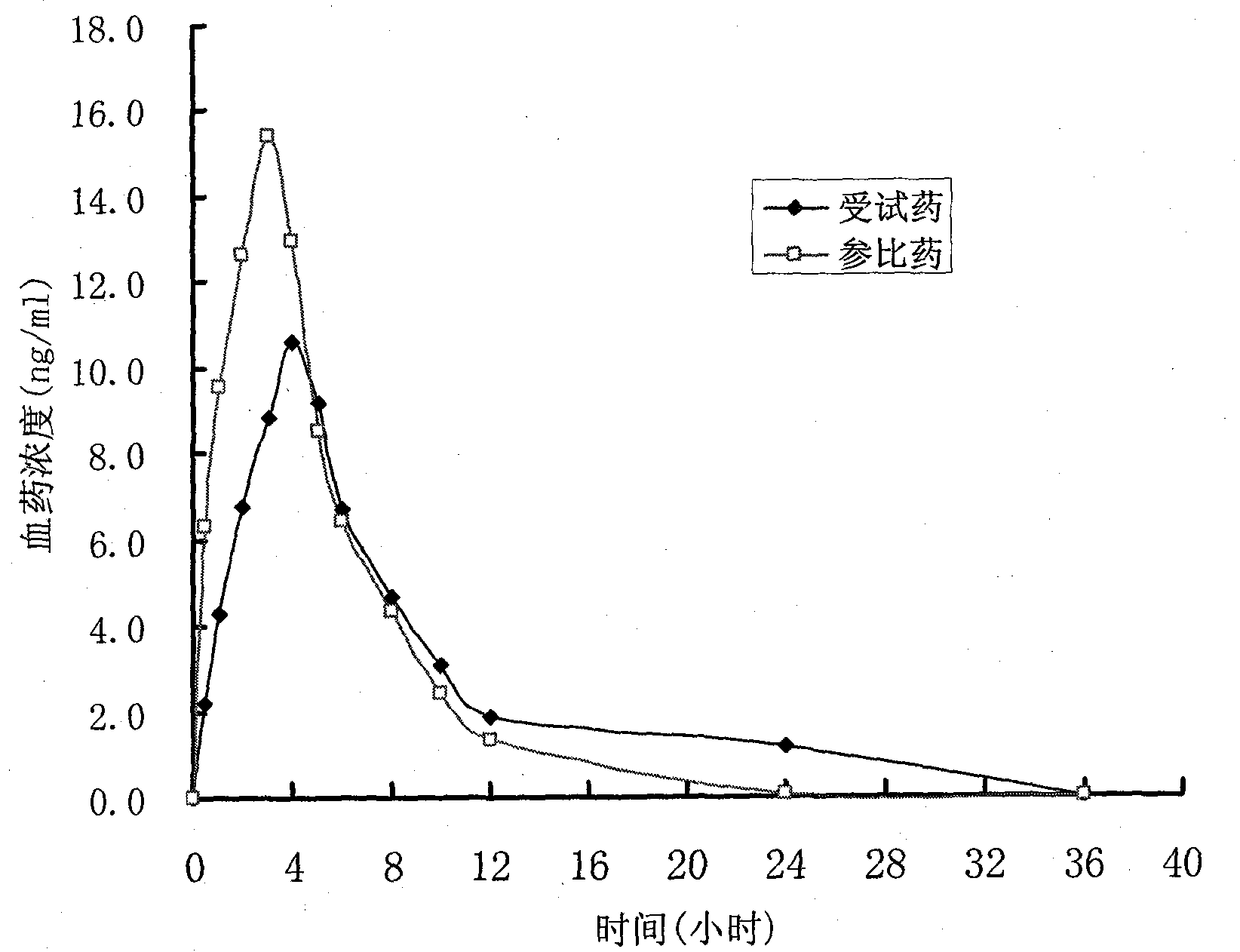
