Preparation method and application of high-purity olmesartan medoxomil intermediate ethyl 4-(1-hydroxy-1-methylethyl)-2-propylimidazolyl-5-carboxylate
A technology of methyl ethyl and propylimidazole, applied in the field of chemical synthesis, can solve problems such as low yield, low product purity, and in-depth research on impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of 4-(1-hydroxy-1-methylethyl)-2-propylimidazole-5-carboxylic acid ethyl ester (1)
[0072] Add 100g (4.16mol) of magnesium powder to tetrahydrofuran (2L), pass in methyl chloride gas at 60℃ until the magnesium powder disappears (about 3 hours), then reflux for 30 minutes, cool to room temperature, and obtain gray methyl magnesium chloride Tetrahydrofuran solution. A 2.1L tetrahydrofuran solution of ethyl 2-propylimidazole-4,5-dicarboxylate (2) (211.9g, 0.83mol) was added dropwise at 30°C. After 2 hours, continue to stir for 30 minutes. The solvent was removed under reduced pressure, and saturated ammonium chloride solution was added dropwise to the residue until the solid was dissolved. 2.5L of ethyl acetate was added, the organic layer was separated, the aqueous layer was extracted with ethyl acetate 500ml×2, and the organic layers were combined. Wash once with saturated brine. Dry with anhydrous magnesium sulfate. The solvent was evaporated to obtain li...
Embodiment 2
