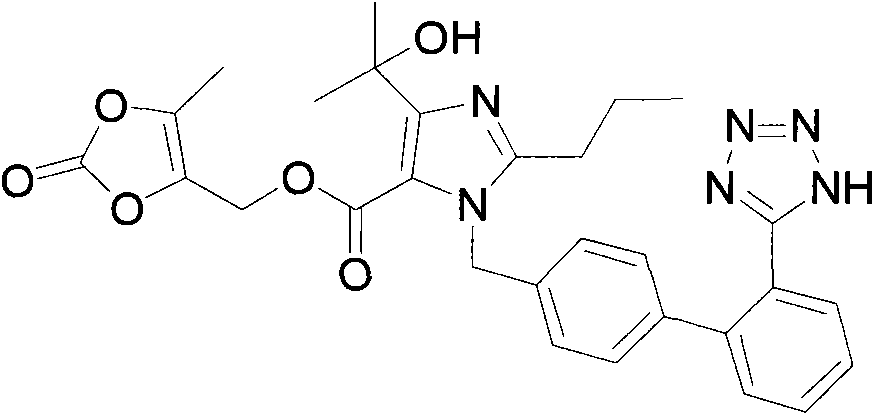
Preparation method of olmesartan medoxomil
A technology of olmesartan medoxomil and methyl ester, which is applied in the field of preparation of olmesartan medoxomil, can solve the problems of complex processing steps and unfavorable industrial production, and achieve the effects of reducing costs, saving time, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
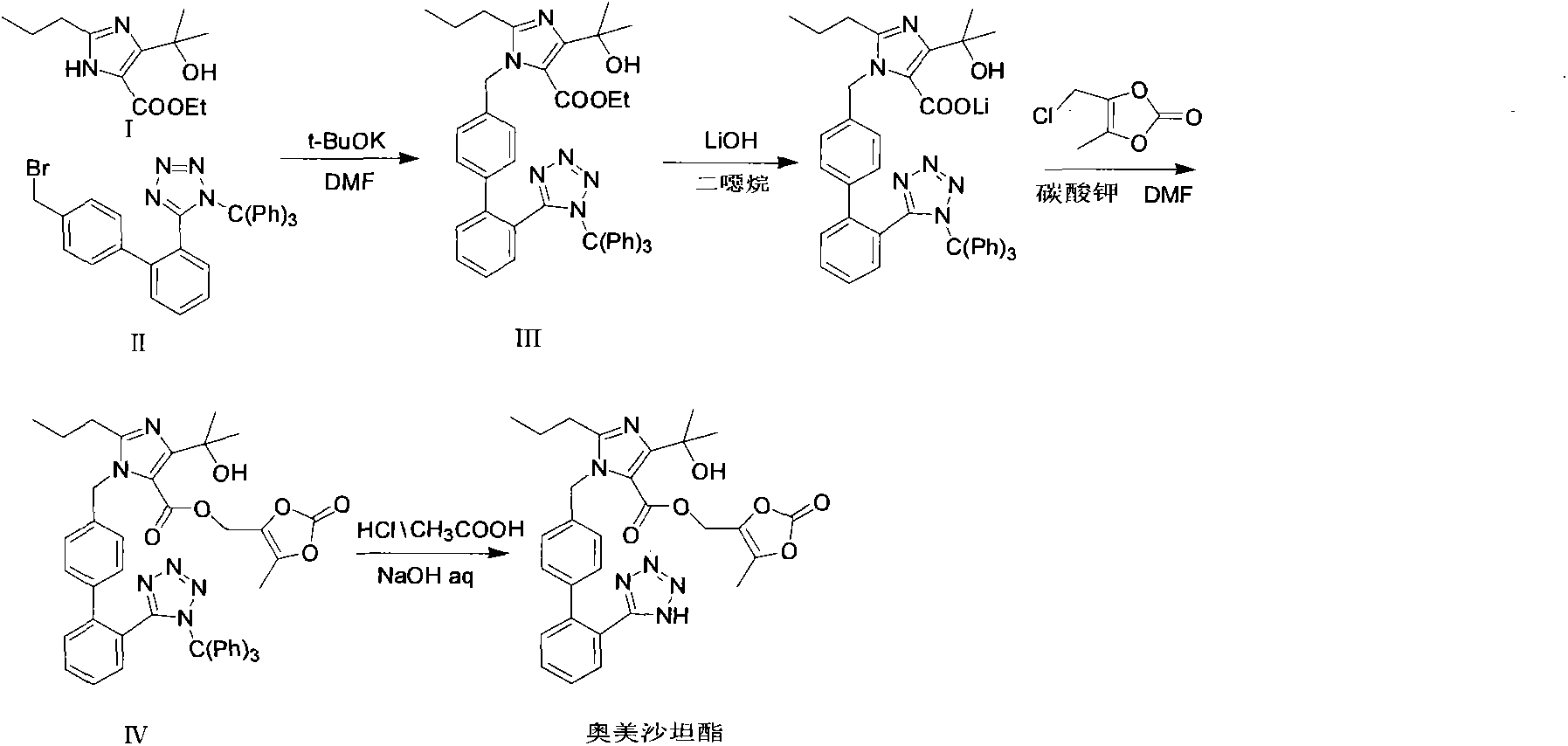
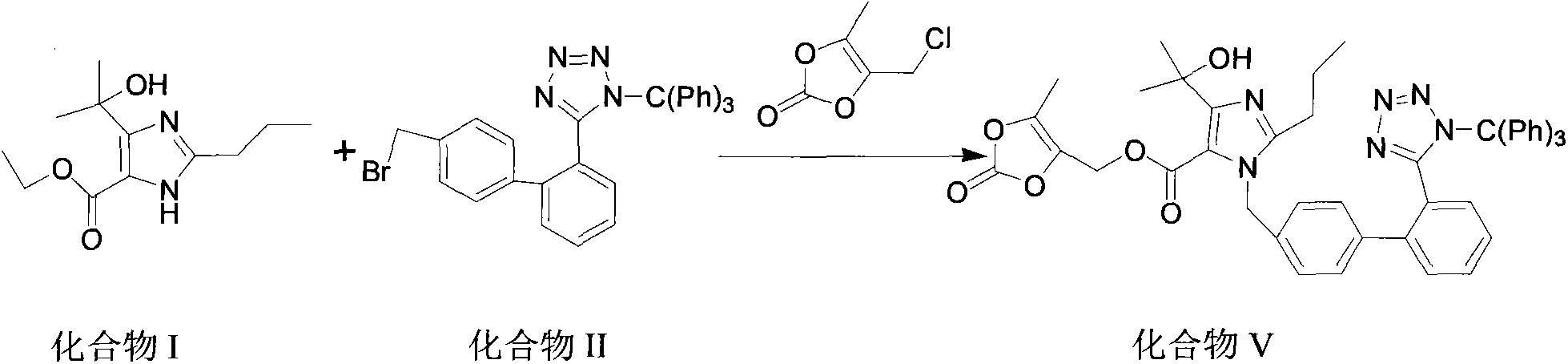
[0036] Add 12g4-(1-hydroxyl-1-methylethyl)-2-propylimidazole-5-carboxylic acid ethyl ester and 3.6g sodium hydroxide, 120ml DMF and 35gN-(triphenylmethyl) in the reaction flask -5-(4'-bromomethylbiphenyl-2-yl)tetrazolium. After the addition, the reaction system was heated up to 75° C., and reacted at this temperature for 10 h. TLC monitored the completion of the reaction, and stopped heating. Add 2.2 g of sodium hydroxide to the above reaction system. After the addition is complete, the temperature rises to 65° C., and the reaction is maintained at this temperature for 3 hours. After the reaction is completed as monitored by TLC, the reaction system is cooled to 10° C.
[0037] Then, 2 g of cesium carbonate and 9 g of 4-chloromethyl-5-methyl-1,3-dioxol-2-one were added to the above system, and the reaction was carried out at room temperature for 2 h. After the reaction was completed, extract with ethyl acetate, wash with saturated aqueous sodium chloride solution twice, dry th...
Embodiment 2
[0040]Add 12g4-(1-hydroxyl-1-methylethyl]-2-propylimidazole-5-ethyl carboxylate and 4g sodium hydroxide in the reaction flask, 120ml DMF, add 31gN-(triphenylmethyl) -5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (II), after the feeding is completed, the reaction system is heated to 70°C, and reacted at this temperature for 11h, TLC monitors that the reaction is complete, stop heating .
