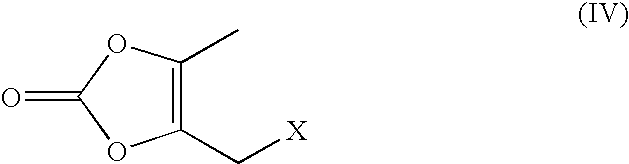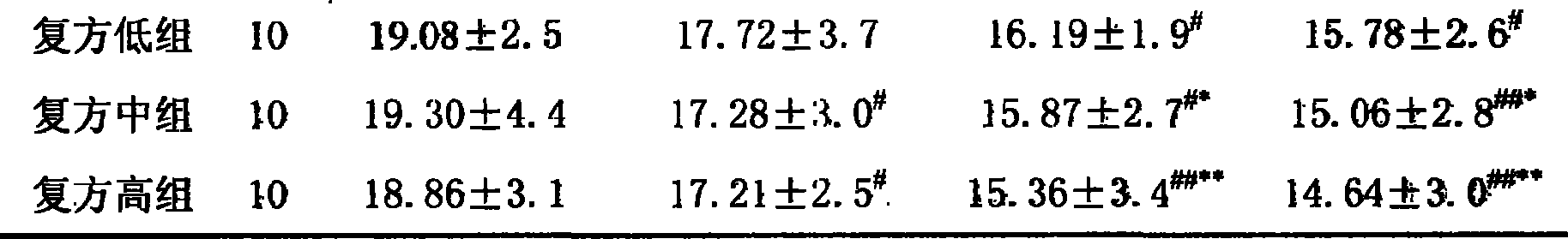Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
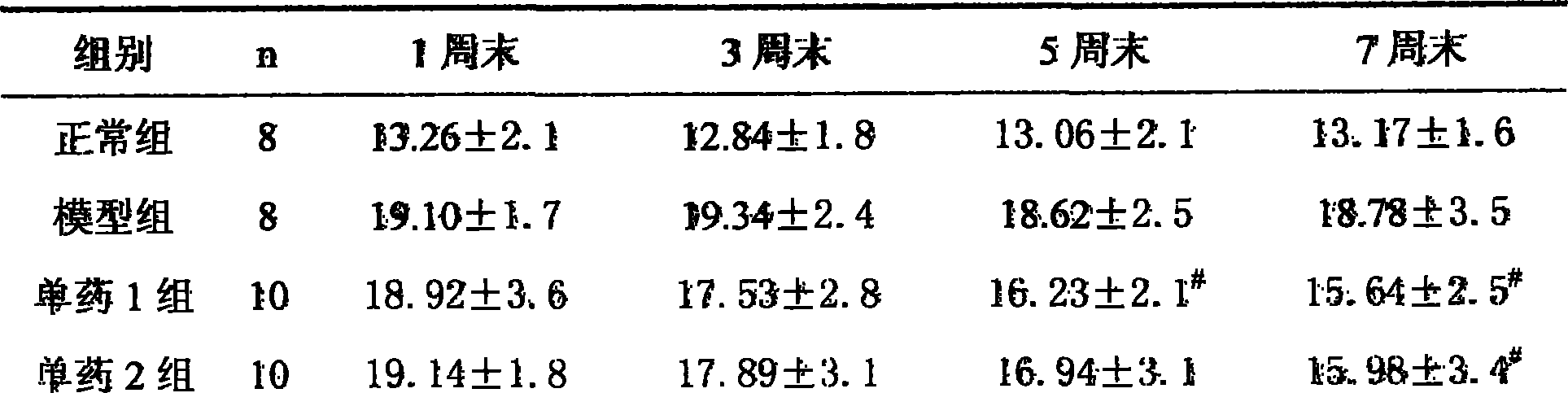
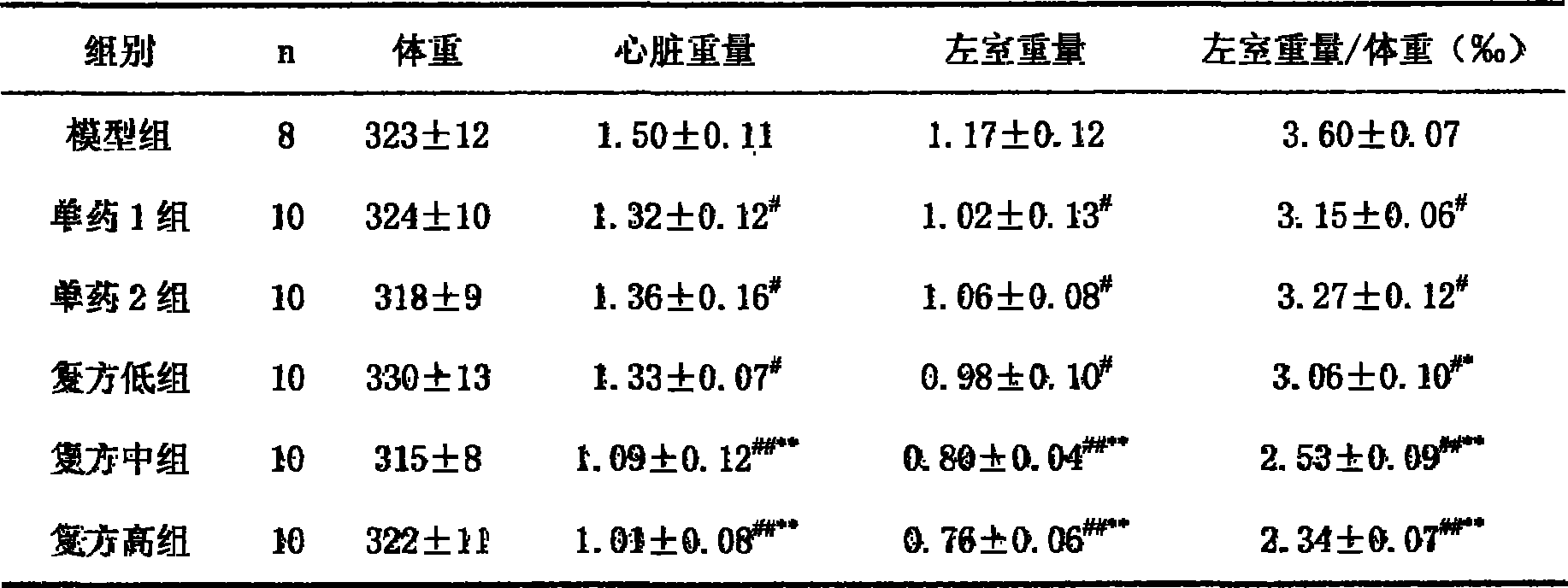
183 results about "Olmesartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
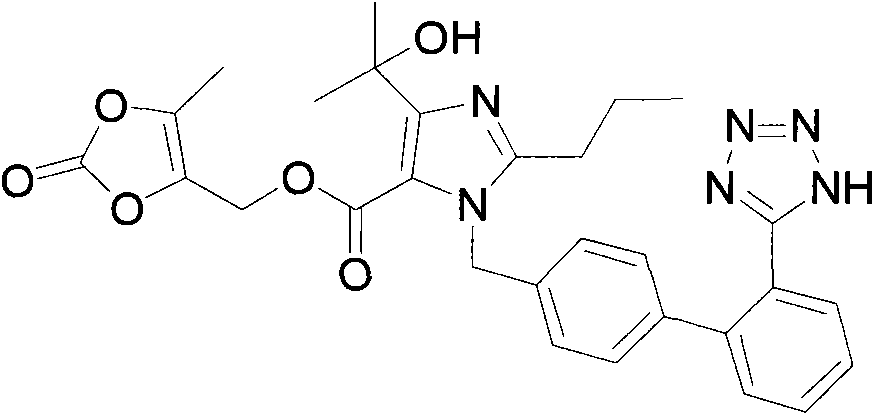
Olmesartan is used to treat high blood pressure (hypertension).
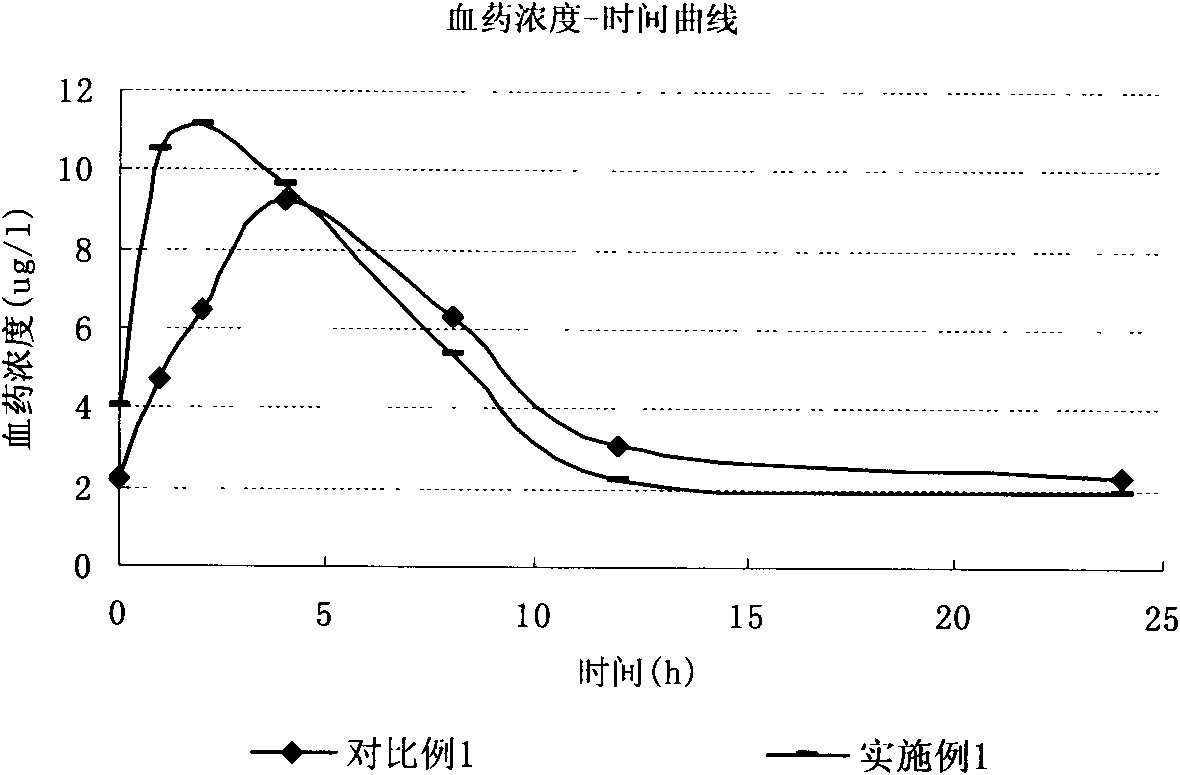
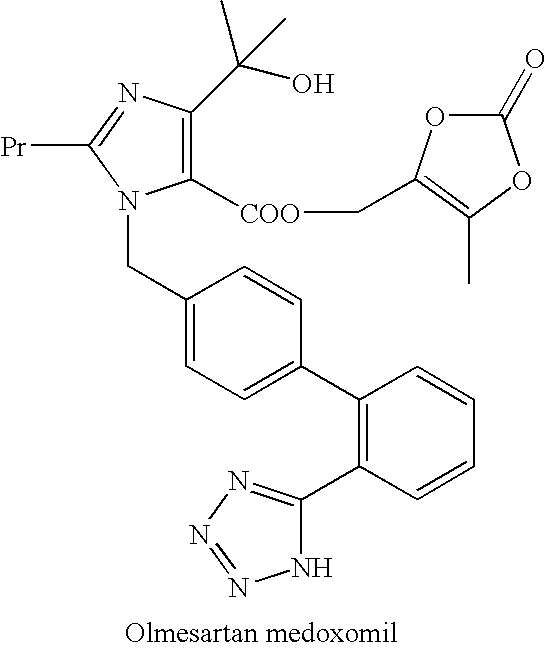
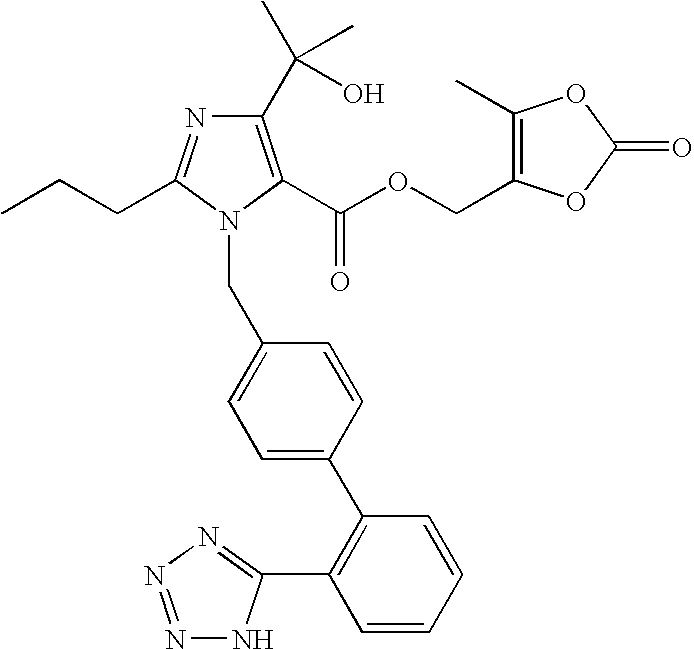
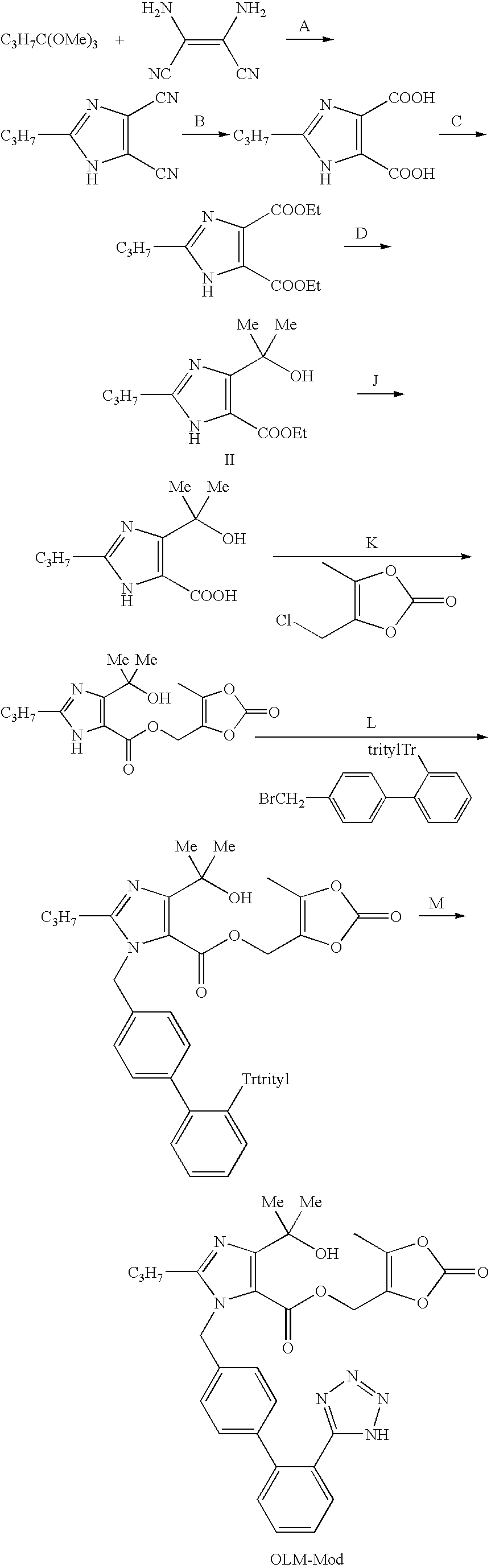
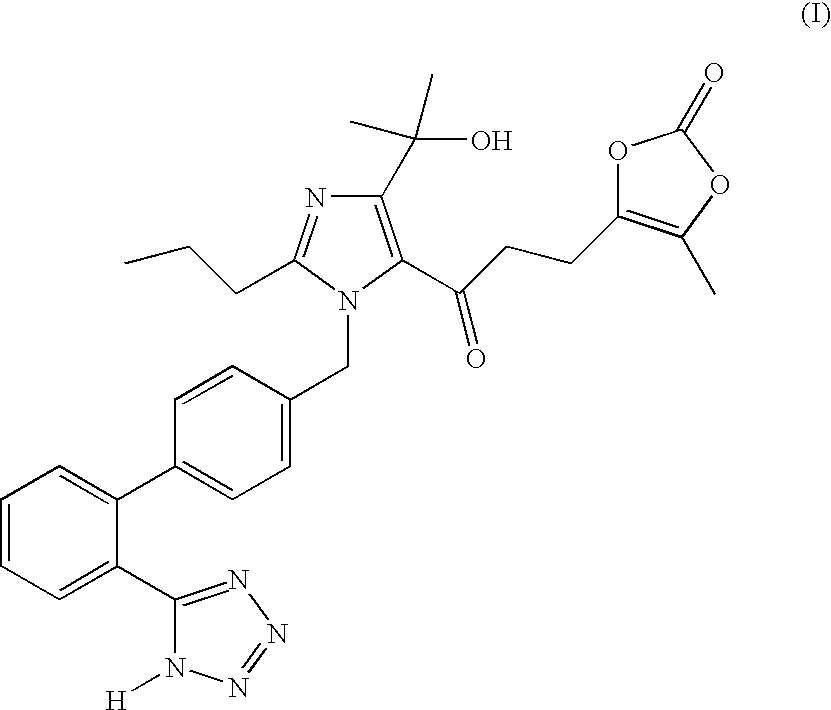
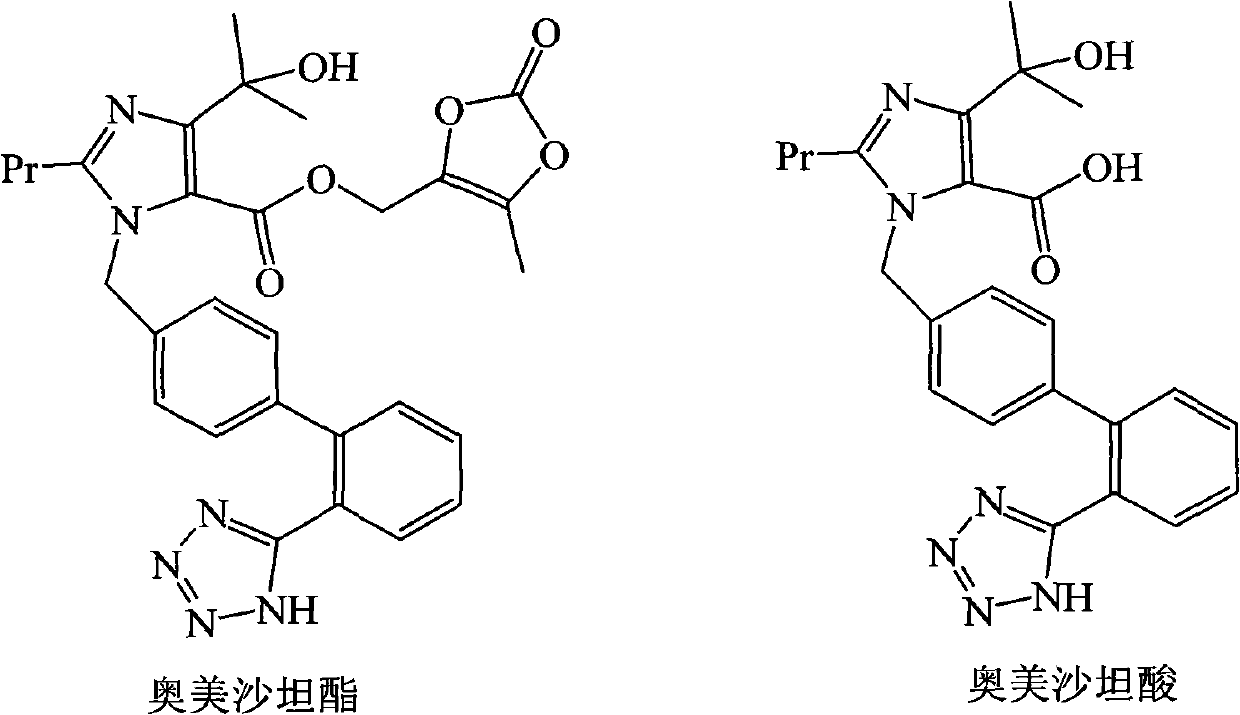
Process for the preparation of olmesartan medoxomil
The present invention relates to an improved process for the manufacture of olmesartan and pharmaceutically acceptable salts and esters thereof as an active ingredient of a medicament for the treatment of hypertension and related diseases and conditions.
Owner:KRKA
Preparation method for olmensartan medoxomil with low-level impurity
InactiveCN102206208AMild conditionsMeet environmental protection requirementsOrganic chemistryBulk chemical productionBenzoyl bromideOlmesartan
The invention relates to the technical field of a preparation method for an olmensartan medoxomil with a low-level impurity. With the invention, 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylic acid ethyl ester and 4-[12-(triphenylmethyl tetrazole-5-yl) phenyl] benzyl bromide are adopted as raw materials, and are sequentially subjected to an alkylating, a hydrolysis and a esterification, and a last process of a protective group removing to obtain the olmensartan medoxomil with high impurity. Content of single impurity of the olmensartan medoxomil is less than 0.1%. In the prior art, the olmensartan medoxomil has disadvantages of a