Preparation method of telmisartan tablets
A technology of telmisartan and tablets, which is applied in the field of preparation of pharmaceutical preparations, can solve the problems of lumping or bed collapse, uneven mixing, and easy adsorption on the wall of the device, and achieves the improvement of stability and dissolution rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
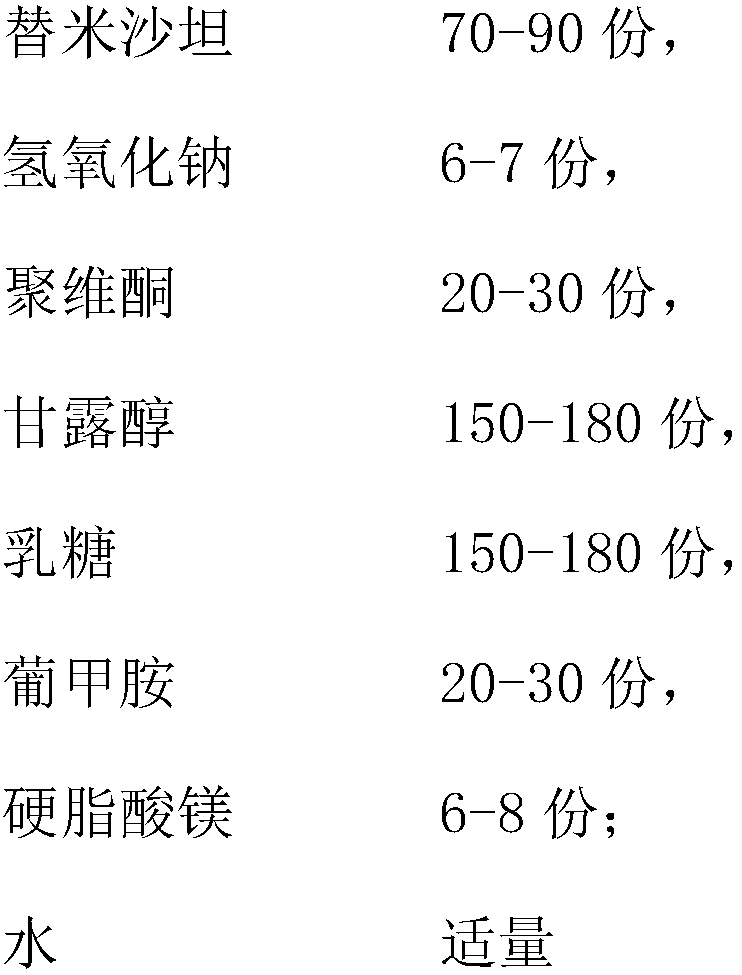
[0050] Prior art prepares telmisartan tablet
[0051]
[0052]
[0053] Preparation:
[0054] Telmisartan, sodium hydroxide, povidone, and meglumine are dissolved in purified water to make slurry and mannitol is granulated in a fluidized bed
[0055] Additional excipients spray lactose, magnesium stearate compressed tablet
Embodiment 2
[0056] Embodiment 2: Telmisartan tablet of the present invention
[0057]
[0058] Preparation:
[0059] Telmisartan, sodium hydroxide, and povidone are dissolved in purified water to make slurry and mannitol is granulated in a fluidized bed
[0060] Additional excipients meglumine, spray lactose, magnesium stearate compressed tablet
[0061] The equipment conditions are as follows:
[0062] The inlet temperature is 90-95°C (the temperature must not exceed 105°C)
[0063] Fluidization air volume 50m 3 / h
[0064] Atomizing air pressure 45psi
[0065] Adhesive flow rate 3r / min
Embodiment 3
[0066] Embodiment 3: prior art prepares telmisartan tablet
[0067]
[0068] Preparation:
[0069] Telmisartan, sodium hydroxide, and povidone are dissolved in purified water to make slurry and mannitol is granulated in a fluidized bed
[0070] Additional excipients spray lactose, magnesium stearate compressed tablet
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com