[0041] Add 2.2 g of potassium hydroxide to the above reaction system. After the addition is complete, the temperature rises to 68° C., and the reaction is maintained at this temperature for 3 hours. After the reaction is monitored by TLC, the reaction system is cooled to 5° C.
[0042] Add 2 g of cesium carbonate and 9 g of 4-chloromethyl-5-methyl-1,3-dioxol-2-one to the above system, and react at room temperature for 3 h. After the reaction was completed, extract with ethyl acetate, wash with saturated aqueous sodium chloride twice, dry the ethyl acetate layer with anhydrous sodium sulfate, eva...
Embodiment 3
[0045] Add 12g4-(1-hydroxyl-1-methylethyl]-2-propylimidazole-5-carboxylic acid ethyl ester and 3.9g sodium hydroxide, 125ml DMF, add 31g N-(trityl )-5-(4'-bromomethylbiphenyl-2-)tetrazolium (II), after the addition, the temperature of the reaction system was raised to 70°C, and the reaction was carried out at this temperature for 10 hours. The reaction was monitored by TLC, and the heating was stopped. .
[0046] Add 2.1 g of potassium hydroxide to the above reaction system. After the addition is complete, the temperature rises to 67° C., and the reaction is maintained at this temperature for 3 hours. After the reaction is monitored by TLC, the reaction system is cooled to 5° C.
[0047] Add 2 g of cesium carbonate and 9 g of 4-chloromethyl-5-methyl-1,3-dioxol-2-one to the above system, and react at room temperature for 2.5 h. After the reaction was complete, extract with ethyl acetate, wash with saturated aqueous sodium chloride twice, dry the ethyl acetate layer with anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com