Method for preparing and purifying olmesartan intermediate
A purification method and intermediate technology, applied in the field of medicine and chemical industry, can solve the problems of large decomposition of vacuum distillation products, difficult to achieve high purity requirements, affecting the production capacity of intermediates, etc., so as to reduce production steps, shorten purification cycle, and shorten production. effect of cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
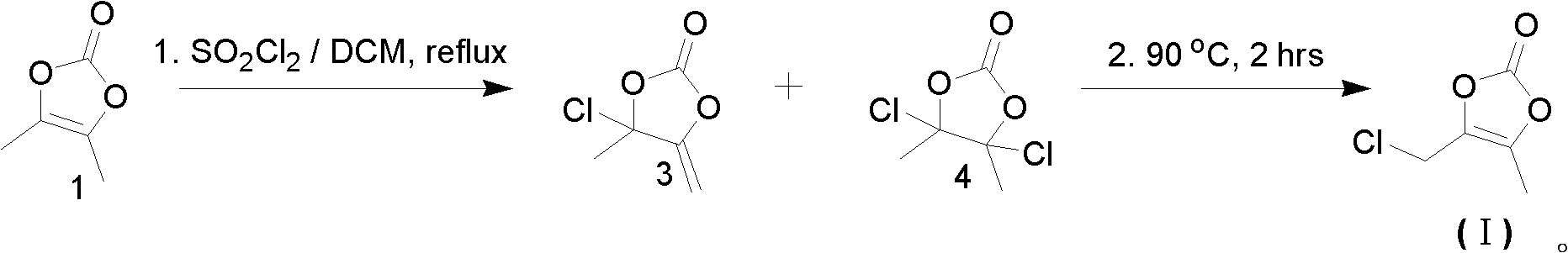
[0028] This embodiment provides a preparation method of 4-chloromethyl-5-methyl-1,3-dioxol-2-one (hereinafter referred to as olmesartan intermediate), which specifically includes the following steps:
[0029] (1), obtain the crude product of olmesartan intermediate
[0030] Add 40g of 4,5-dimethyl-1,3-dioxol-2-one and 320ml of dichloromethane into a 1L three-necked flask, stir well to form a clear solution, and slowly heat up to reflux at a temperature of 40°C At ~42°C, add 49.7g of sulfonyl chloride (1.05 equivalents) dropwise, after dropping, reflux for 2 hours, then start distillation until the temperature of the reaction system rises to 90°C, keep the temperature for 2 hours, cool down to below 50°C, and concentrate until there is no solvent. Obtain 38g of crude product of olmesartan intermediate. The GC purity of the crude product is 65%-73% (the area calculated from the GC spectrum, not the real content of the olmesartan intermediate).
[0031] (2), recrystallization p...
Embodiment 2
[0037] This embodiment provides a preparation method of 4-chloromethyl-5-methyl-1,3-dioxol-2-one (hereinafter referred to as olmesartan intermediate):
[0038] (1), obtain the crude product of olmesartan intermediate: with embodiment 1.
[0039] (2), recrystallization purification treatment
[0040]Take 38g of the crude product of olmesartan intermediate, add 20mL n-heptane, stir and cool down to -10°C~-20°C, crystallize for 2~48h to obtain a white suspension, filter with suction, and wash the filter cake with 20mL n-heptane , to obtain the product, weighing 36g (yield 69.4%, GC purity 99.4%). The crystallization mother liquor can be applied mechanically three times.
Embodiment 3
[0042] Take 38g of the crude product of olmesartan intermediate (GC content is 65%-73%), add 20mL of n-hexane, stir and cool down to -10°C-20°C, crystallize for 2-48h to obtain a white suspension, suction filter, filter The cake was washed with 20 mL of n-heptane to obtain the product, weighing 36 g (69.4% yield, 99.4% GC purity). The crystallization mother liquor can be applied mechanically three times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com