polyvidone and 10 parts of magnesium stearate. By selecting and using the combination of sorbierite and calcium hydrophosphate as filler, the composition can obviously improve the stability of the preparation and is favorable for production and application of the preparation.
Owner:BEIJING JINGFENG PHARMA GRP
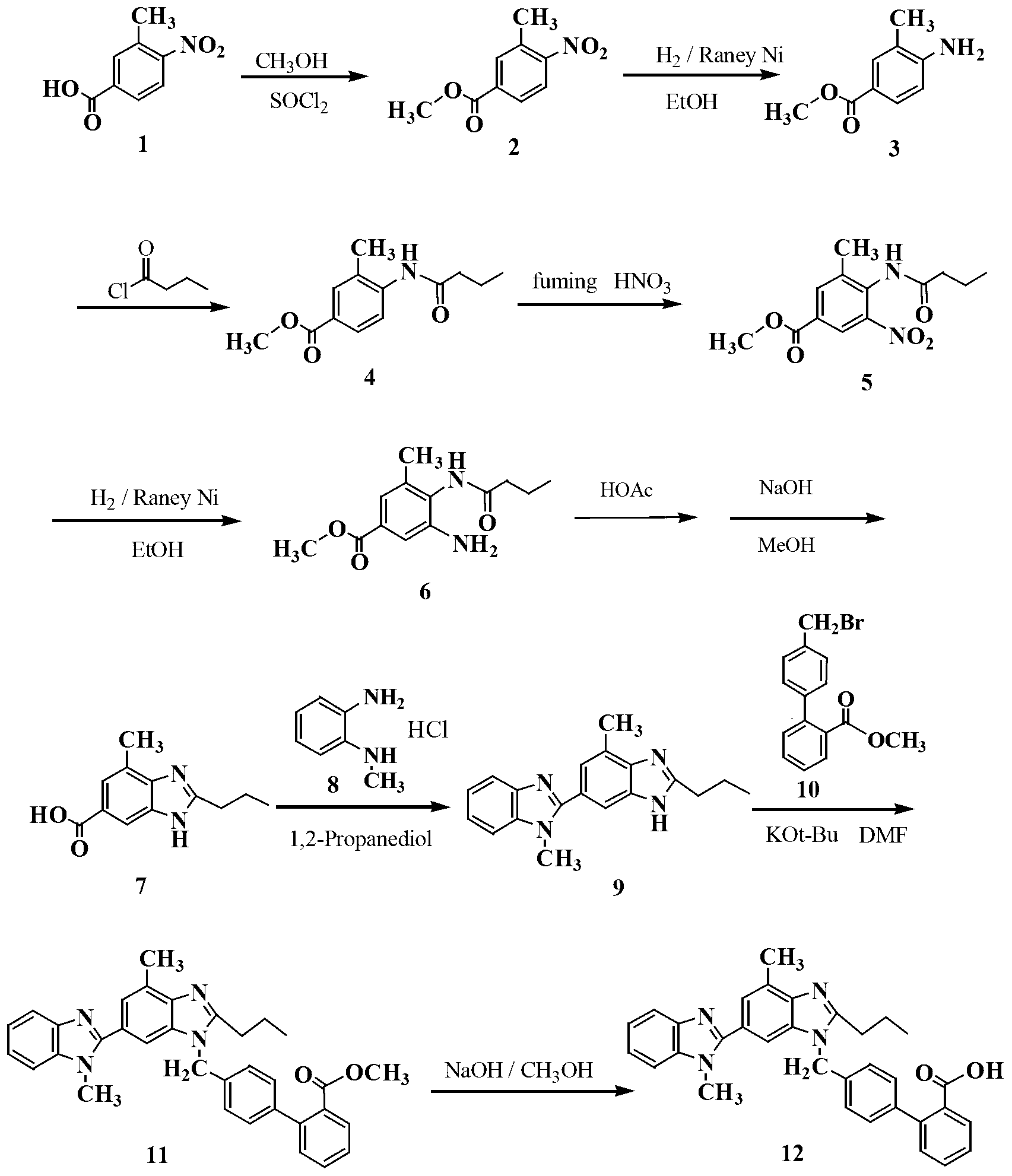
Preparation method for telmisartan
The invention discloses a preparation method for telmisartan, and belongs to the technical field of pharmaceutical synthesis. The key points of the technical scheme lie in that in the preparation method for the telmisartan takes ionic liquid as a reaction medium, and 3-methyl-4-nitryl-benzoic acid as a raw material, 2-n-propyl-4-methyl-6- carboxyl benzimidazole is obtained successively through an esterification reaction, a reduction reaction, an acylation reaction, a nitratlon reaction, a reduction reaction and a cyclization reaction, then under the action of a polyphosphoric acid, the 2-n-propyl-4-methyl-6-carboxyl benzimidazole and N-methyl-o-phenlenediamine dihydrochloride are condensed to obtain 2-n-propyl-4-methyl-6-[1-methylbenzimidazole-2-yl] benzimidazole, 2-n-propyl-4-methyl-6-[1-methylbenzimidazole-2-yl] benzimidazole is condensed with 4,-bromine biphenyl-2-carboxylic acid methyl ester, and hydrolysis is carried out to obtain the telmisartan. According to the method, the ionic liquid is used for replacing methanol with larger toxicity in an original technology to be used as the reaction medium, raw materials are easy to obtain, the operation is simple and convenient, the yield is high, the purity of products is high, the residual quantity of an organic solvent is small, and the method is environmentally-friendly and is suitable for industrialized production.
Owner:HENAN NORMAL UNIV
Compound preparation of telmisartan and amlodipine and preparation method thereof
ActiveCN101780078AOvercome the defect that the compound cannot be completely separatedOrganic active ingredientsGranular deliveryAmlodipineChemistry
The invention provides a compound preparation of telmisartan and amlodipine and a preparation method thereof. The compound preparation consists of first mini-pills containing the telmisartan and second mini-pills containing the amlodipine. The preparation has high stability and convenient production. The invention also relates to a preparation method for the compound preparation of telmisartan and amlodipine.