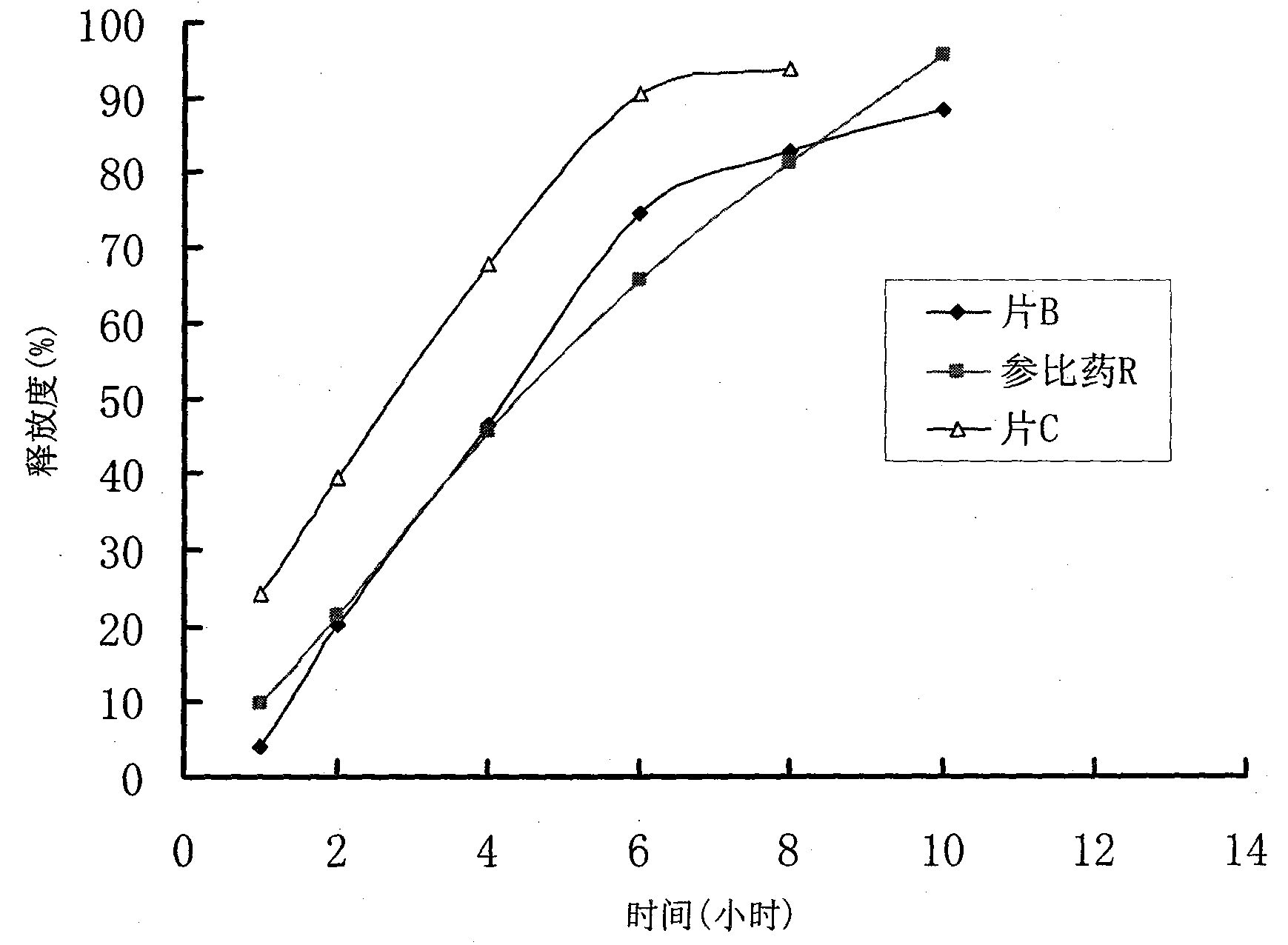
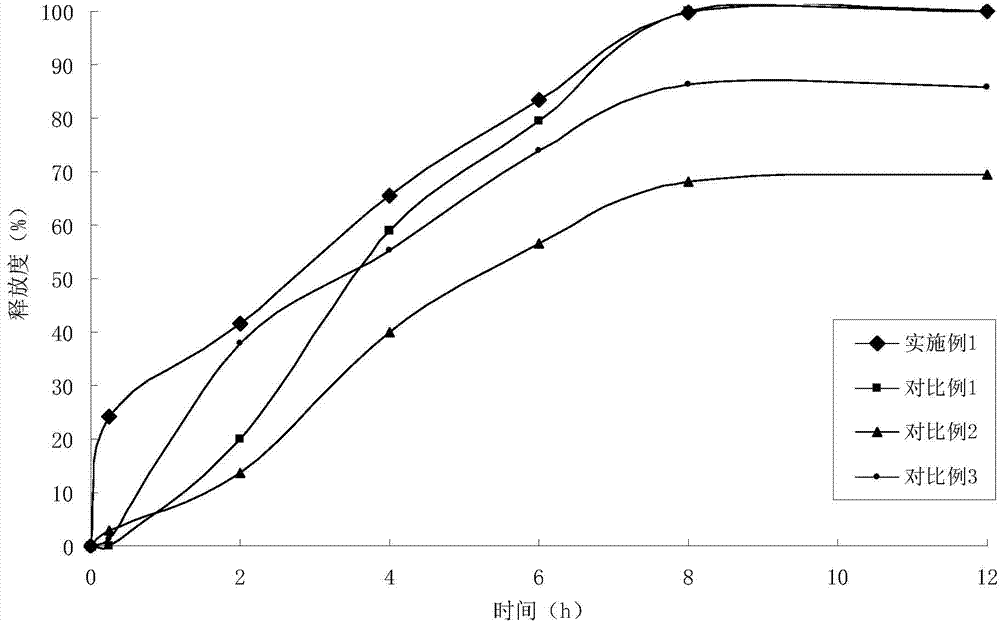
