Olmesartan medoxomil tablet and preparation technology thereof
A technology for the preparation of olmesartan medoxomil, which is applied in the field of drug preparation, can solve problems such as unfavorable industrialization, high production cost, complex process, etc., and achieve cost reduction and labor intensity, time and energy saving, and simple production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A kind of preparation technology of olmesartan medoxomil tablet, comprises steps as follows:
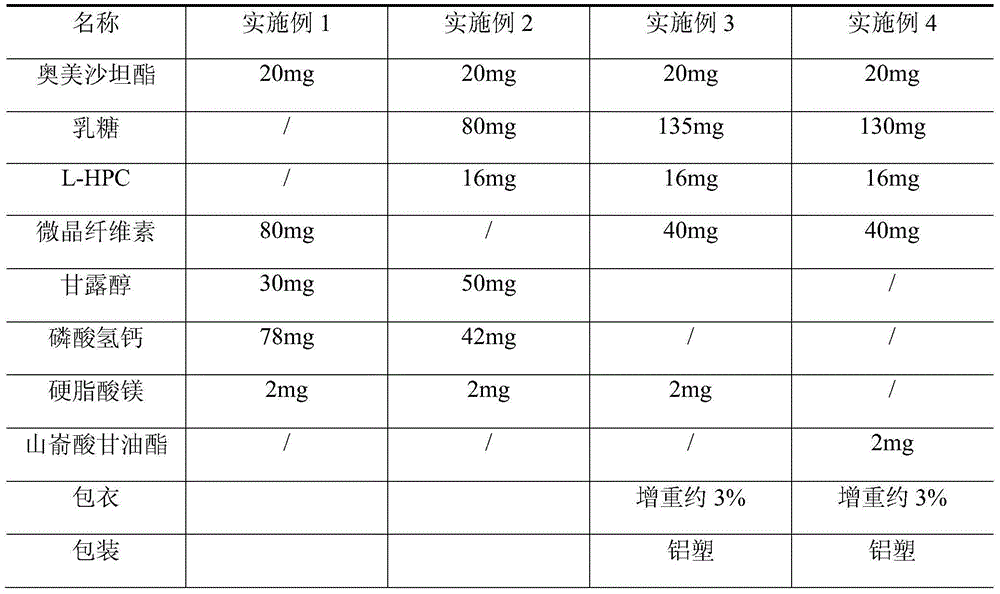
[0047] (1) Selection of tablet core raw materials: the tablet core is composed of olmesartan medoxomil and pharmaceutical excipients, the pharmaceutical excipients include fillers, disintegrants and lubricants, and the weight ratio composition of raw materials in the tablet core is shown in Table 1;
[0048] (2) Raw material pulverization: olmesartan medoxomil is pulverized by airflow pulverization, and the particle size at 90% cumulative volume is controlled at 1-100 microns;
[0049](3) Mixing: mixing the pulverized olmesartan medoxomil with the filler and disintegrant in the pharmaceutical adjuvant, and finally adding lubricant and mixing;
[0050] (4) Compress the mixed raw materials into tablets according to the particle content;
[0051] (5) Coating.
Embodiment 2
[0053] A kind of preparation technology of olmesartan medoxomil tablet, step is the same as embodiment 1, and the weight ratio composition of raw material in tablet core is shown in Table 1.
Embodiment 3
[0055] A kind of preparation technology of olmesartan medoxomil tablet, comprises steps as follows:
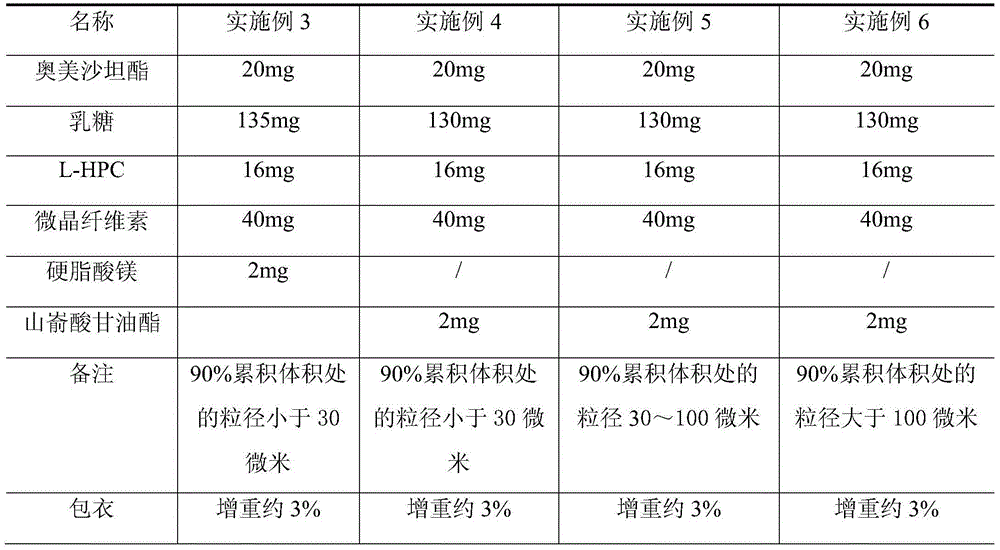
[0056] (1) Selection of tablet core raw materials: the tablet core is composed of olmesartan medoxomil and pharmaceutical excipients, the pharmaceutical excipients include fillers, disintegrants and lubricants, and the weight ratio composition of raw materials in the tablet core is shown in Table 1;
[0057] (2) Raw material pulverization: olmesartan medoxomil is pulverized by airflow pulverization, and the particle size at 90% cumulative volume is controlled to be less than 30 microns;
[0058] (3) Mixing: first mix the pulverized olmesartan medoxomil with lactose, then add microcrystalline cellulose, low-substituted hydroxypropyl cellulose and mix, and finally add lubricant and mix;
[0059] (4) Compress the mixed raw materials into tablets according to the particle content;
[0060] (5) Coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com