Owner:湖南威特制药股份有限公司
Amorphous telmisartan
Amorphous telmisartan and combinations of amorphous telmisartan with one or more pharmaceutical carriers.
Owner:DR REDDYS LAB LTD +1
Improved telmisartan preparation process
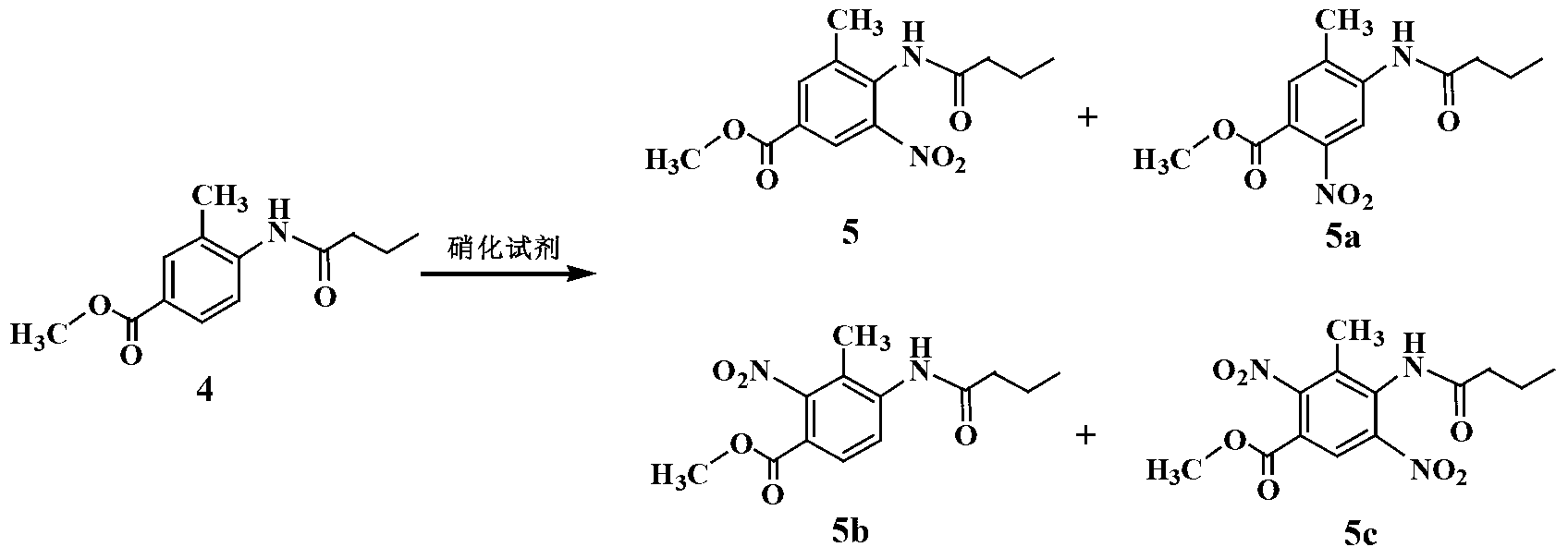
InactiveCN103319414AEasy to operateShorten the production cycleOrganic chemistryChromatographic separationNitration
The invention provides an improved process for preparing a telmisartan raw material medicament. The improved process comprises the following step: preparing an intermediate 2-n-propyl-4-methyl-benzimidazole-6-carboxylic acid by using methyl 4-butylacetamino-3-methylbenzoate as a raw material through four steps, namely nitration, reduction, cyclization and hydrolysis. According to the process, crude products in nitrification, reduction and cyclization reactions are not subjected to purification and separation, and a target product can be separated by regulating the pH of a reaction liquid obtained in a hydrolysis reaction to be between 6 and 7, so that the problems of complicated operation and yield loss caused by separation of nitrified and reduced products by recrystallization and chromatographic separation of a cyclized product by a column are solved. Therefore, the purity of the prepared telmisartan crude product is over 99 percent after recrystallization. The improved process is simple to operate, high in yield and low in cost, and is advantageous to industrial production.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Solid oral preparation containing telmisartan and preparation method thereof
ActiveCN102114015ARapid dissolutionQuality improvementOrganic active ingredientsPill deliverySURFACTANT BLENDReagent