variety of the impurities, high content of the impurities, and a requirement of a purification by a column chromatography so as to go against an industrial production. According to the present invention, problems of the prior art are solved. In addition, the preparation method provided by the present invention is mild, and avoids using solvents with high toxicity so as to comply with environmental requirements of the industrial production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Olmesartan medoxomil with reduced levels of impurities
The present invention provides the preparation of olmesartan medoxomil containing less than about 0.1% of one or more of the impurities OLM-Me, OLM-Cl, and OLM-eliminate.
Owner:TEVA PHARM USA INC
Process for the preparation of olmesartan medoxomil
The present invention relates to an improved process for the manufacture of olmesartan and pharmaceutically acceptable salts and esters thereof as an active ingredient of a medicament for the treatment of hypertension and related diseases and conditions.
Owner:KRKA
Substantially pure olmesartan medoxomil and processes for its preparation
A process for purifying olmesartan medoxomil is provided comprising (a) dissolving olmesartan medoxomil in a solvent system comprising a ketone and at least one solvent selected from the group consisting of an alcohol-containing solvent, an ester-containing solvent and mixtures thereof to obtain a solution; and (b) recovering substantially pure olmesartan medoxomil. Also disclosed is substantially pure olmesartan medoxomil and pharmaceutical compositions containing same.
Owner:GLENMARK GENERRICS LTD
A process for the preparation of olmesartan medoxomil
The present invention relates to a process for the preparation and purification of trityl olmesartan medoxomil and olmesartan medoxomil.
