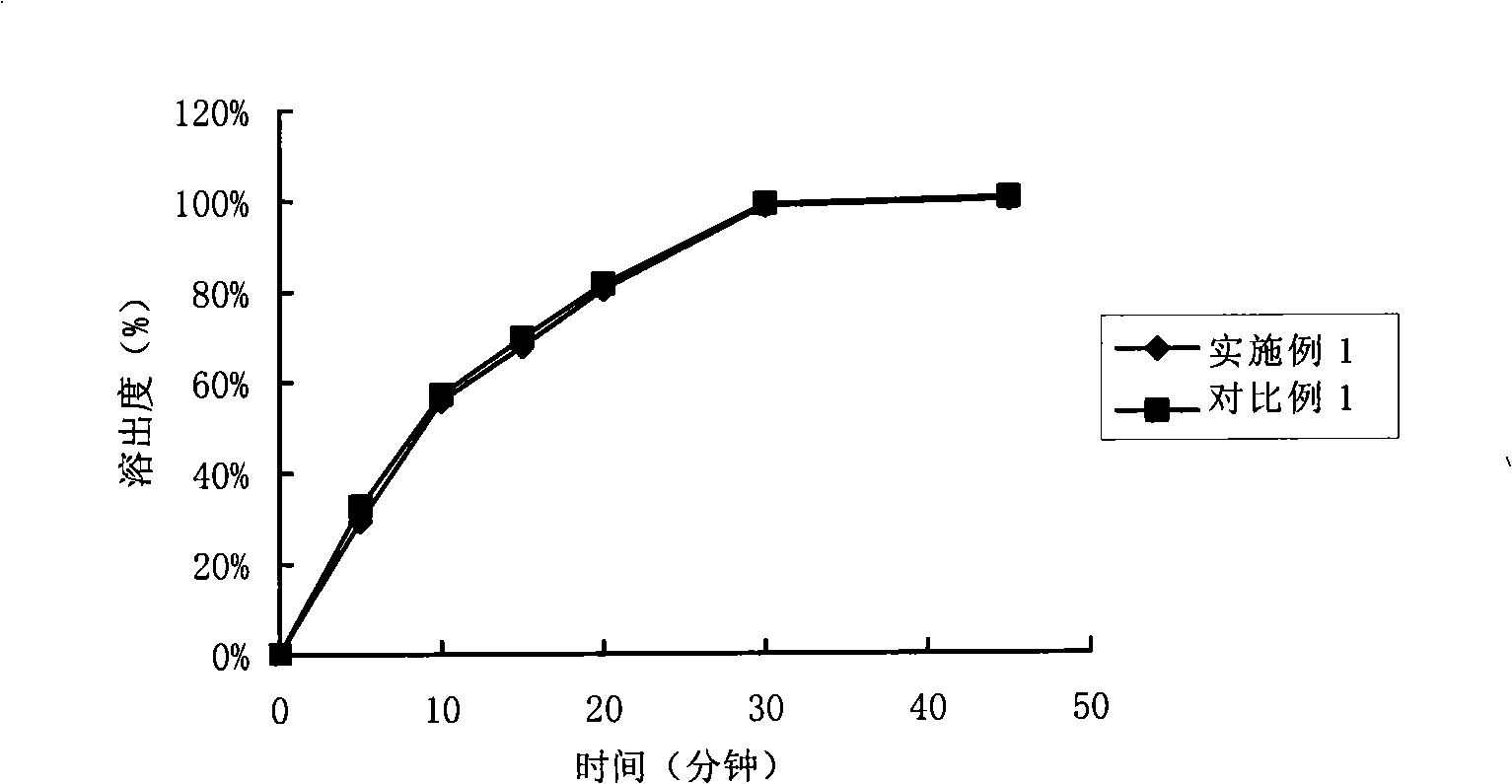
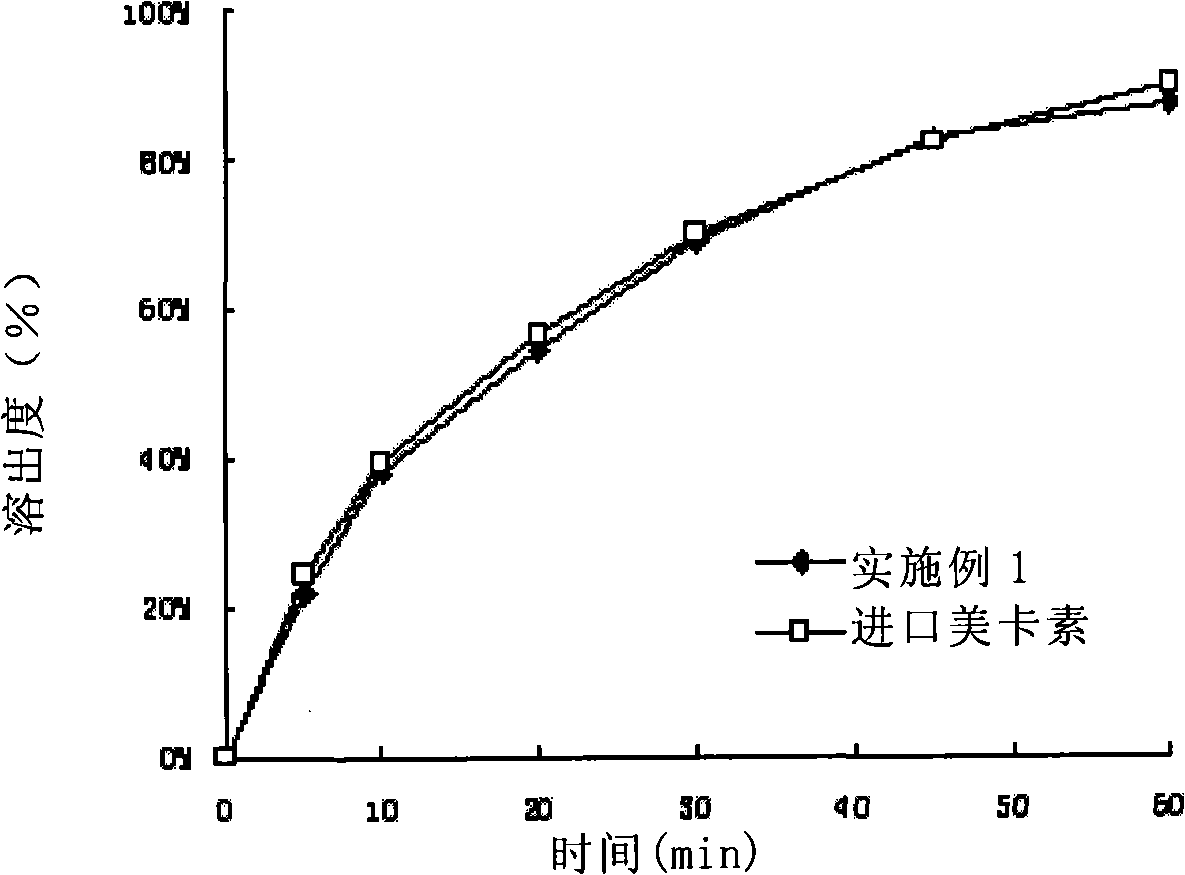
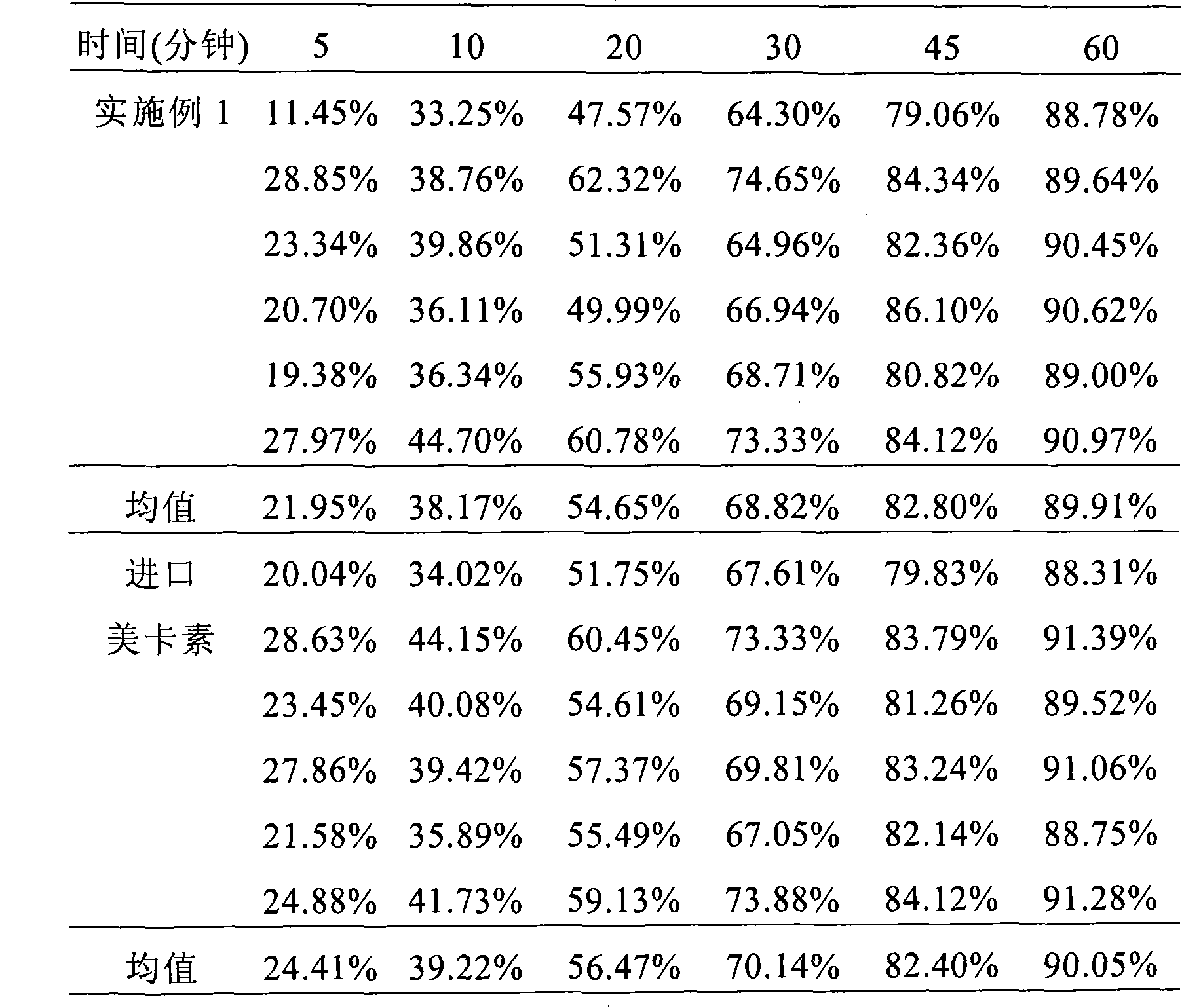
Composition containing telmisartan and preparing method thereof
A technology for telmisartan and a composition, which is applied to the field of tablets containing telmisartan, can solve problems such as time-consuming and laborious spray drying methods, and achieve the effects of improving efficiency, mature technology and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Telmisartan 40g
[0050] Polyvinylpyrrolidone 12g
[0051] Meglumine 12g
[0053] Microcrystalline Cellulose 80g
[0054] Mannitol 90g
[0055] Cross-linked polyvinylpyrrolidone 40g
[0057] Dissolve the above amount of telmisartan, meglumine, sodium hydroxide and polyvinylpyrrolidone in 95% ethanol-water solution (4:1) (about 110 ml) to obtain a uniform solution. Pass the above amount of mannitol, microcrystalline cellulose and cross-linked polyvinylpyrrolidone through a 60-mesh sieve, and then mix uniformly to obtain a granular mixture. The resulting particle mixture is placed in a fluidized bed and sprayed