The invention provides a solid oral preparation without a surfactant. The solid oral preparation comprises the following components in percentage by weight: 5-25% of telmisartan, an alkaline reagent and the balance of pharmaceutically acceptable vectors, wherein the weight of the alkaline reagent is equal to 7-20% of that of telmisartan. The invention also provides a method for preparing the solid oral preparation. The preparation prepared by the method can be quickly and sufficiently released in the psychophysical pH range of gastrointestinal tracts; and compared with the prior art, the preparation method has the advantages that the process is simpler, the quality of the soli oral preparation is more stable, the cost is greatly saved, and the efficiency is improved.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
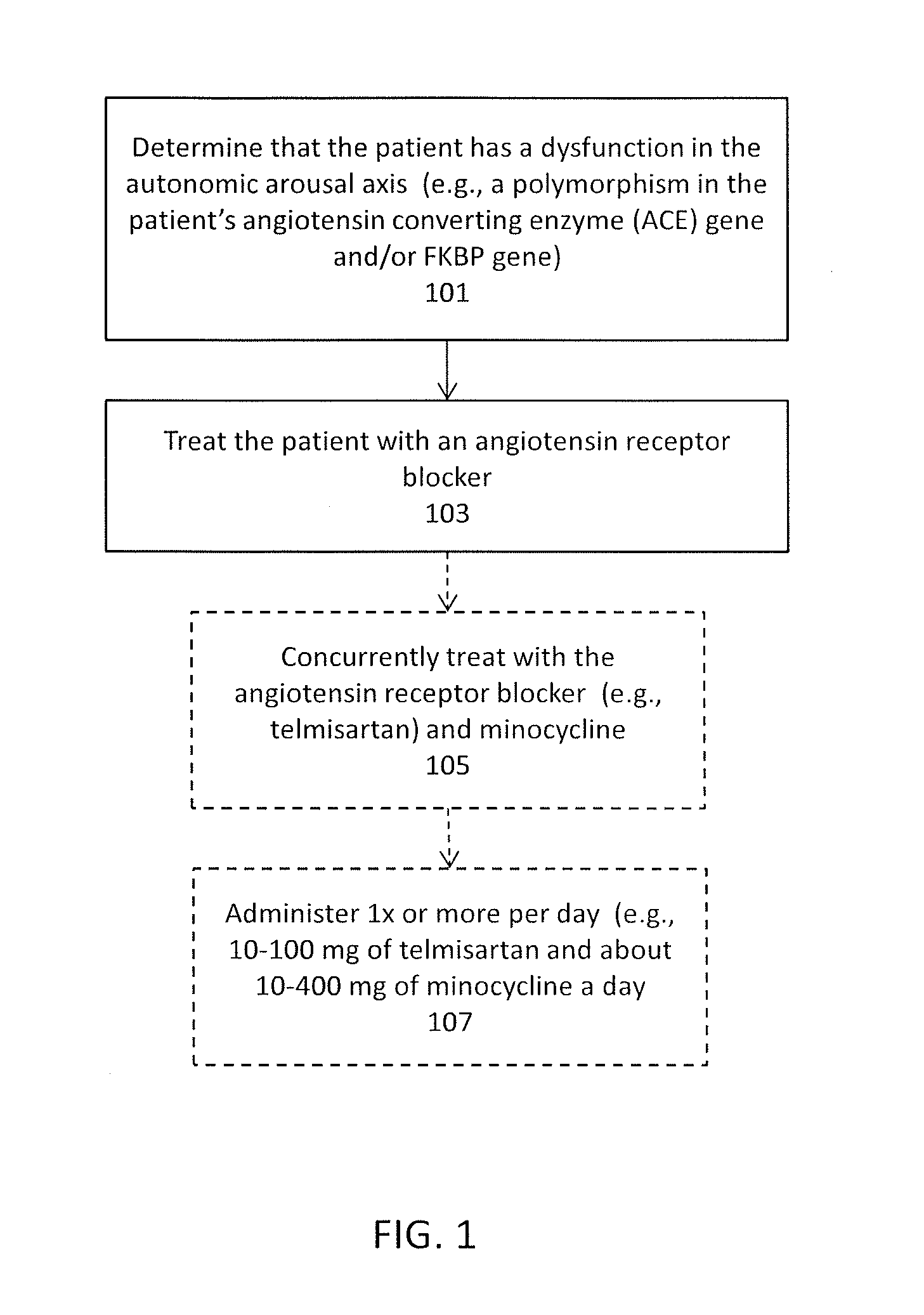
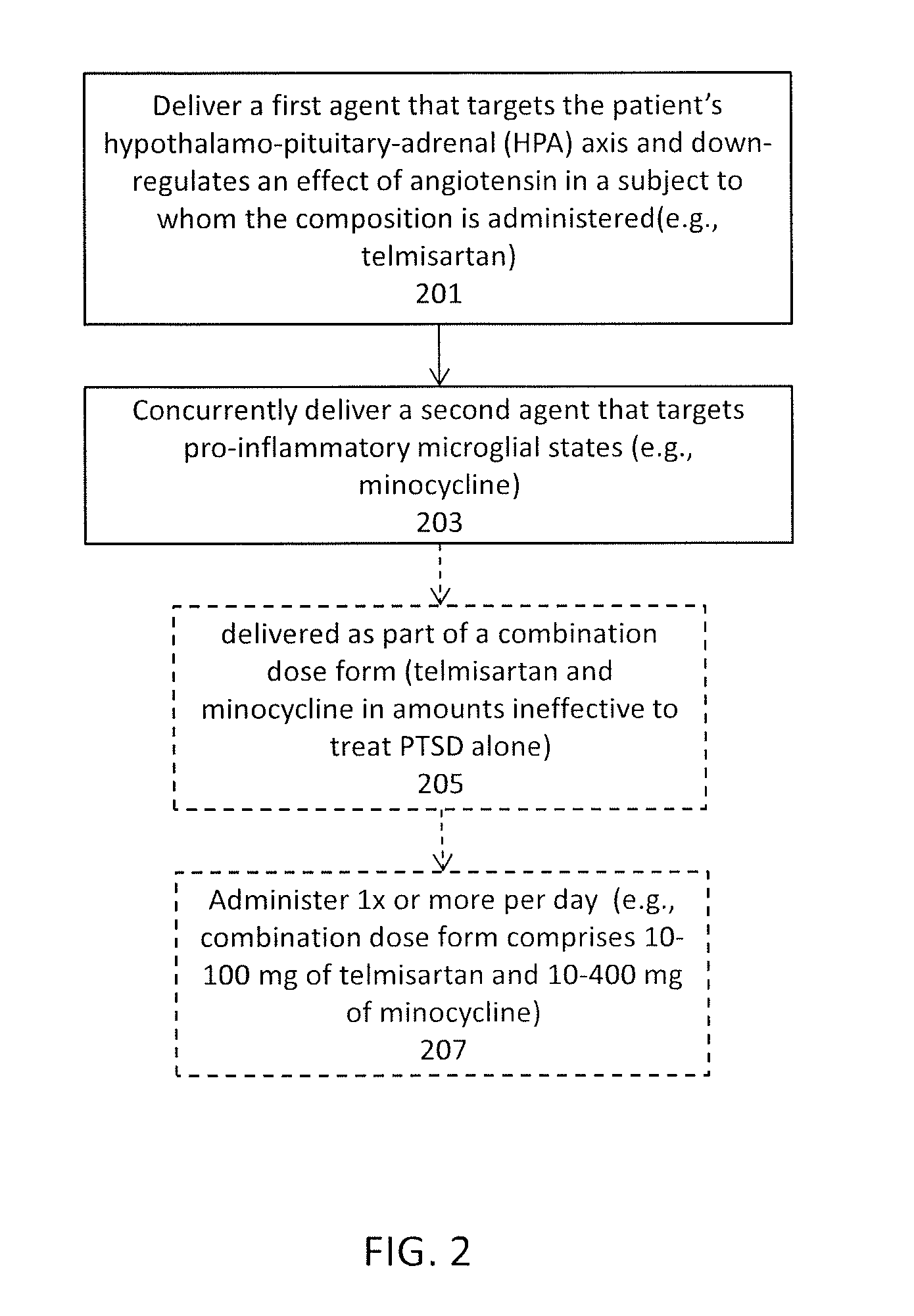
Methods and compositions for the treatment of post-traumatic stress disorder
InactiveUS20170029892A1Improve blood-brain barrier permeabilityReduce hyperactivityTetracycline active ingredientsMicrobiological testing/measurementAngiotensin receptorAngiotensin Receptor Blockers
Methods and compositions are disclosed to treat neuropsychiatric disorders post-traumatic stress disorder (PTSD). In particular, described herein are angiotensin receptor blockers (ARBs), and in particular the combination of one or more ARB (such as telmisartan) and an agent that enhances the delivery of the ARB across the blood-brain barrier (such as minocycline). PTSD may be treated using a combination of telmisartan and minocycline at levels of each that are, by themselves, infective to treat PTSD. Also described herein are methods for treating PTSD by first identifying patents for whom the use of an ARB treatment would be effective, by determining that patient has a dysfunction in their angiotensin converting enzyme and / or other genes in the autonomic arousal axis.
Owner:GENOMIND
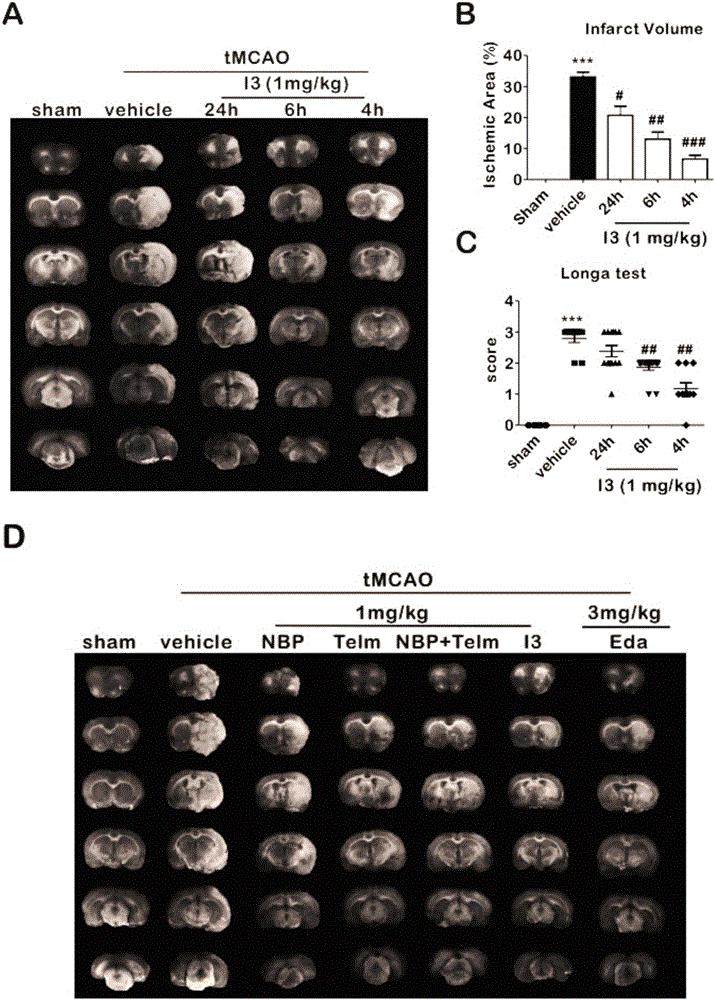
Butylphthalide-telmisartan heterocomplex and preparation method and application thereof
ActiveCN106800537AStrong anti-ischemic activityOrganic active ingredientsNervous disorderDiseaseMedicine