Owner:LEK PHARMA D D
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Olmesartan medoxomil tablets and preparation method thereof
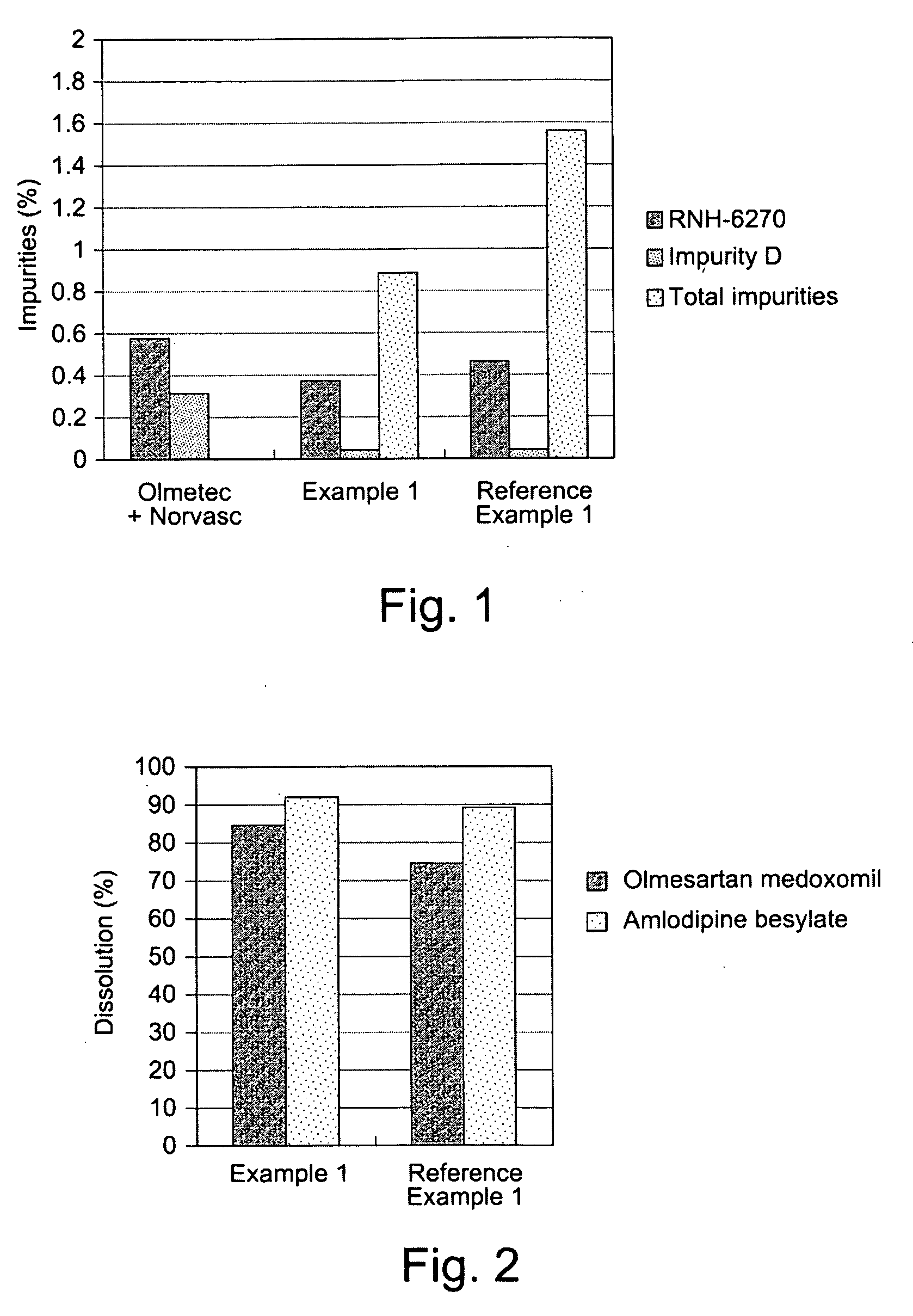
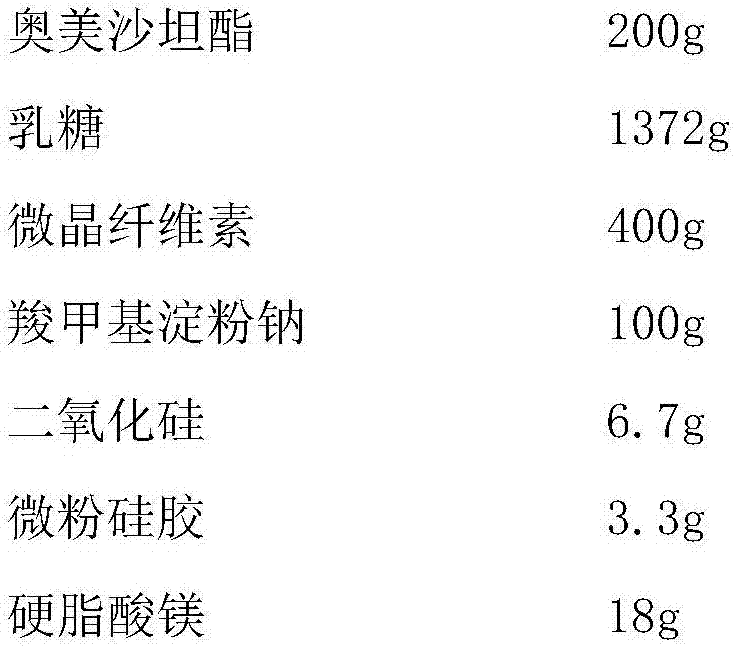
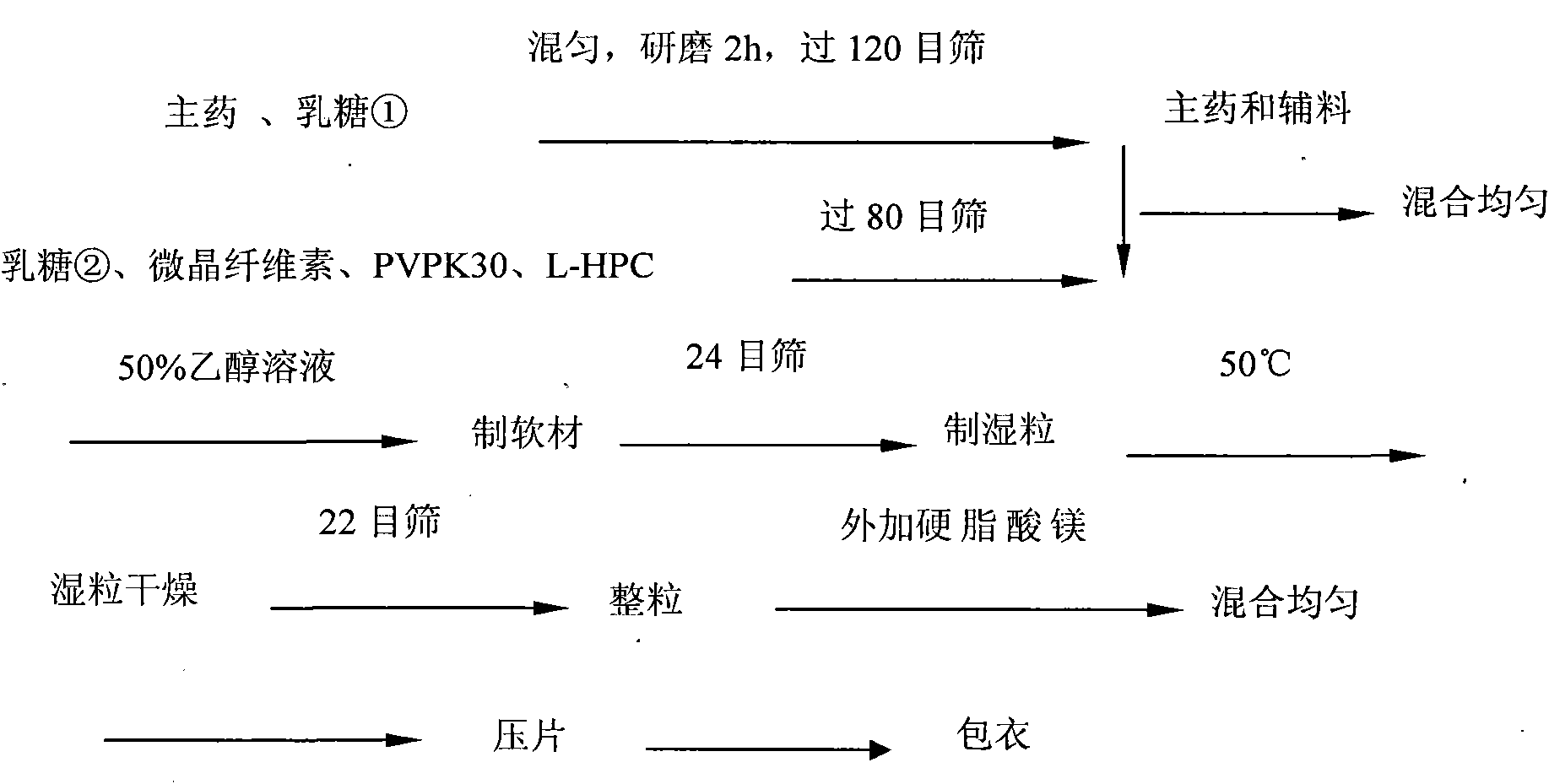
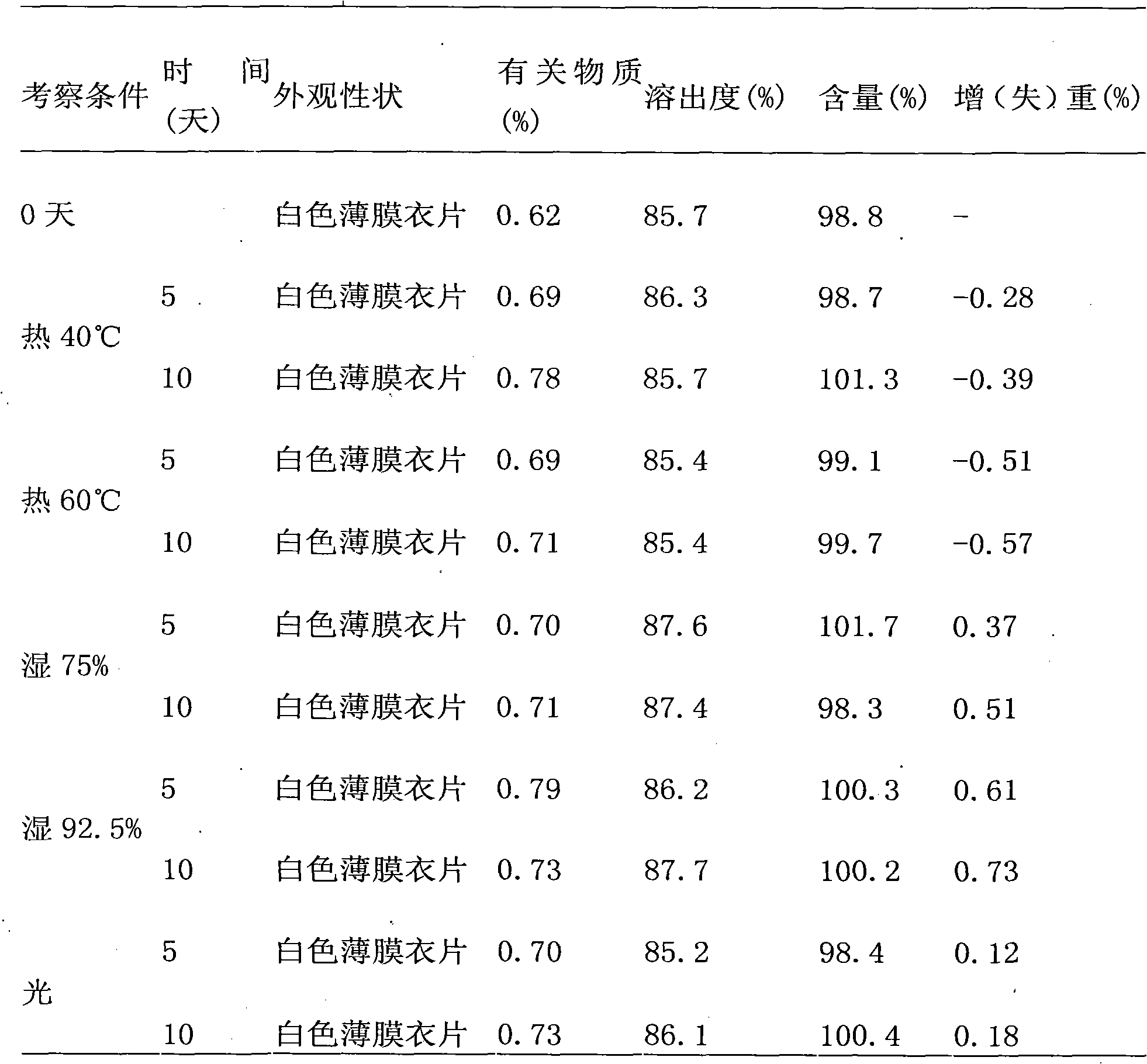
The invention discloses olmesartan medoxomil tablets and a preparation method thereof. The cores of 1000 olmesartan medoxomil tablets comprise 20g of olmesartan medoxomil, 60-95g of lactose (1), 30g of lactose (2), 45-50g of microcrystalline cellulose, 5g of polyvinylpyrrolidone K30 (PVPK30), 8g of low-substituted hydroxypropyl cellulose (L-HPC), 1g of magnesium stearate and an ethanol water solution for preparing a soft material. The invention solves the problem that no olmesartan medoxomil tablets can be used clinically at present.