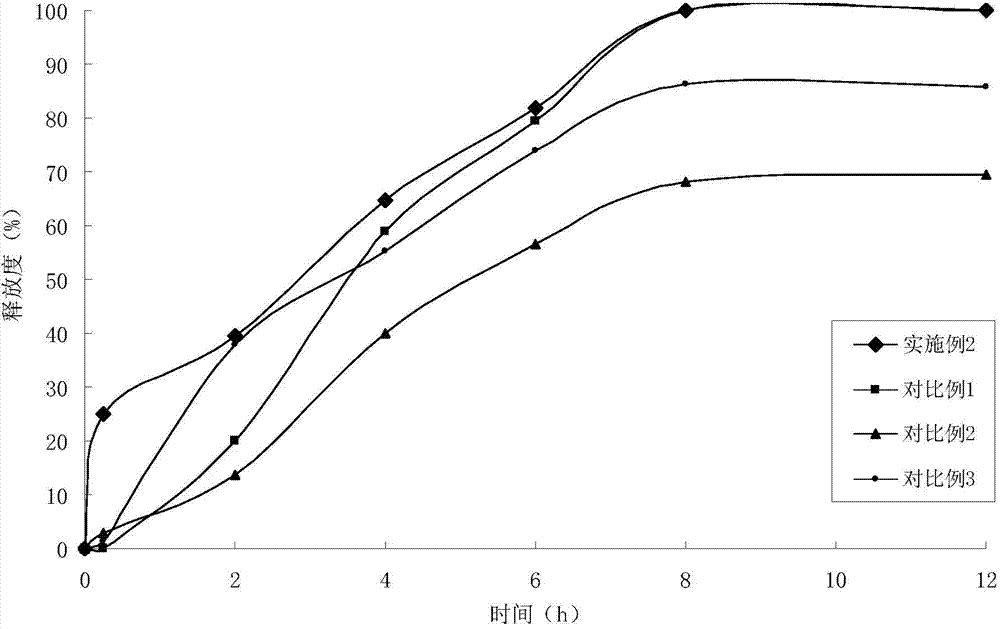