The invention relates to the fields of pharmaceutical chemistry and pharmaceutical therapeutics, and more particularly to an optically active ring-opening butylphthalide-telmisartan heterocomplex or a pharmaceutically acceptable salt or ester thereof, preparation methods thereof, pharmaceutical compositions containing the compounds, and pharmaceutical application of the pharmaceutical compositions, particularly application in prevention and treatment of neuroinflammation-related diseases, including ischemic stroke, alzheimer's disease, brain trauma, Parkinson's disease, multiple sclerosis and depression.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Telmisartan medicinal composition, telmisartan medicinal composition tablets and preparation method for telmisartan medicinal composition tablets
ActiveCN102526037AGood water solubilityHas a diuretic effectOrganic chemistryHydroxy compound active ingredientsTherapeutic effectMagnesium stearate
The invention provides a telmisartan medicinal composition, telmisartan medicinal composition tablets and a preparation method for the telmisartan medicinal composition tablets. The composition consists of the following components: telmisartan sodium salt, sorbitol, polacrilin potassium, anhydrous calcium phosphate, lactose, microcrystalline cellulose and magnesium stearate. The invention provides a preparation method for the telmisartan sodium salt, reaction conditions are mild, few side reactions are performed, and the generated sodium salt has higher water solubility; meanwhile, the sorbitol in the medicine has a diuresis effect, and has a synergistic antihypertensive effect with telmisartan in human bodies, so that the therapeutic effect of the product is strengthened; and after the telmisartan is taken, the urinary albumin excretion rate (UAER) of patients with early diabetic nephropathy is remarkably reduced, so the telmisartan has a kidney protection effect.