Owner:FUJIAN TIANQUAN PHARMA
Process for refining olmesartan medoxomil by adopting mixed solution of acetone and water
The invention discloses a process for refining olmesartan medoxomil by adopting a mixed solution of acetone and water. The process comprises the steps that the olmesartan medoxomil is dissolved in the acetone or the mixed solution of the acetone and the water; then an obtained olmesartan medoxomil solution is added to hot water to be stirred and crystallized; and after the olmesartan medoxomil solution is filtered and dried, the olmesartan medoxomil is obtained. The process has the characteristics of good refining effect and high yield; and the acetone residual amount is small and can be controlled at about 1,000 ppm.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
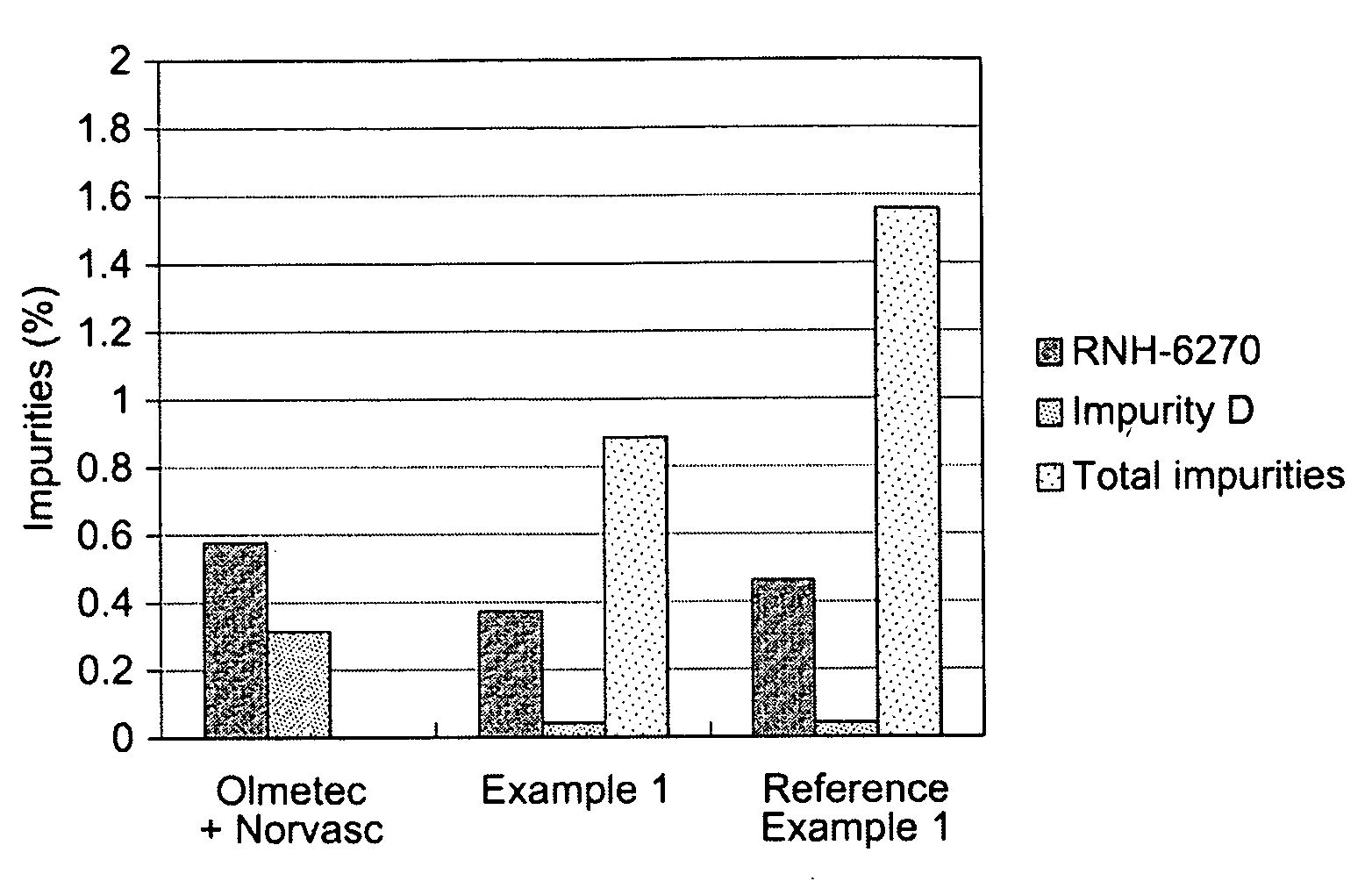
Medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide
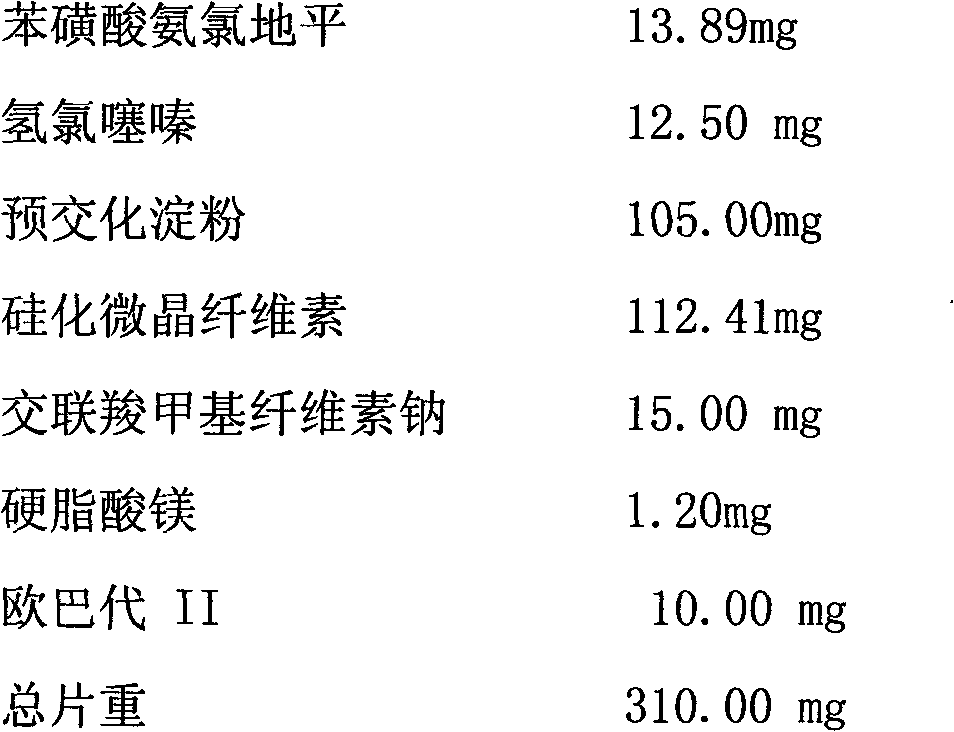
The invention relates to a medicament compound preparation formed by mixing olmesartan medoxomil with benzene sulfonic acid amlodipine and hydrochlorothiazide. Both the olmesartan medoxomil and amlodipine are unstable compounds, and are difficultly prepared into mixed medicaments with stable active ingredients. According to the invention, the adjuvant of the olmesartan medoxomil and amlodipine compound preparation is regulated, so that the amounts of degradation products and impurities of the olmesartan medoxomil are effectively reduced. The invention provides a method for preparing the fixed-dose compound preparation which takes the olmesartan medoxomil, the amlodipine and hydrochlorothiazide as the active ingredients.
Owner:ZHUHAI EBANG PHARMA
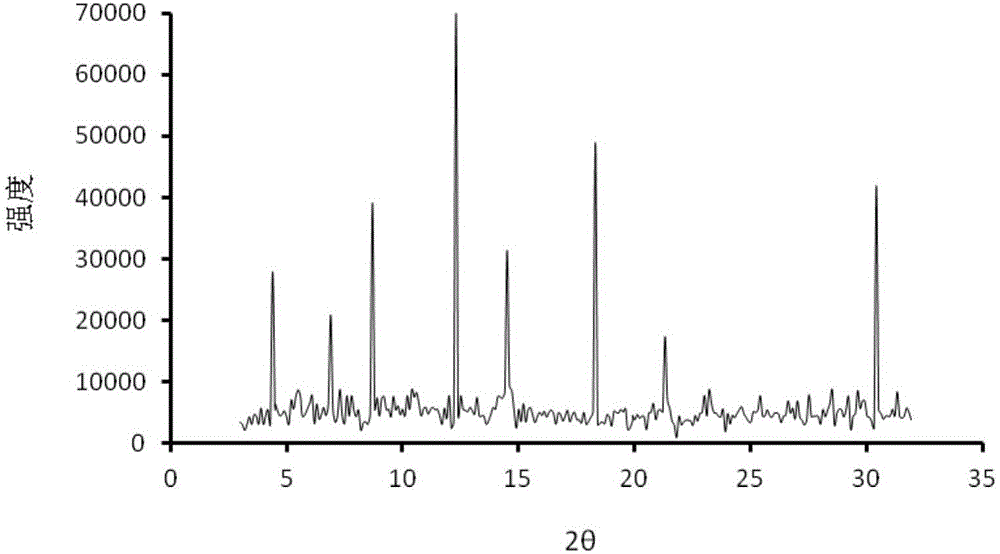
Olmesartan medoxomil crystal and preparation method thereof
InactiveCN102850333AGood dissolution effectHigh crystallinityOrganic active ingredientsOrganic chemistryOlmesartanOrganosolv
The invention provides a new olmesartan medoxomil crystal and a preparation method thereof; the crystal has good crystallinity and stability; the preparation method is realized by recrystallization of olmesartan medoxomil in a mixed solvent. The olmesartan medoxomil crystal prepared by the method has good crystallinity and stability; the method is simple; the used organic solvent is a Class III solvent, which has low toxicity, and is beneficial to environmental protection; and the method is suitable for large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Olmesartan medoxomil tablet and preparation method thereof
ActiveCN104398485ASolve the problem of powder direct compression olmesartan medoxomil tabletsOrganic active ingredientsPharmaceutical delivery mechanismAlcoholOlmesartan
The invention relates to the field of medicines, and particularly relates to an olmesartan medoxomil tablet and a preparation method thereof. Tablet cores of every 1,000 pieces of olmesartan medoxomil tablets consist of the following raw materials according to the weight: 18-22 g of olmesartan medoxomil, 80-120 g of lactose, 30-45 g of microcrystalline cellulose, 30-50 g of low substituted hydroxypropy cellulose, 8-12 g of polylactic acid and 0.5-1.5 g of magnesium stearate. After being tabletted, the components are wrapped by thin film coats; thin film coat liquid is prepared from the following materials: 20 g of opadry and 5 g of polylactic acid and is prepared by dissolving the raw materials in 200 g of ethyl alcohol with the concentration of 80 percent for uniform mixing.
Owner:哈药集团股份有限公司 +1
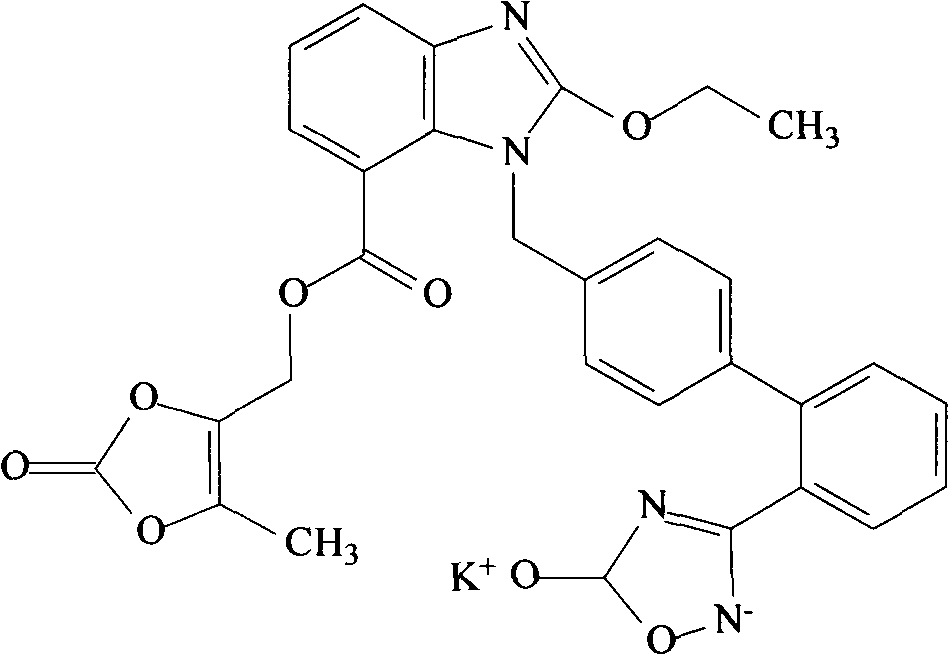
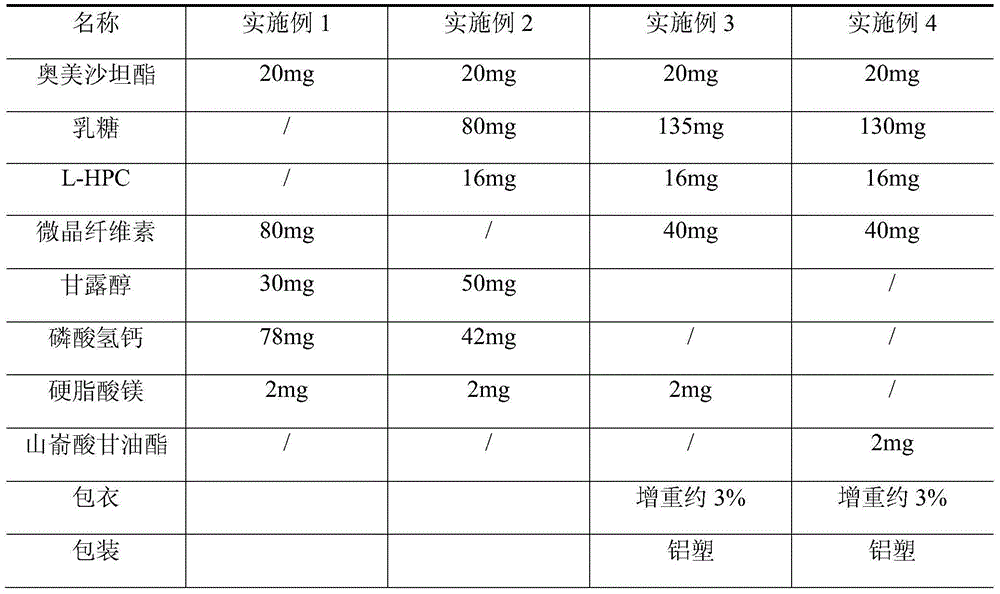
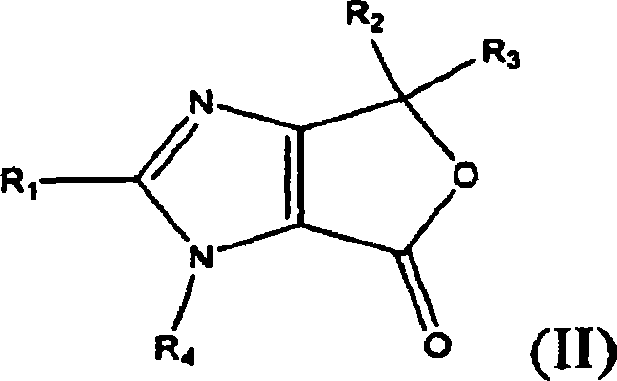
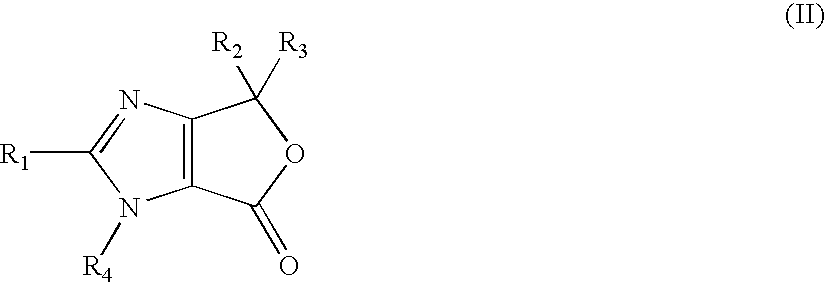
4,6-dihydrofuran [3,4-d] imidazole-6- ketone derivative and salt and preparation method thereof
A 4,6-dihydrofurano[3,4-d] imidazole-6-ketone derivative, its pharmacological receptible salt, and the preparing process of said derivatives are disclosed. Said compound can be used as the intermediate for synthesizing the antagon of angiotensin II receptor.