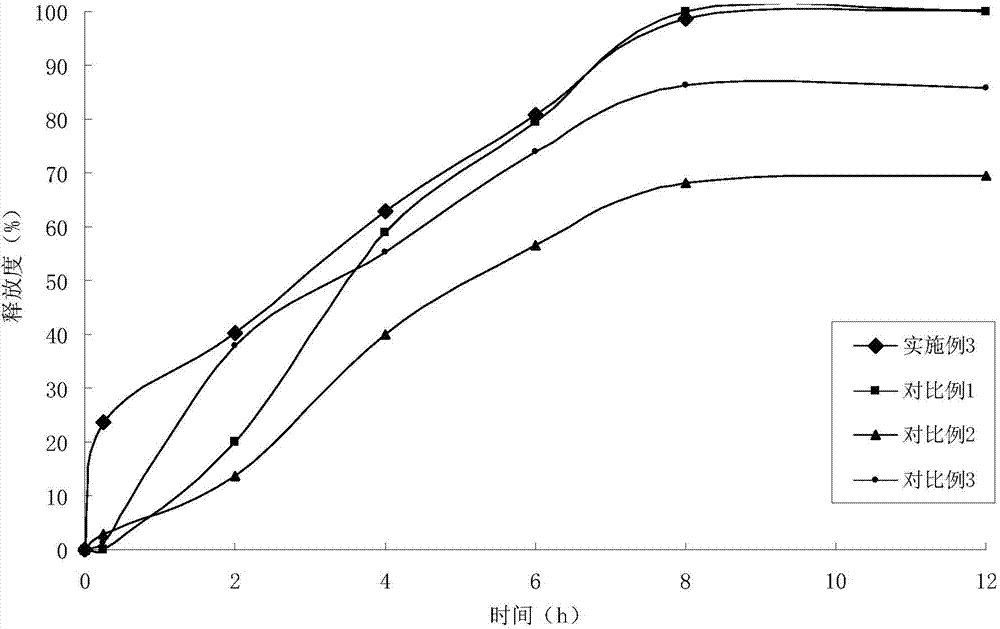