Owner:CHONGQING CONQUER PHARML
Preparation technology of telmisartan active pharmaceutical ingredient
InactiveCN101983962AThe preparation process route is simpleThe preparation process is matureOrganic chemistryBenzoic acidNitration
The invention relates to a preparation technology of a telmisartan active pharmaceutical ingredient, which comprises the technological routes: taking 3-methyl-4-nitryl-benzoic acid as an initial raw material; preparing 4-amino-3-methyl benzoic acid methyl ester through esterification and reduction; preparing 4-methyl-2-propyl-benzimidazole-6-carboxylic acid through acylation, nitration of fuming nitric acid, reduction, cyclization and hydrolysis; producing 4-methyl-2-propyl-6-(1-benzimidazole-methylbenzimidazole-2-yl)-benzimidazo through the condensation with N-methy-o-phenylenediamine; and condensing with 4'-brooethyl biphenyl-2-carboxylic methyl ester in the presence of potassium tert-butoxide and preparing a telmisartan crude product through the hydrolysis of a sodium hydroxide solution-methanol system. The technological routes are simple and mature. The total molar yield can finally reach 23.2% through improving the traditional technological routes; and reaction operation is more easier, the process is simpler, serious three-waste pollution is not generated and the preparation technology is convenient for industrial production.
Owner:FUZHOU NEPTUNUS FUYAO PHARMA
Compound tablet containing telmisartan and hydrochlorothiazide
ActiveCN102512423AEvenly mixedAvoid delamination during tablet compressionOrganic active ingredientsPharmaceutical delivery mechanismHydrochlorothiazideMedicine
The invention discloses a compound tablet containing telmisartan and hydrochlorothiazide. The tablet is prepared by uniformly mixing telmisartan particles and hydrochlorothiazide particles, and tabletting the mixture. According to the weight of the hydrochlorothiazide particles, the hydrochlorothiazide particles contain 6 to 50 percent of disintegrant. The compound tablet is a single-layer tablet. The telmisartan / hydrochlorothiazide tablet is quickly dissolved and is stable in quality. The invention also provides a preparation method for the compound tablet, which is simple in process, low in cost and suitable for industrial mass production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
New process for preparing telmisartan
InactiveCN102267949ASimple processThe synthesis process steps are simpleOrganic chemistryHydrolysisMethyl group
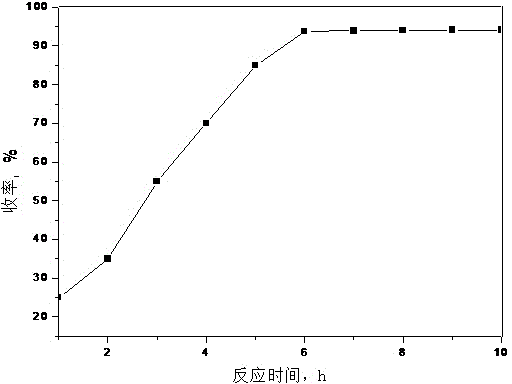
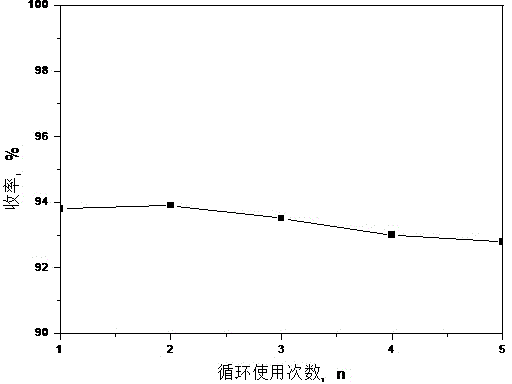