Owner:SHANGHAI INST OF PHARMA IND
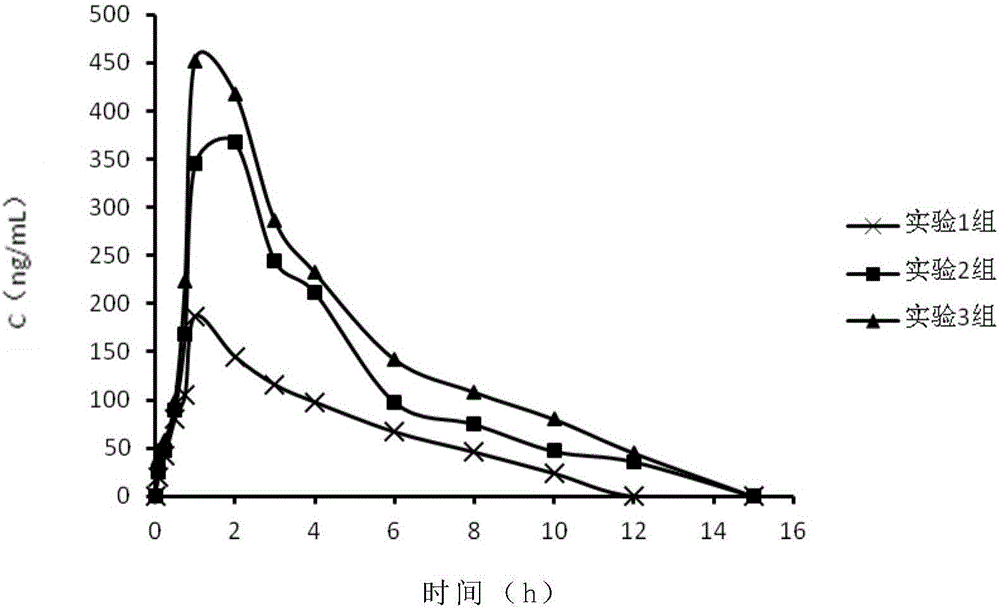
Preparation method of levamlodipine and olmesartan medoxomil tablet
The invention relates to a preparation method of a compound tablet prepared from levamlodipine and olmesartan medoxomil as well as auxiliary materials. The preparation method is characterized in that the compound tablet is prepared by adopting a mode of non-wet granulation and has better quality and stability.
Owner:北京迈劲医药科技有限公司
Method for preparing and purifying olmesartan intermediate
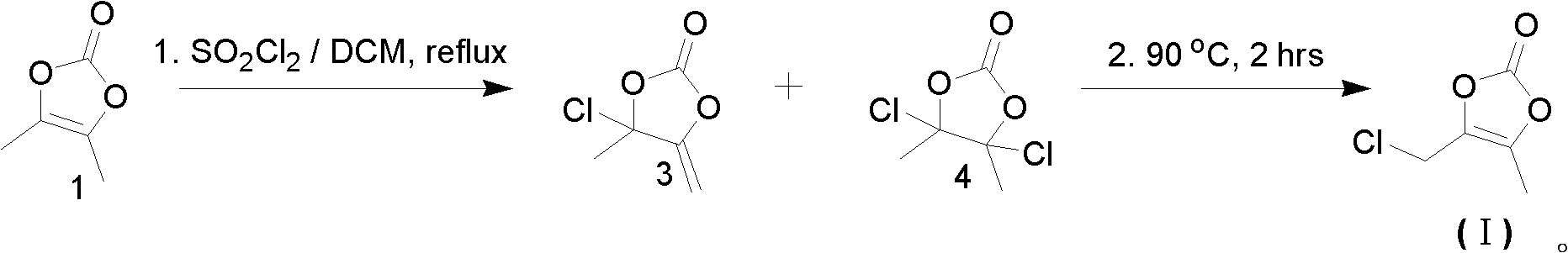
The invention relates to a method for preparing and purifying an olmesartan intermediate. The preparation method comprises the following steps of: chlorinating 4,5-dimethyl-1,3-dioxa-cyclopentene-2-ketone, distilling under reduced pressure to obtain 4-chloro-4-methyl-5-methylene-1,3-dioxolane-2-ketone, and performing rearrangement reaction to generate the olmesartan intermediate; reducing the temperature to be below 50 DEG C, concentrating until a solvent is removed completely to obtain a crude product; and recrystallizing and purifying the crude product at the temperature of between -20 and 0 DEG C for 1 to 48 hours, wherein a recrystallization solvent may be one or a mixed solvent of more of an alkane solvent and an ether solvent. The preparation method is low in production cost, mild in reaction condition and easy to operate, raw materials are wide in sources, and high-content 4-chloromethyl-5-methyl-1,3-dioxa-cyclopentene-2-ketone can be obtained directly, so the method is particularly suitable for large-scale industrial production.
Owner:SHANGHAI SYNCORES TECH INC
Olmesartan liposome solid preparation
InactiveCN102138899AHigh encapsulation efficiencyImprove stabilityOrganic active ingredientsPill deliverySide effectSterol
The invention discloses an olmesartan liposome solid preparation prepared by the following raw and supplementary material components in parts by weight: 1 part of olmesartan, 2.5-14 parts of dioleoyl-phosphatidylcholine, 0.5-6 parts of cholesterol, 0.8-10 parts of sodium glycyl-cholate, 0.2-5 parts of soy sterol and 2-20 parts of pharmaceutically acceptable carriers or excipients. The liposome solid preparation provided by the invention has high entrapment rate, even grain size, long medicament retention time in blood circulation, simple equipment, easiness in operation, improved product quality of the preparation and lessened toxic and side effects and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Olmesartan medoxomil with reduced levels of impurities