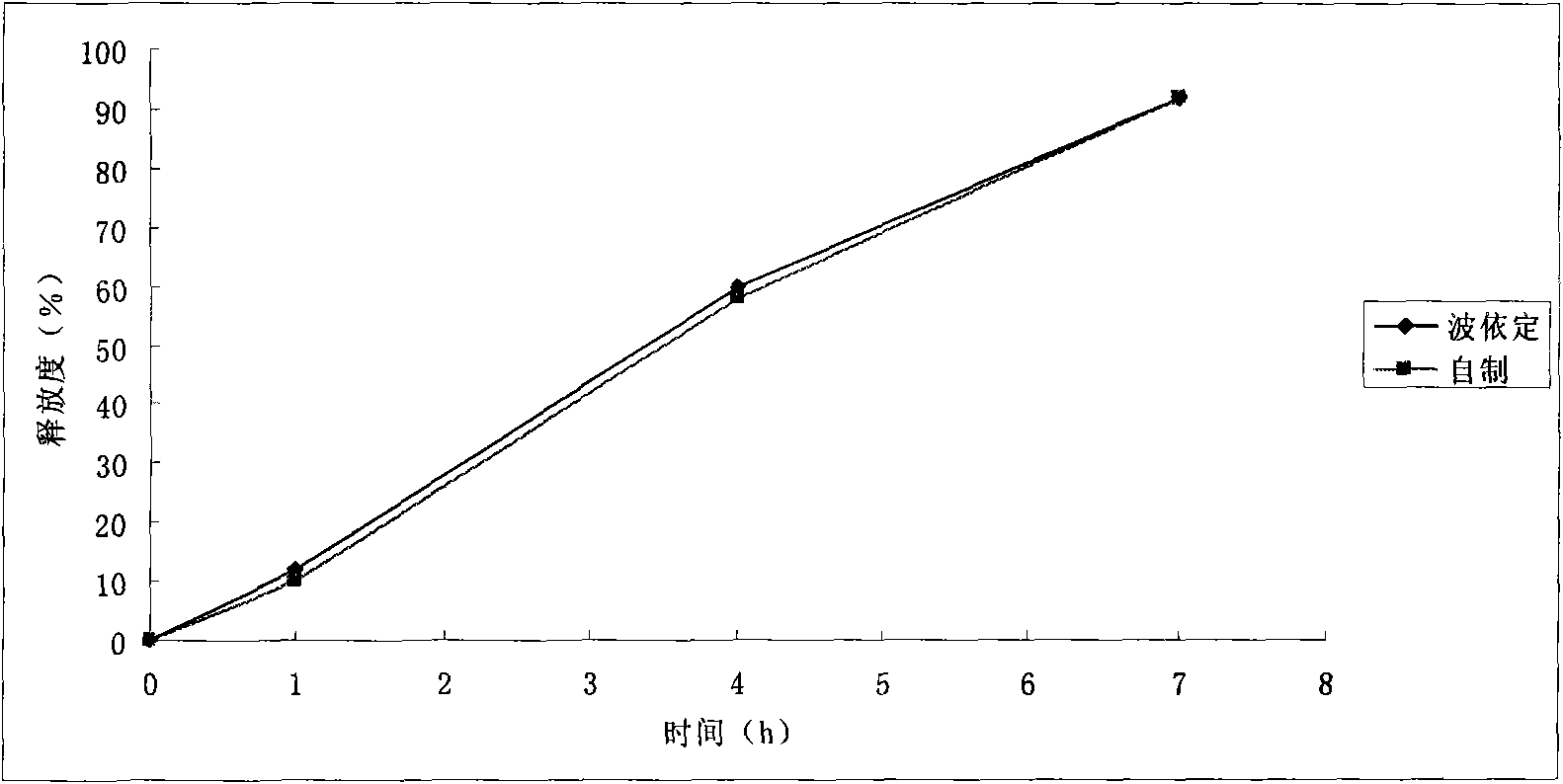