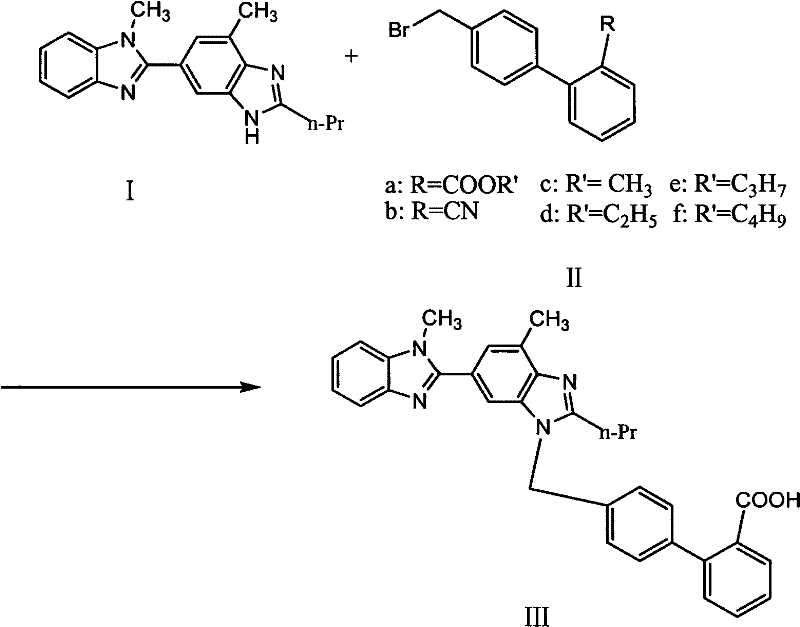
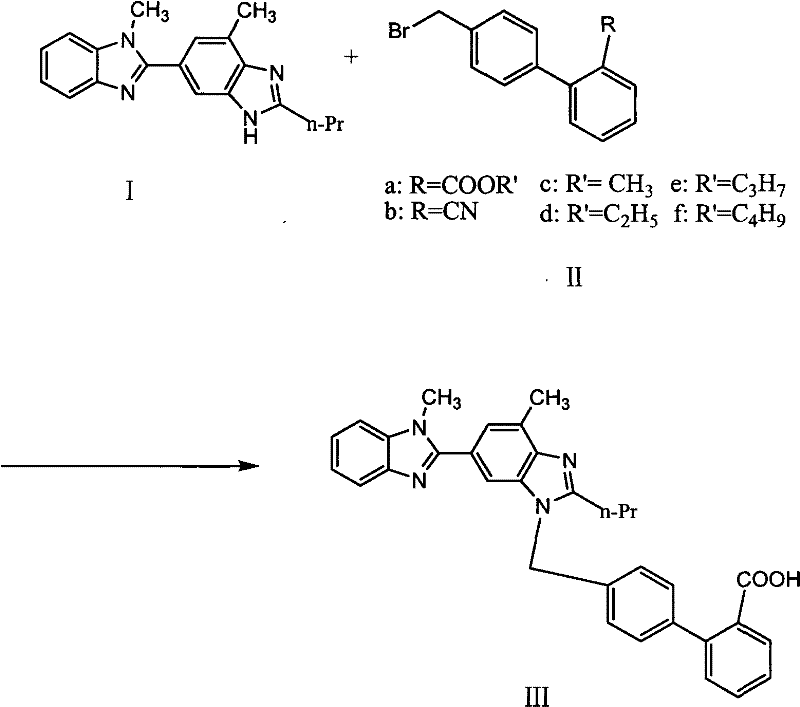
The invention relates to a new process for preparing telmisartan, which uses 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2'-yl)-benzimidazole and 4' -Bromomethyl-biphenyl-2-carboxylate alkyl ester or 4'-bromomethyl-biphenyl-2-carbonitrile undergoes a one-step reaction to complete the two-step synthesis reaction of condensation and hydrolysis to directly obtain Telmisartan. Compared with other processes, it has the advantages of solving the technical difficulties of industrial production, simple operation, green synthesis, high purity of telmisartan, high yield and low cost, and has been successfully applied in industrial production.
Owner:张长利
Telmisartan solid dispersion and preparation method thereof
InactiveCN102178642AImprove solubilityImprove bioavailability in vivoOrganic active ingredientsPharmaceutical delivery mechanismMass ratioDissolution
The invention relates to the technical field of medicines, in particular to a telmisartan solid dispersion as a specific angiotensin II receptor antagonist (ATI type) and a preparation method thereof. The telmisartan solid dispersion comprises medicine telmisartan, a carrier and an alkali matter, and also comprises a surfactant, wherein the carrier is a hydrophilic polymeric carrier, and the mass ratio of the medicine telmisartan to the hydrophilic polymeric carrier to the alkali matter to the surfactant is 1:(1-9):(0.1-0.5):(0.1-0.5). The telmisartan solid dispersion has better dissolution rate during the use, and is convenient for gastrointestinal tract absorption, thereby improving bioavailability.
Owner:SUZHOU UNIV
Composition containing telmisartan and preparing method thereof
InactiveCN101313905AEasy to prepareMature technologyOrganic active ingredientsInorganic non-active ingredientsPharmacologyReagent
The invention provides a medicine composition, which comprises 100 weight portions of telmisartan, 25 to 45 weight portions of alkaline reagents, 15 to 40 weight portions of polyvinylpyrrolidone, and pharmacologically acceptable carriers. The invention also provides a preparation method for the medicine composition.
Owner:上海信谊嘉华药业有限公司
Telmisartan production process
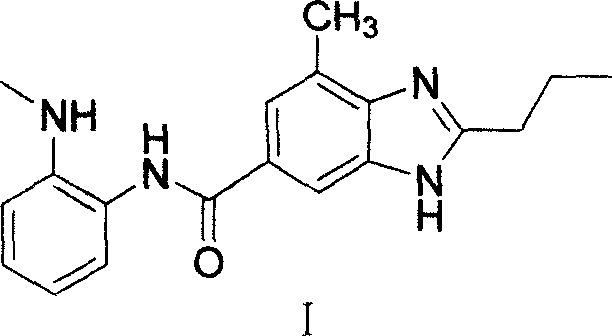
InactiveUS20060264491A1High yieldOvercome limitationsBiocideOrganic active ingredientsChemistryTelmisartan
The present invention provides an intermediate and a process for preparing Telmisartan, which overcomes the drawbacks of conventional methods and produces Telmisartan in high purity and yield.