The present invention provides the preparation of olmesartan medoxomil containing less than about 0.1% of one or more of the impurities OLM-Me, OLM-Cl, and OLM-eliminate.
Owner:TEVA PHARM USA INC
Process for preparing trityl olmesartan medoxomil and olmesartan medoxomil
InactiveUS8076492B2Suitable can be appliedCost-effective processOrganic chemistryOlmesartanCarboxylic acid
Owner:CIPLA LTD
Process for the preparation of olmesartan medoxomil
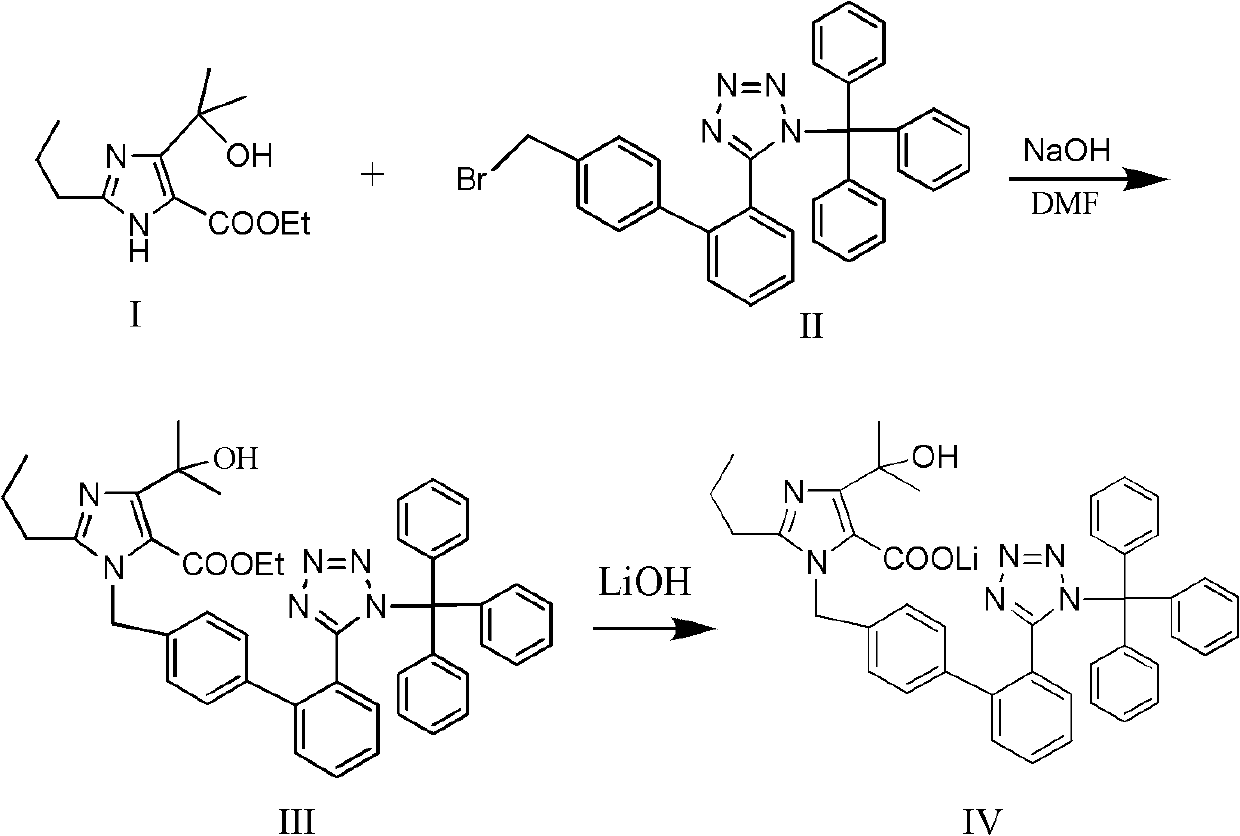
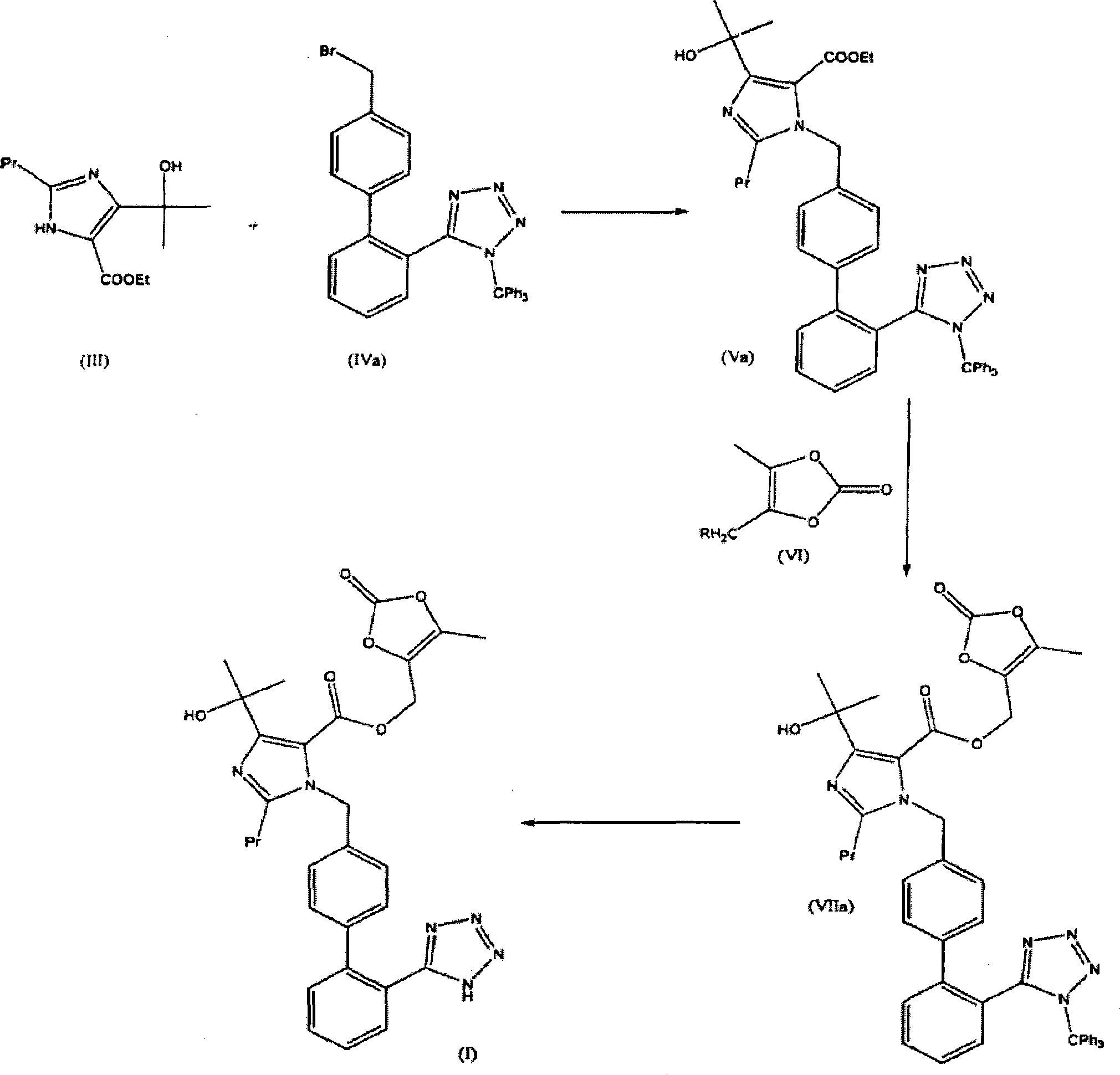
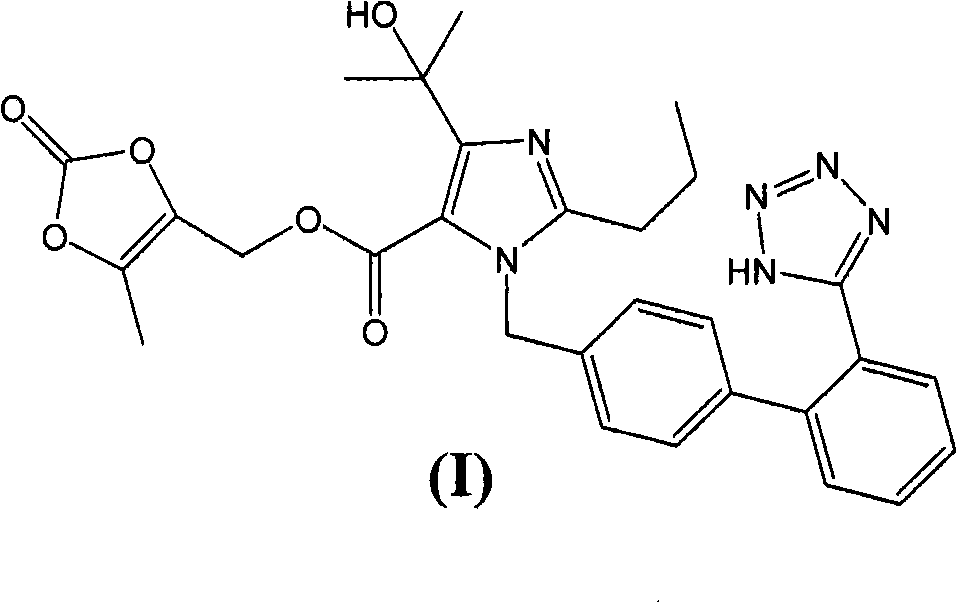
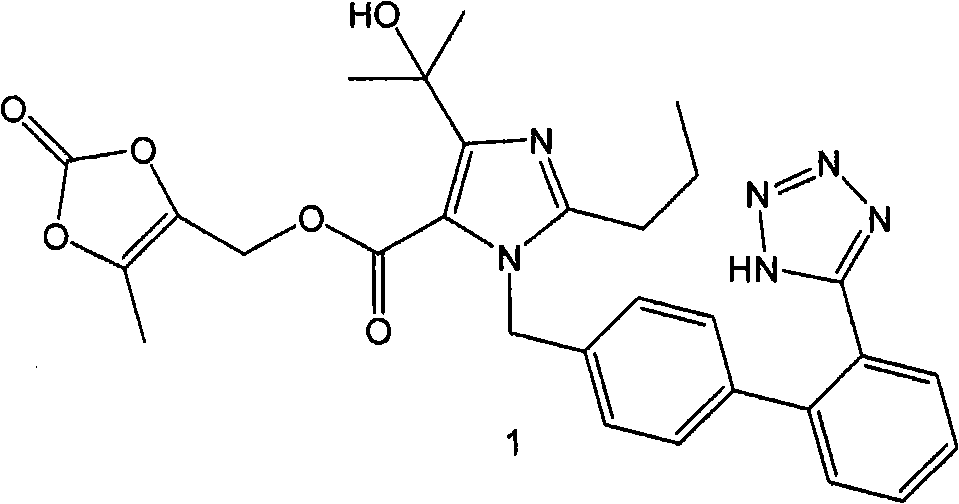
The present invention provides a process for the preparation of Olmesartan medoxomil] by condensing the ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate with 4-[2-(trityl tetrazol-5-yl)phenyl]benzyl bromide to obtain ethyl 4-(1-hydroxy-1-methyl ethyl)-2-propyl-1-{4-[2-(trityl tetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylate and then hydrolyzing ethyl 4-(1-hydroxy-1-methyl ethyl)-2-propyl-1-{4-[2-(trityl tetrazol-5-yl)phenyl]phenyl}methyl imidazole-5-carboxylate to obtain trityl Olmesartan dihydrate followed by reacting trityl Olmesartan dihydrate with 4-chloromethyl-5-methyl-2-oxo-1,3-dioxolene to obtain trityl Olmesartan medoxomil and then deprotecting trityl Olmesartan medoxomil to obtain Olmesartan medoxomil.
Owner:MYLAN LAB
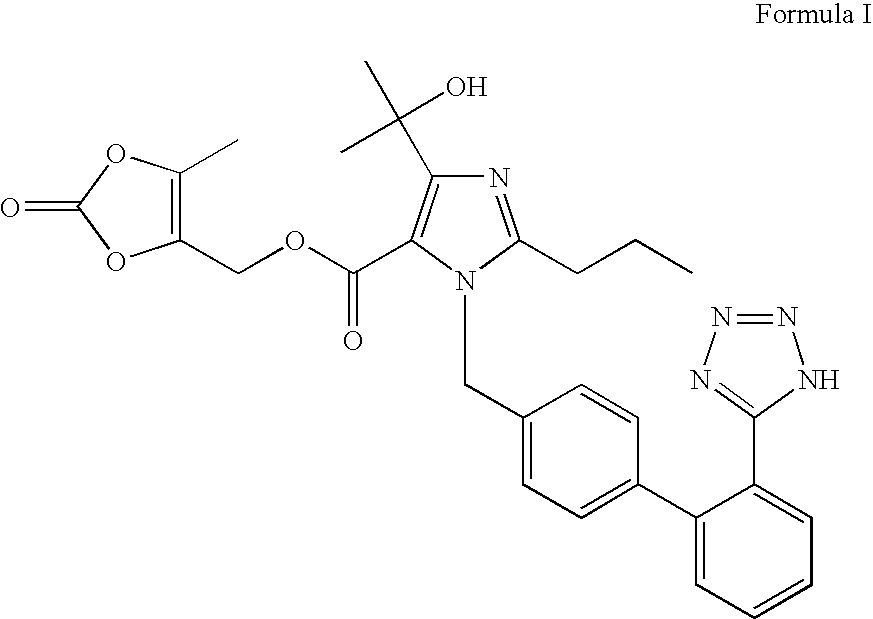
Preparation method and application of high-purity olmesartan medoxomil intermediate ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazolyl-5-carboxylate
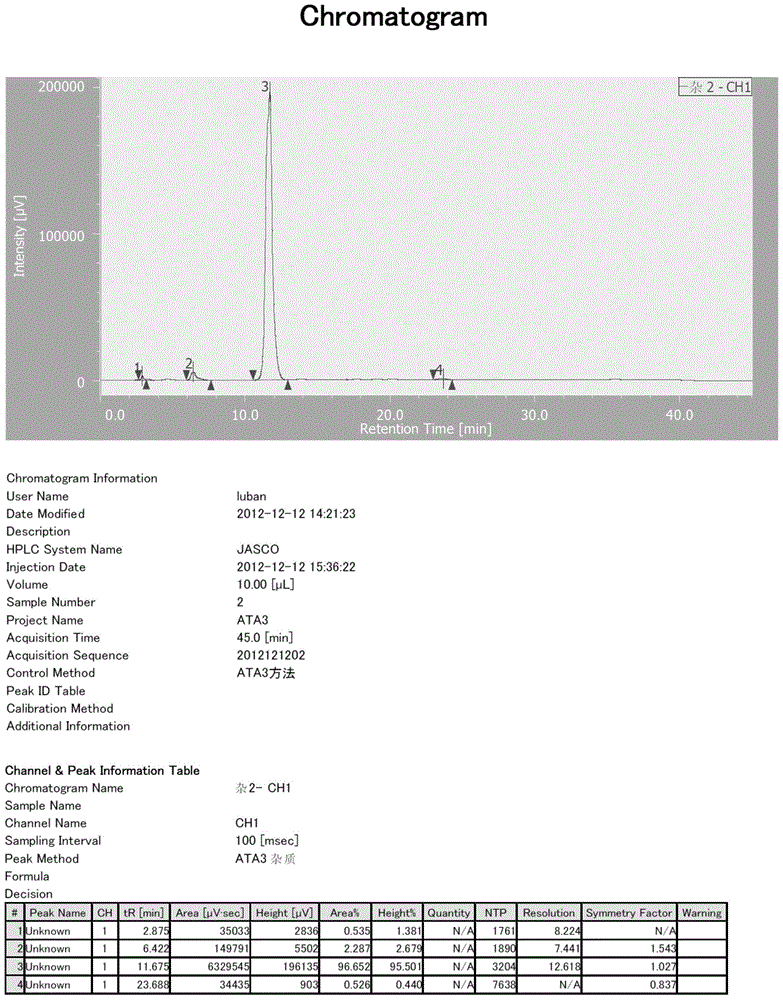
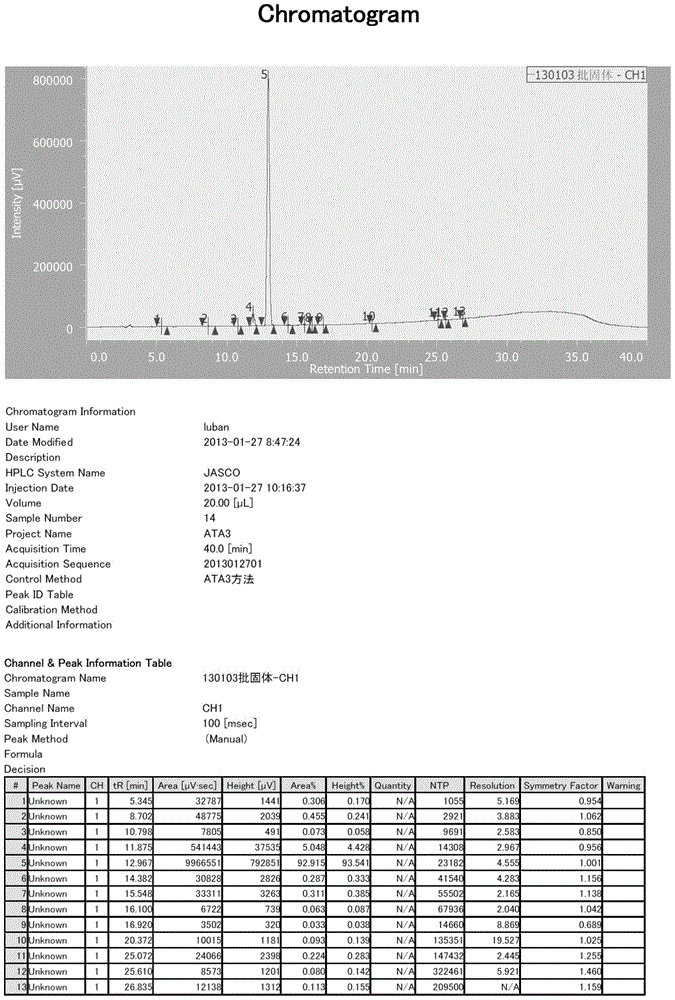
The invention provides a preparation method of ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazolyl-5-carboxylate (1). The method accurately controls the reaction material ratio, solvent composition, solvent consumption, reaction temperature, reaction time and other technological parameters, and obviously lowers the content of the impurity compound disclosed as Formula 3, thereby obtaining the high-purity olmesartan medoxomil. The invention also provides application of the compound disclosed as Formula 3 as a quality control standard substance in preparing the ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazolyl-5-carboxylate (1).