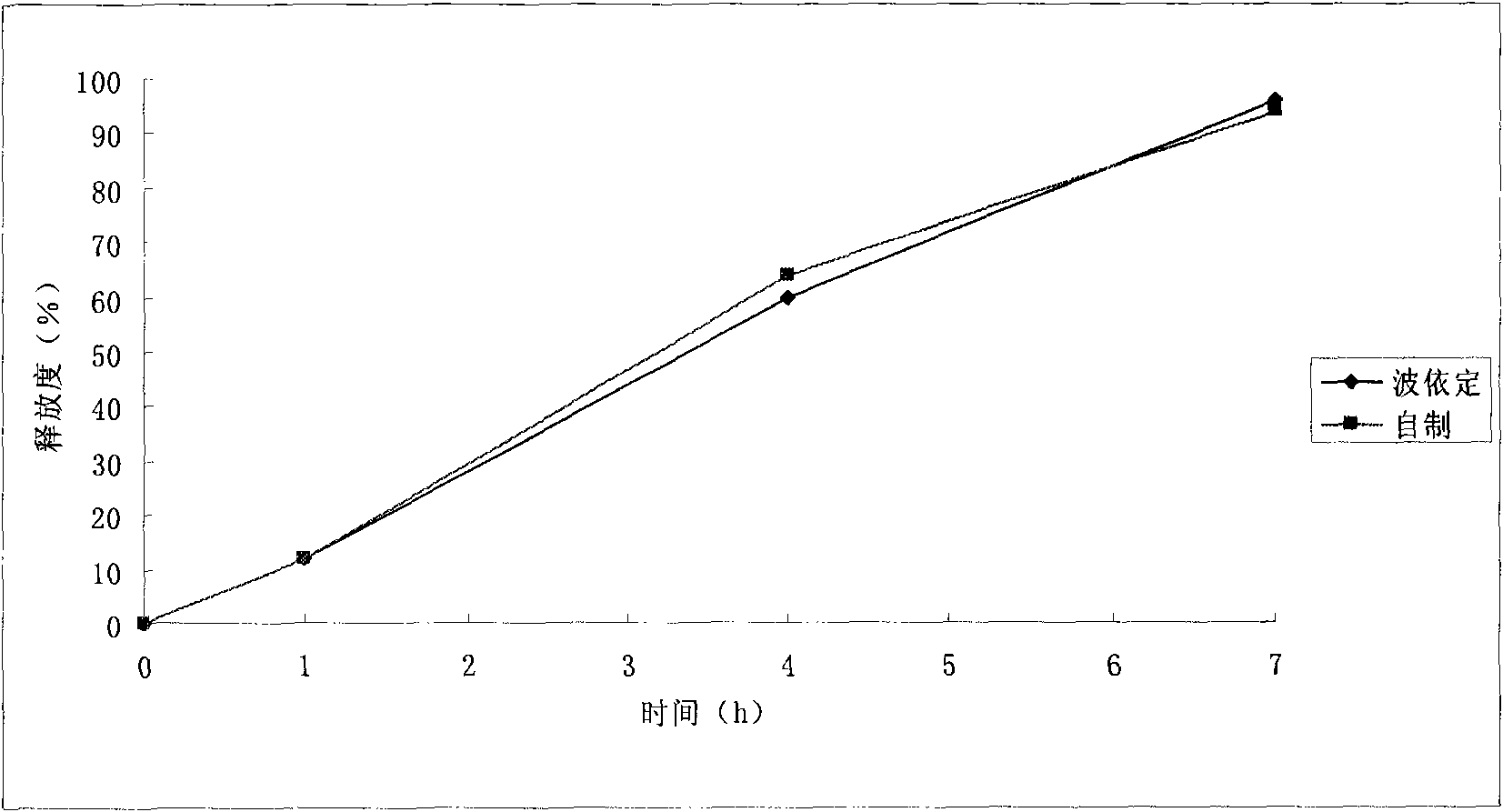
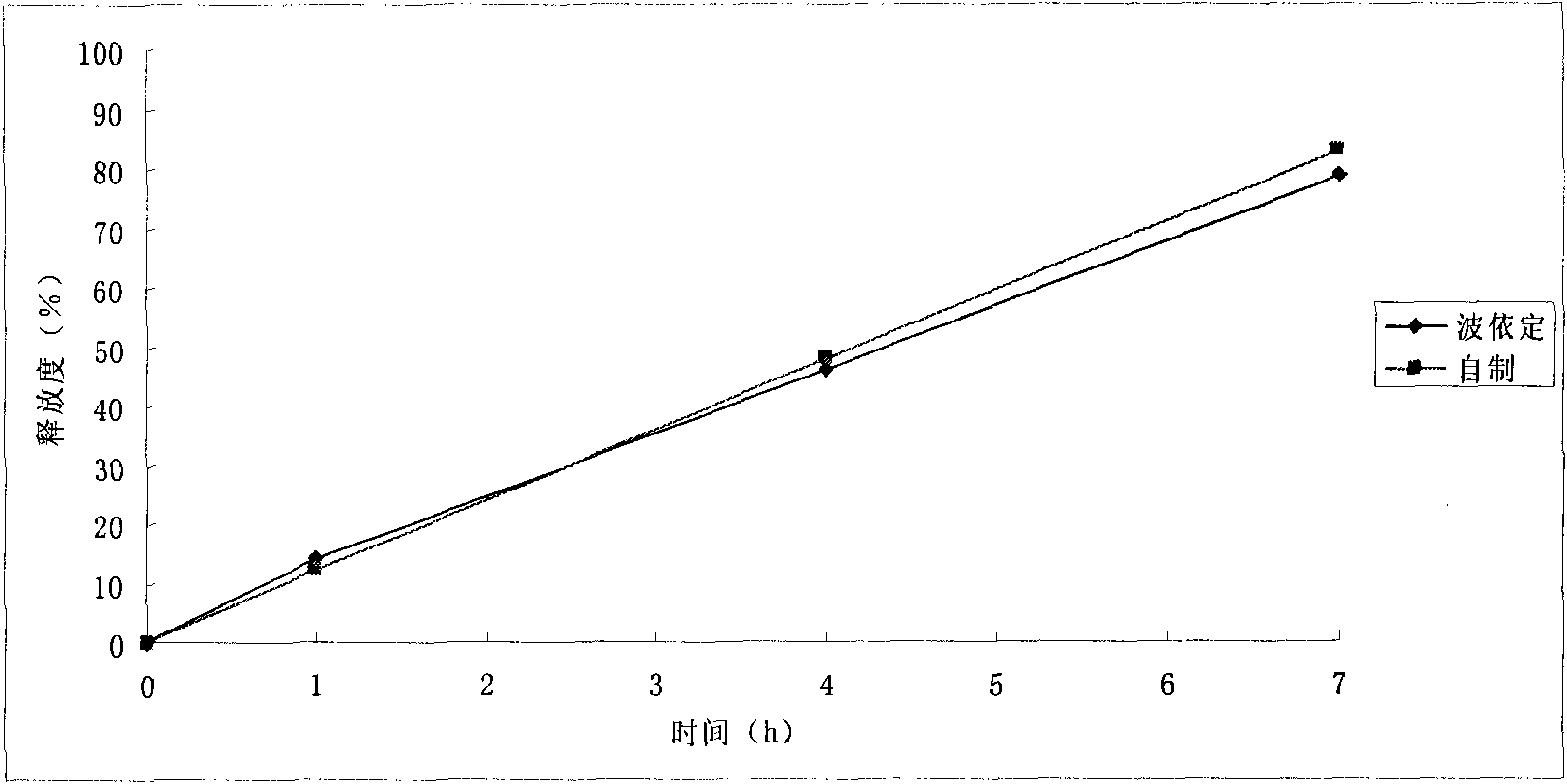