Owner:CHEMAGIS
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
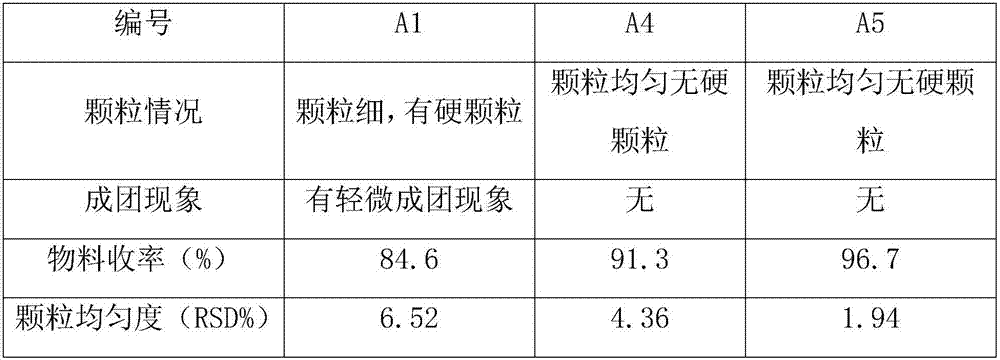
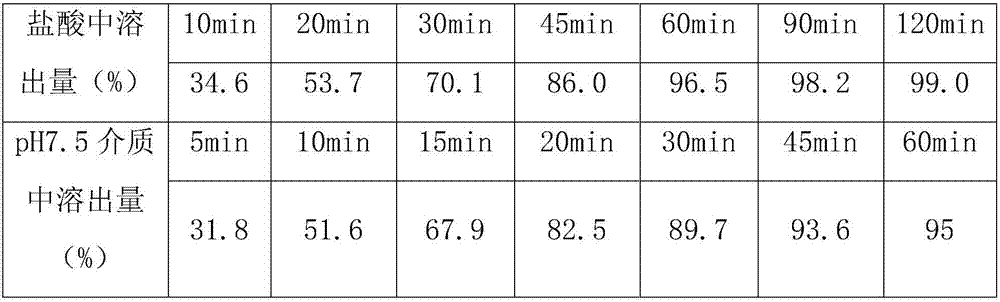
Preparation method of telmisartan tablets
InactiveCN107811984ANo lumpsNo bed collapseOrganic active ingredientsPill deliveryAir volumeAdhesive
The invention relates to a preparation method of telmisartan tablets. The preparation method comprises the following steps: dissolving telmisartan, sodium hydroxide and povidone in purified water forpulping, pelleting with mannitol in a fluidized bed to obtained dried particles, and adding lactose, meglumine and magnesium stearate tablets by adopting an addition method. Equipment conditions adopted according to the invention are as follows: the inlet temperature is 90-95 DEG C (the temperature does not exceed 105 DEG C), the fluidization air volume is 50m<3> / h, the atomizing air pressure is 45psi, and the flow rate of an adhesive is 3r / min.
Owner:南京双科医药开发有限公司
Telmisartan tablet preparation and preparation method thereof
InactiveCN103263395AHigh dependencePromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsLactoseExcipient
The invention discloses a telmisartan tablet preparation and a preparation method thereof. The tablet preparation is prepared through uniformly mixing drug-containing granules with a pharmaceutically acceptable excipient and then tabletting, wherein the drug-containing granules are prepared through uniformly mixing telmisartan and sodium dodecyl sulfate, micronizing the mixture, uniformly mixing the mixture with micronized lactose and then carrying out wet granulation. The preparation disclosed by the invention does not contain alkaline materials, is rapid in drug dissolution and is simple in preparation process, thereby being suitable for being produced in a large scale.
Owner:NANJING ZHENGKUAN MEDICAL TECH
Telmisartan tablet and preparation method thereof
InactiveCN107982232AWell mixedNo obvious agglomerationOrganic active ingredientsInorganic non-active ingredientsParticulatesFiller Excipient
The invention discloses a telmisartan tablet and a preparation method thereof. The preparation method comprises the following steps: 1) in the presence of a solvent, mixing telmisartan with an acid and alkaline regulator to prepare a telmisartan sodium salt; 2) adding a binding agent into the telmisartan sodium salt to prepare slurry, adding a part of a filling agent into the slurry, drying and pelletizing, thereby preparing particulate matters; and 3) adding the rest of the filling agent and a lubricating agent into the particulate matters to tablet, thereby preparing the telmisartan tablet,wherein the acid and alkaline regulator comprises at least sodium hydroxide. The preparation method is used for firstly mixing the telmisartan with the acid and alkaline regulator in the existence ofthe solvent, adding the binding agent, then adding one part of the filling agent, drying and pelletizing to prepare the particulate matters, and further, adding the rest of the filling agent and all the lubricating agent into the particulate matters to mix and tablet, so that the telmisartan in the telmisartan tablet prepared through the steps is uniformly mixed without an obvious clustering phenomenon, and therefore, the material yield is relatively high.
Owner:湖南威特制药股份有限公司
Levamlodipine and telmisartan compound preparation
InactiveCN102058591AImprove toleranceSuppress heart rateOrganic active ingredientsPill deliveryHigh dosesLevamlodipine
The invention discloses a levamlodipine and telmisartan compound preparation and an optimal compound formulation. In the invention, the combination of the levamlodipine and the telmisartan is used for treating hypertension, good synergic antihypertension effect is obtained between the levamlodipine and the telmisartan, the doses of the levamlodipine and the telmisartan can be reduced while the equivalent and even better antihypertension effect is achieved, and the adverse effect caused by high dose of single drug is reduced. Besides, the telmisartan can obviously improve the tolerance of the levamlodipine, can inhibit heart rate increase caused by the levamlodipine and can reduce the incidence of peripheral edema.