[0077] Preparation of ketone ethyl ester impurity 3 reference substance
[0078] Take 10.0 g of the oily substance prepared in Example 1 and carry out column chromatography separation, eluting with dichloromethane:methanol=25:1 eluent, collect the eluent rich in ketone ethyl impurity 3, and remove the solvent. , Get 0.3 g of ketone ethyl ester impurity 3.
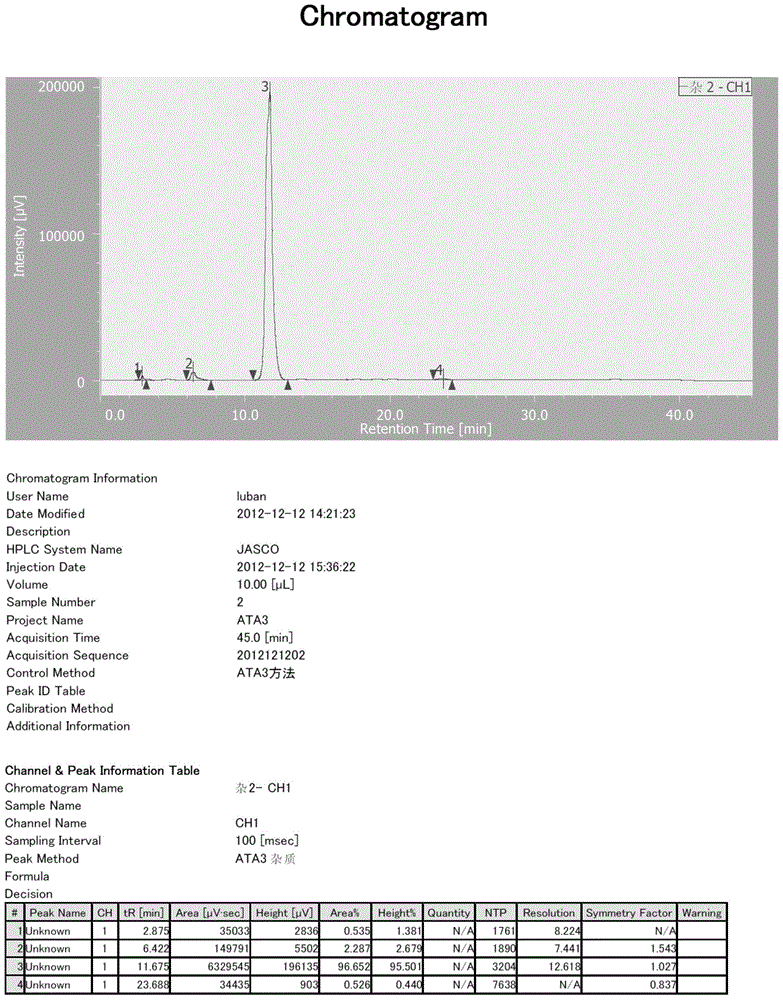
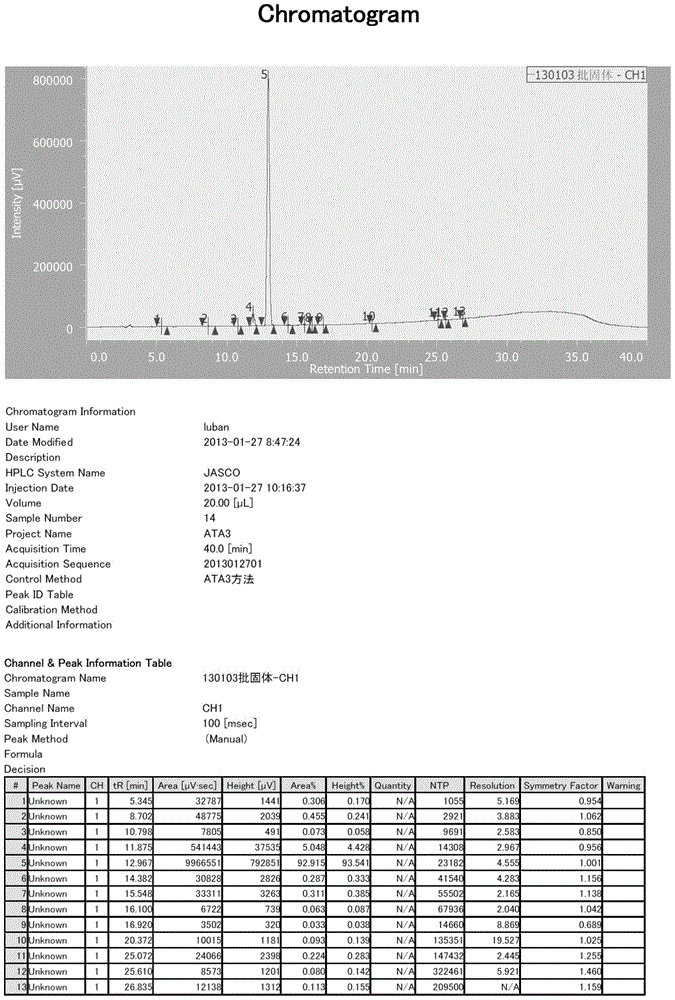
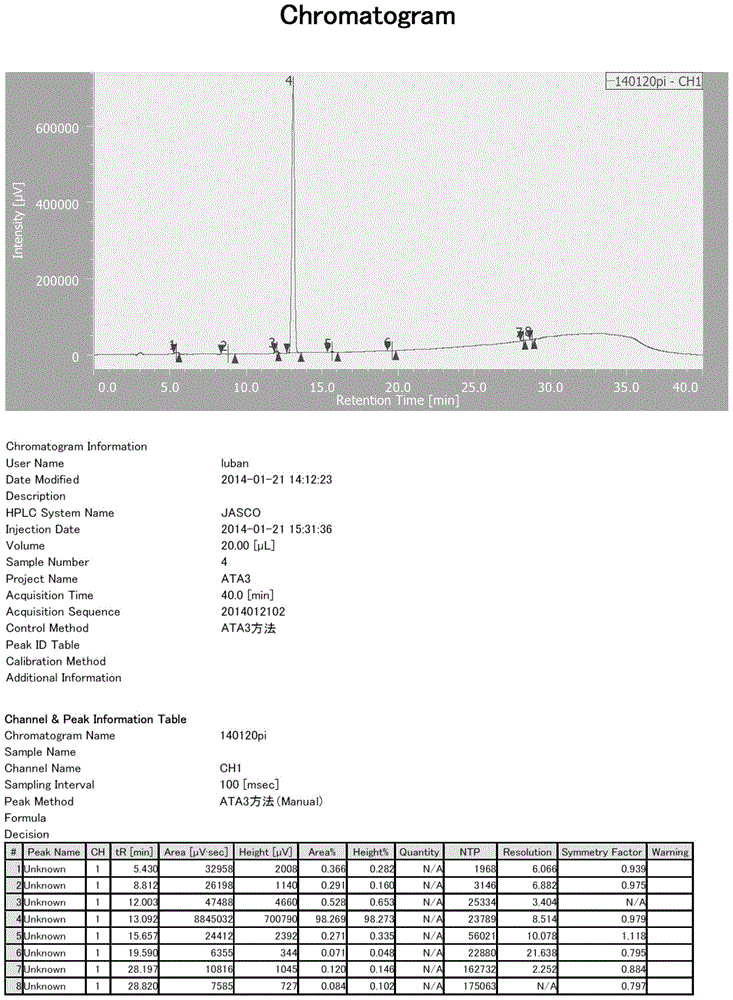
[0079] HPLC conditions: chromatographic column: C184.6*250mm 5um; mobile phase: 0.02M tetrabutylammonium bromide + 1% triethylamine aqueous solution (pH=6.0): acetonitrile=78:22; column temperature: room temperature; detection wavelength : 254nm; flow rate: 1ml / min; sample concentration: 45mg-25ml (acetonitrile: water=1:4), injection volume: 2ul; retention time of ketone ethyl impurity 3: 11.67min.
[0080] MS (Q-TOF micro, ESI + ):247.10[M+Na] + .
[0081] 1 HNMR(CDCl 3 )δ:9.8~10.5(1H,br,N H ,),4.45(2H,q,OC H 2 CH 3 ),2.76(2H,t,CH 3 CH 2 C H 2 ),2.74(3H,s,-C H 3 ),2.02(2H,m,CH 3 C H 2 CH 2 ),1.42(3H,t,OCH 2 C H 3 ),0.98(3H,t,C H ...
Embodiment 3
[0083] Preparation of ketone ethyl ester impurity 3 reference substance
[0084] According to the literature ARKIVOC (2010), (2), 292-302 method to prepare ketone ethyl impurity 3 reference substance 15.2 g.
[0085] MS (Q-TOF micro, ESI + ):247.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com