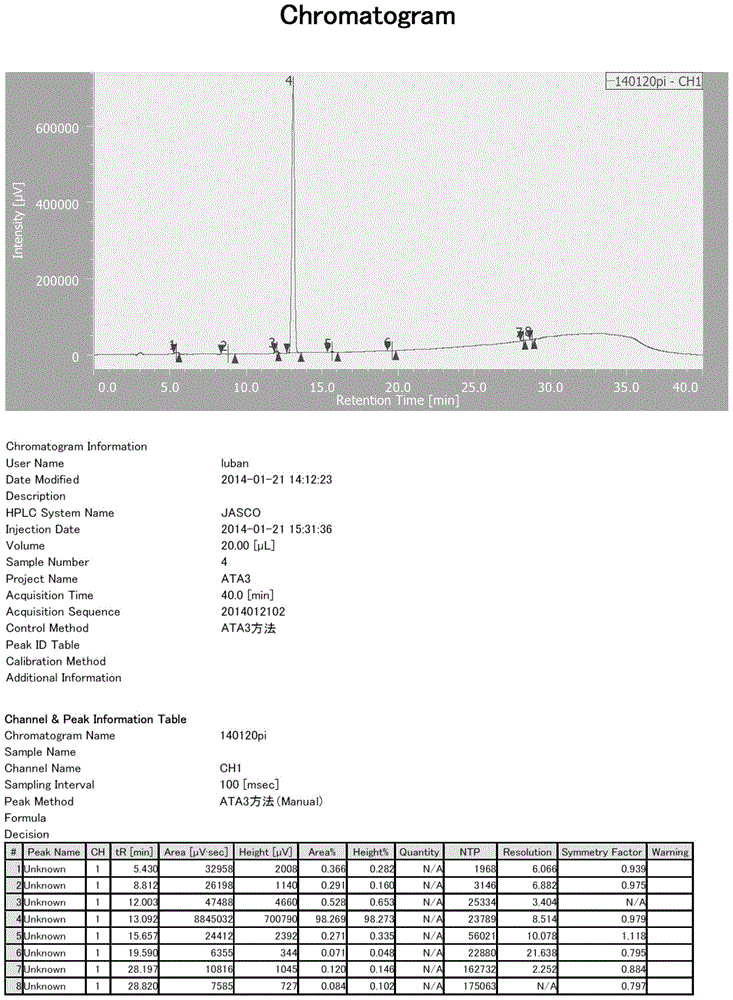
Owner:HUANGGANG LUBAN PHARM
Angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof
ActiveCN106474479AGood treatment effectStable in natureMetabolism disorderGroup 5/15 element organic compoundsTasosartanValsartan
The present invention provides a an angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof, wherein the complex comprises an angiotensin receptor antagonist and creatine phosphate sodium, a molar ratio of the angiotensin receptor antagonist to the creatine phosphate sodium is 1:1-2, and the angiotensin receptor antagonist is selected from valsartan, losartan, irbesartan, telmisartan, eprosartan, candesartan, olmesartan, saprisartan, tasosartan, and elisartan. According to the present invention, the complex is formed by compounding the angiotensin receptor antagonist and the creatine phosphate sodium, and provides the unexpected double effect and the synergistic effect for treatment of heart failure and high blood pressure, the cocrystallization salt hydrate formed by linking the hydrogen bond has the stable characteristic, the pharmacokinetic property is significantly provided, and the positive application prospects are provided in the fields of anti-high blood pressure treatment and anti-heart failure treatment.
Owner:珠海赛隆药业股份有限公司(长沙)医药研发中心
Solid Dosage Form of Olmesartan Medoxomil And Amlodipine
InactiveUS20090175942A1Improve stabilityReduce weightBiocideAnimal repellantsHydrochlorothiazideOlmesartan
The invention relates to a stable solid dosage form comprising olmesartan medoxomil and amlodipine or a pharmacologically acceptable salt thereof. In particular, it relates to solid dosage forms free from reducing sugars. The stable solid dosage form may optionally further comprise hydrochlorothiazide or a pharmacologically acceptable salt thereof.
Owner:DAIICHI SANKYO CO LTD
Olmesartan medoxomil tablet and preparation technology thereof
ActiveCN104398483AImprove stabilityGood particle size dissolutionOrganic active ingredientsOil/fats/waxes non-active ingredientsOlmesartanDissolution
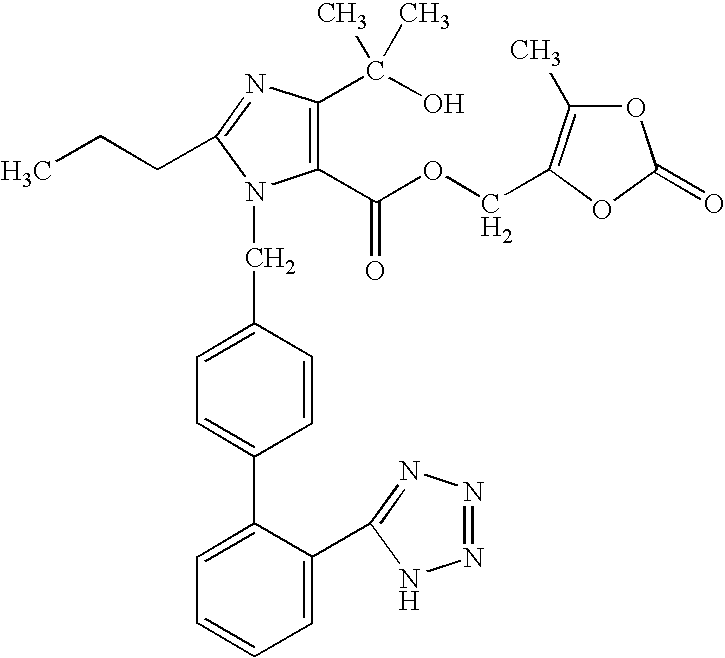
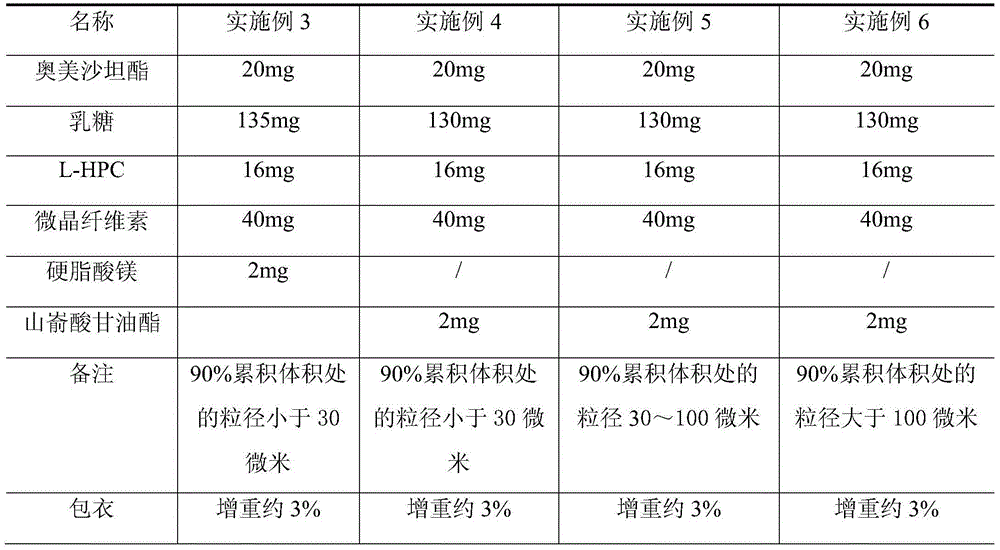
The invention relates to an Olmesartan medoxomil tablet and preparation technology thereof. The core of the Olmesartan medoxomil tablet is prepared by directly pressing Olmesartan medoxomil and medicinal assistants, and the particle size of a position accumulating 90% of Olmesartan medoxomil is 1-100[mu]m; and the medicinal assistants comprise a filler, a disintegrating agent and a lubricant, wherein the lubricant is one or more of neutral or inert lubricants. Raw materials of the core of the tablet comprise, by weight, 20 parts of Olmesartan medoxomil, 80-180 parts of the filler, 10-20 parts of the disintegrating agent and 2-10 parts of the lubricant. The Olmesartan medoxomil is crushed in an airflow crushing mode, the particle size is controlled, and the preparation technology adopts a direct powder pressing method, so the introduction of water in a wet granulation process and the degradation of active substances in a wet particle drying process are avoided, and the incidence of a hydrolysis reaction is prevented. The Olmesartan medoxomil tablet has the advantages of stability, good dissolution and simple preparation.
Owner:SHANGHAI PHARMA GRP QINGDAO GROWFUL PHARMA CO LTD
Tablet containing olmesartan medoxomil and amlodipine and preparation method of tablet
ActiveCN103006651AIncrease dissolution rateHigh dissolution rateOrganic active ingredientsPill deliveryOlmesartanMethyl cellulose
The invention discloses a tablet containing olmesartan medoxomil and amlodipine. The tablet is prepared from amlodipine besylate solid dispersion, olmesartan medoxomil and pharmaceutic adjuvants, wherein the amlodipine besylate solid dispersion consists of amlodipine besylate and hydroxypropyl methyl cellulose according to the weight rate of 1: (7-12). The amlodipine besylate in the compound tablets can be rapidly dissolved out and absorbed by organisms; and after orally taken by hypertensive, the olmesartan medoxomil and amlodipine-containing tablet plays the roles of enhancing the synergistic hypotensive effect of two active ingredients and remarkably reducing the adverse drug reaction.