Owner:SHIHUIDA PHARMA GRP (JILIN) LTD
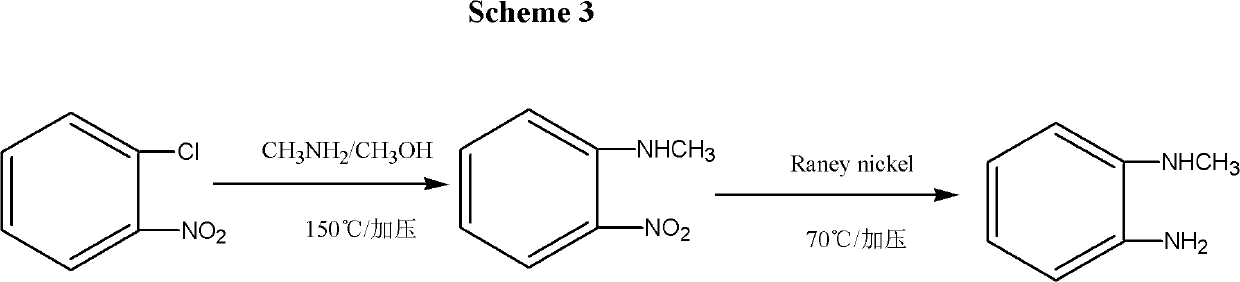
Synthesis method for N-Methyl-o-Phenylenediamine (salt) and isomeride thereof
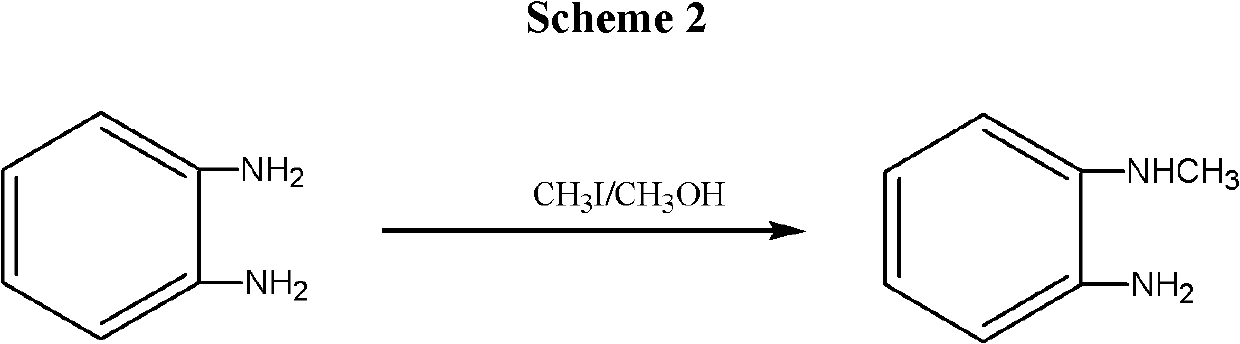
InactiveCN102557964ARaw materials are cheap and easy to getHigh selectivityOrganic compound preparationAmino compound preparationSynthesis methodsUnit operation
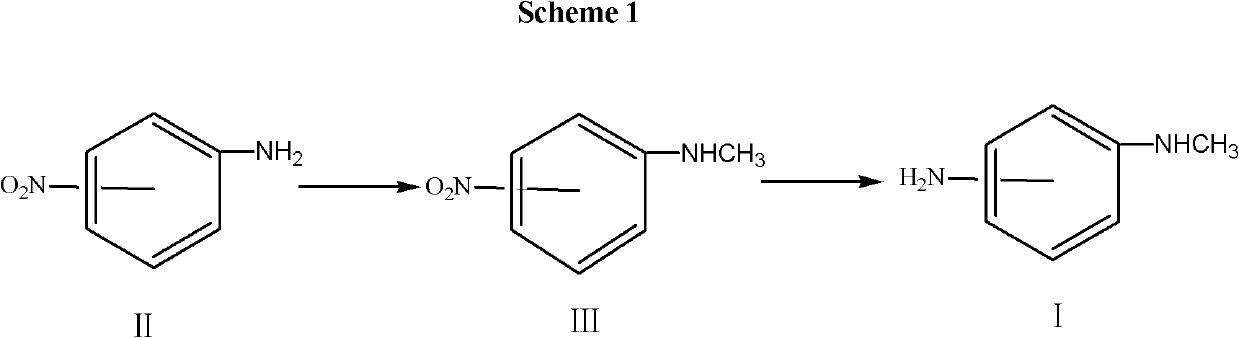
The invention discloses a synthesis method for a telmisartan intermediate, N-Methyl-o-phenylenediamine (salt), and isomeride thereof. According to the synthesis method, o (m or p)-nitroaniline is subjected to methylation under the action of a methylation reagent and a catalyst so as to obtain N-Methyl-o (m or p)-nitroaniline; and then under the action of a reducing agent, the N-Methyl-o (m or p)-nitroaniline is used to prepare the N-Methyl-o (m or p)-phenylenediamine. According to the synthesis method, the o (m or p)-nitroaniline is adopted as an initial material and is cheap and easy to get, the selectivity of actions of the methylation reagent is strong, and by-product of N, N-dimethyl is avoided. The reaction in the two steps are all subjected to conventional unit operation, the reaction is simple and convenient, and the yield and quality are high, so that the synthesis method is applicable to mass industrial production.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
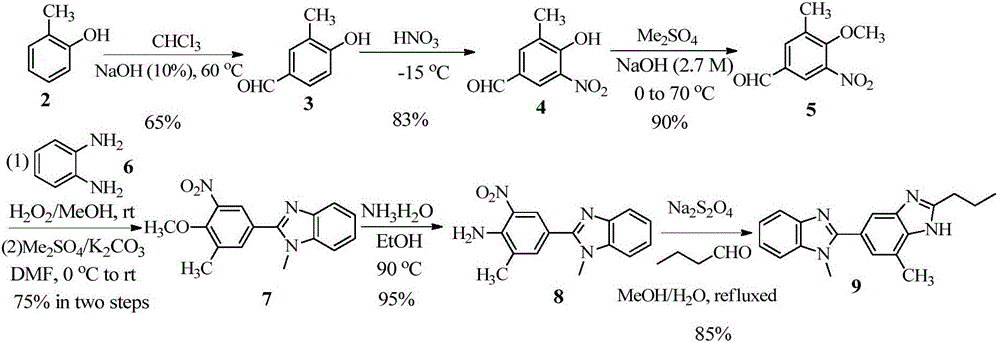
Benzimidazole compound and preparation method thereof
The invention discloses a benzimidazole compound and a preparation method thereof, and applications of the benzimidazole compound in preparation of telmisartan. According to the present invention, the prepared benzimidazole compound can be used for preparing telmisartan, and the preparation method has advantages of low raw material cost, simple operation, safety, controllability, high product purity and high total yield, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com