with the above homogeneous solution. The granules sprayed with the solution are dried and passed through a 24-mesh sieve for sizing, then the above-mentioned amount of magnesium stearate is added, mixed evenly, and compressed into tablets to obtain 1000 tablets of the preparation.
Embodiment 2
[0059] Telmisartan 40g
[0061] Meglumine 8.5g
[0062] Polyvinylpyrrolidone (K30) 16.0g
[0063] Microcrystalline Cellulose 70g
[0064] Cross-linked polyvinylpyrrolidone 34g
[0065] Mannitol: 40g
[0067] Dissolve the above amount of telmisartan, meglumine, sodium hydroxide and polyvinylpyrrolidone in 95% ethanol-water solution (4:1) (about 110 ml) to obtain a uniform solution. Pass the above amount of mannitol, microcrystalline cellulose and cross-linked polyvinylpyrrolidone through a 60-mesh sieve, and then mix uniformly to obtain a granular mixture. The resulting particle mixture is placed in a fluidized bed and sprayed with the above homogeneous solution. The granules sprayed with the solution are dried and passed through a 24-mesh sieve for sizing, then the above-mentioned amount of magnesium stearate is added, mixed evenly, and compressed into tablets to obtain 1000 tablets of the preparation.
Embodiment 3
[0069] Telmisartan 40g
[0070] Sodium hydroxide 6g
[0071] Meglumine 12g
[0072] Polyvinylpyrrolidone (K30) 8.0g
[0073] Microcrystalline Cellulose 80g
[0074] Cross-linked polyvinylpyrrolidone 40g
[0075] Mannitol 14g
[0076] Magnesium stearate 0.2g
[0077] Dissolve the above amount of telmisartan, meglumine, sodium hydroxide and polyvinylpyrrolidone in 95% ethanol-water solution (4:1) (about 110 ml) to obtain a uniform solution. Pass the above amount of mannitol, microcrystalline cellulose and cross-linked polyvinylpyrrolidone through a 60-mesh sieve, and then mix uniformly to obtain a granular mixture. The resulting particle mixture is placed in a fluidized bed and sprayed with the above homogeneous solution. The granules sprayed with the solution are dried and passed through a 24-mesh sieve for sizing, then the above-mentioned amount of magnesium stearate is added, mixed evenly, and compressed into tablets to obtain 1000 tablets of the preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com