Owner:NANJING CHIA TAI TIANQING PHARMA
Preparation method of olmesartan medoxomil
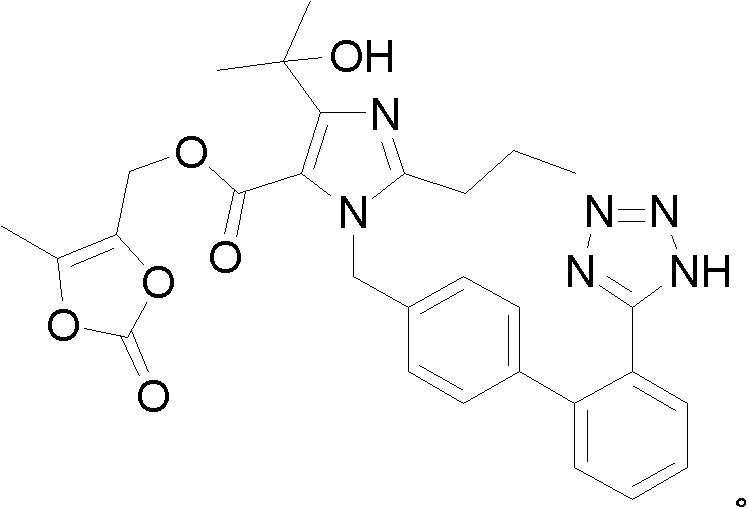
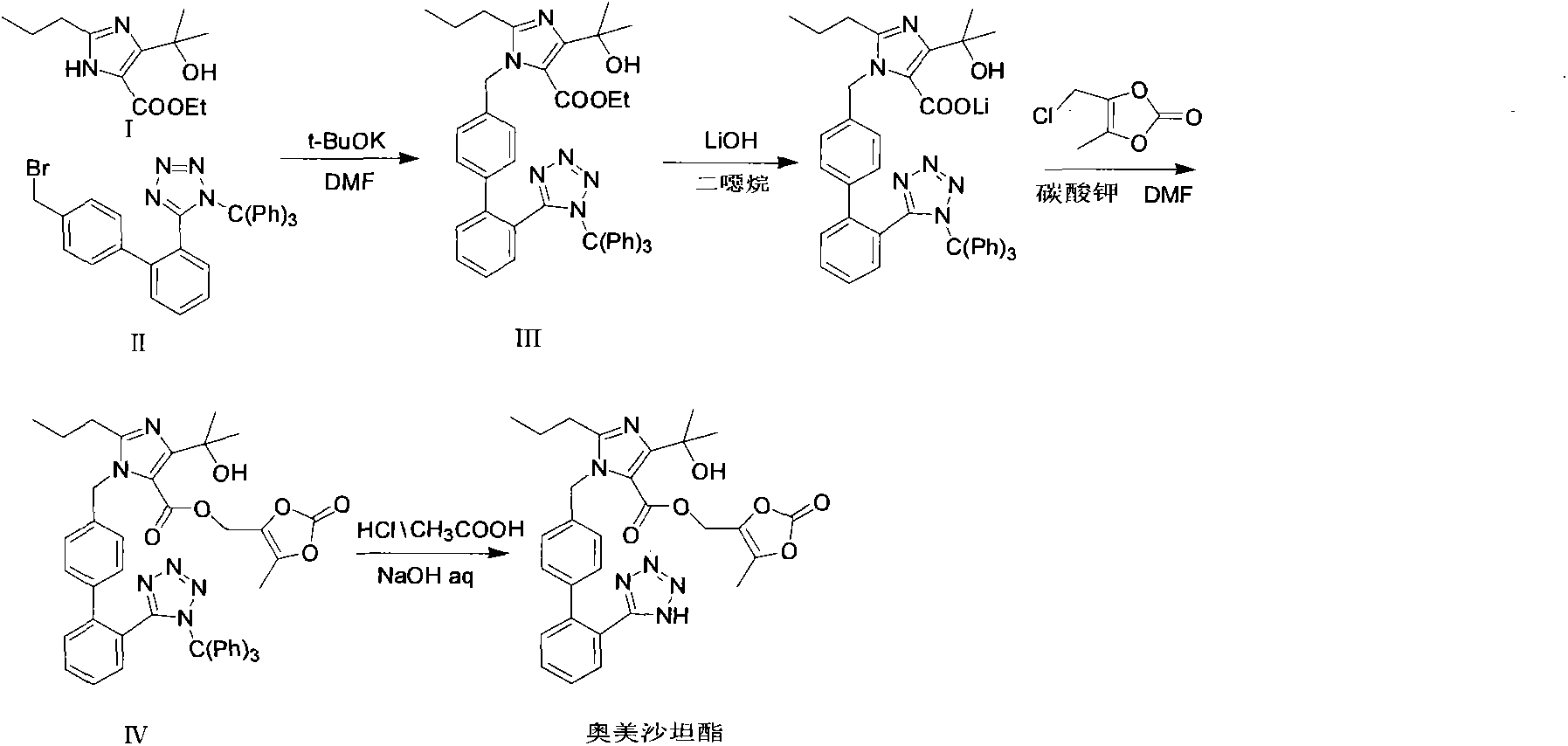
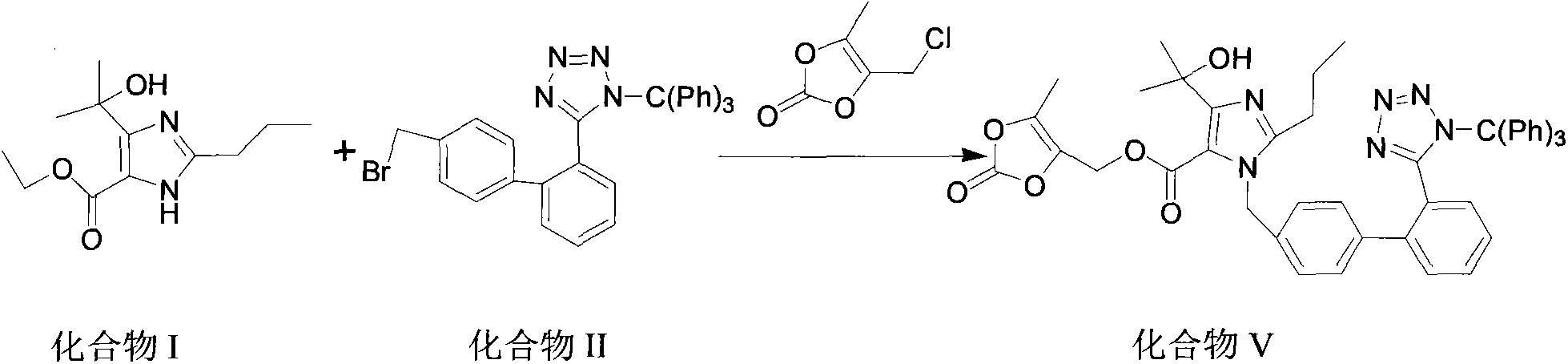
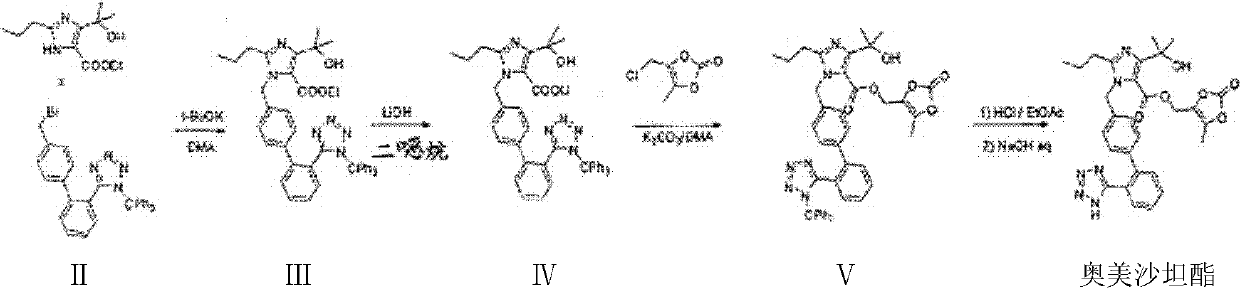
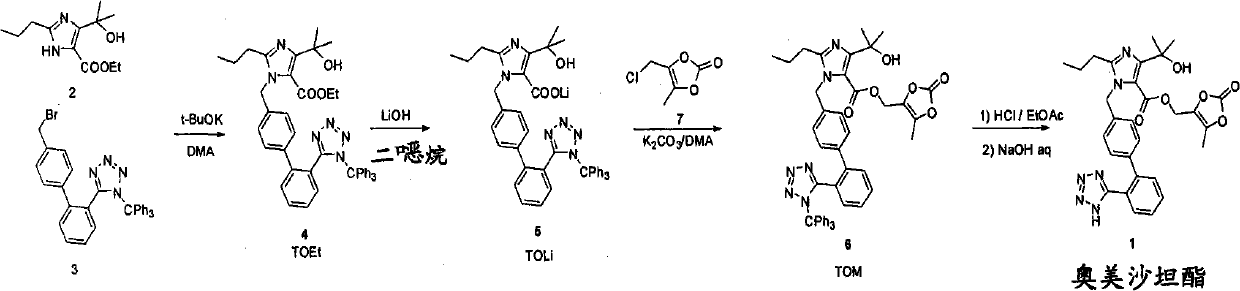
The invention discloses a preparation method of olmesartan medoxomil and relates to the technical field of medicine. The method comprises the following steps: using 4-(1-hydroxy-1-methylethyl)-2-propylimidazol-5-ethylcarboxylate and N-(triphenylmethyl)-5-(4'-bromomethyl diphenyl-2-yl)tetrazole as raw materials, adopting a one-pot method to directly obtain 1-[[[27-(triphenylmethyl)-2H-tetrazole-5-yl]dipheny-4-yl]methyl]-2-propyl-4-(1-hydroxy-1-methylethyl)imidazol-5-carboxylic acid (5-methyl-2-oxo-1,3-dioxycyclopentene-4-yl)methyl ester. Compared with the prior art, the preparation method has the characteristics of simple preparation technology, high yield, low cost and the like, and the method is more beneficial to implementation of large-scale industrial production.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Process for the preparation of olmesartan medoxomil
Owner:MYLAN LAB
Preparation method of olmesartan medoxomil
The invention discloses a preparation method of olmesartan medoxomil,Which is characterized by reacting ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylate (I) and N-(triphenylmethyl)-5-(4'-bromoethylbiphenyl-2-)tetrazole (II) by a one-pot process to directly obtain lithium 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(triphenylmethyltetrazolyl-5-yl)phenyl]phenyl}methylimidazol-5-carboxylate (IV). The invention has the advantages of mild reaction conditions, fewer byproducts, high purity of the end product, high total yield, high safety and environmental protection, and is suitable for industrial production; and by using the one-pot process, the invention is simpler to operate, saves the time and lowers the cost.
Owner:山东安信制药有限公司
Tablet containing olmesartan medoxomil and preparation method of tablet
ActiveCN107998097AFast dissolution rate in vitroImprove in vitro dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsOlmesartanPolyethylene glycol
The invention discloses a tablet containing olmesartan medoxomil and a preparation method of the tablet. The tablet is composed of olmesartan medoxomil, a disintegrating agent, a filling agent, a diluent, an anti-sticking agent and a lubricating agent. The preparation method disclosed by the invention comprises the following steps: performing jet milling treatment on olmesartan medoxomil, and mixing with the anti-sticking agent to pass through a 30-mesh sieve; mixing with other medicinal auxiliary materials, and directly tabletting; performing film coating on the tablet core after tabletting,wherein the film coating premixed agent is composed of the following materials: hydroxypropyl methylcellulose, titanium dioxide and polyethylene glycol. The olmesartan medoxomil tablet provided by theinvention is high in in-vitro dissolution rate, high in bioavailability, excellent in stability and mechanical strength, less in odor, sticking-free and suitable for large-scale mass production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Compressed preparation
[Object]The present invention provides an olmesartan medoxomil-containing drug product having an improved dissolution property.[Means for Solution]A production method for an olmesartan medoxomil-containing drug product characterized in that the method comprises a process of compressing a composition.
Owner:DAIICHI SANKYO CO LTD
Medical composition capable of treating hypertension
ActiveCN101416966AImprove protectionReduce incidenceOrganic active ingredientsCardiovascular disorderUse medicationChemical composition
The invention provides a novel medical composition used for treating hypertension and more particularly relate to levamlodipine and olmesartan medoxomil, wherein, the weight ratio of the levamlodipine to the olmesartan medoxomil is 1 to (1-20). The levamlodipine disclosed by the invention comprises salts accepatable in pharmacy, such as benzene sulfonate, maleate and other salts. The medical composition provided by the invention has the advantages of obvious therapeutic effect, convenient usage and low cost.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






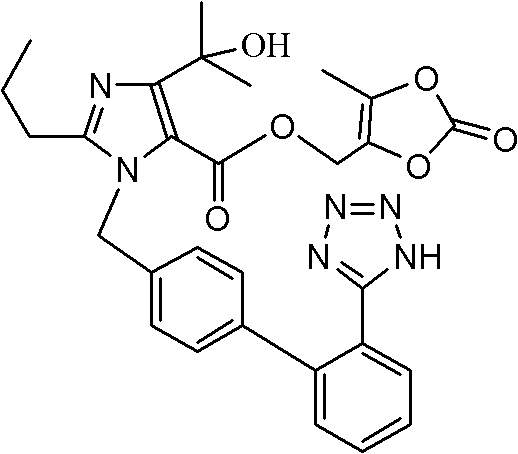
![4,6-dihydrofuran [3,4-d] imidazole-6- ketone derivative and salt and preparation method thereof 4,6-dihydrofuran [3,4-d] imidazole-6- ketone derivative and salt and preparation method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/bfd17bcd-a2ae-48f3-b13d-4154667a2200/03115940.PNG)