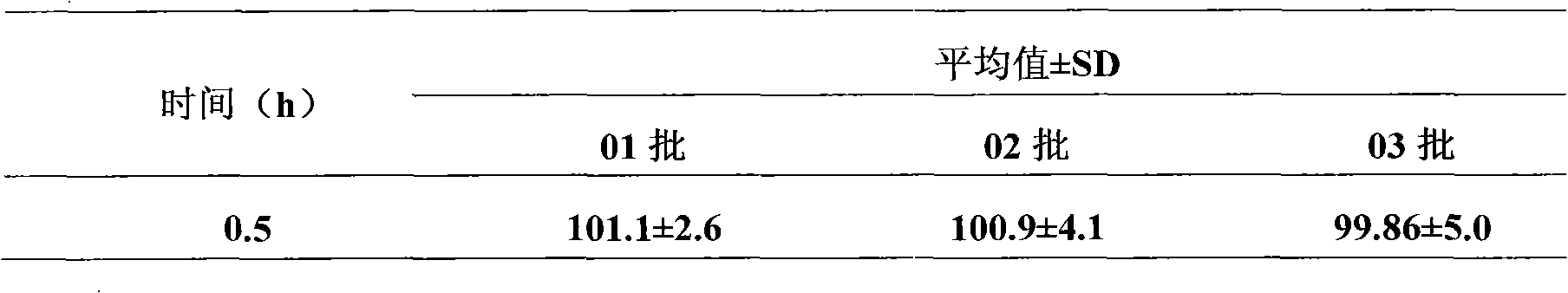
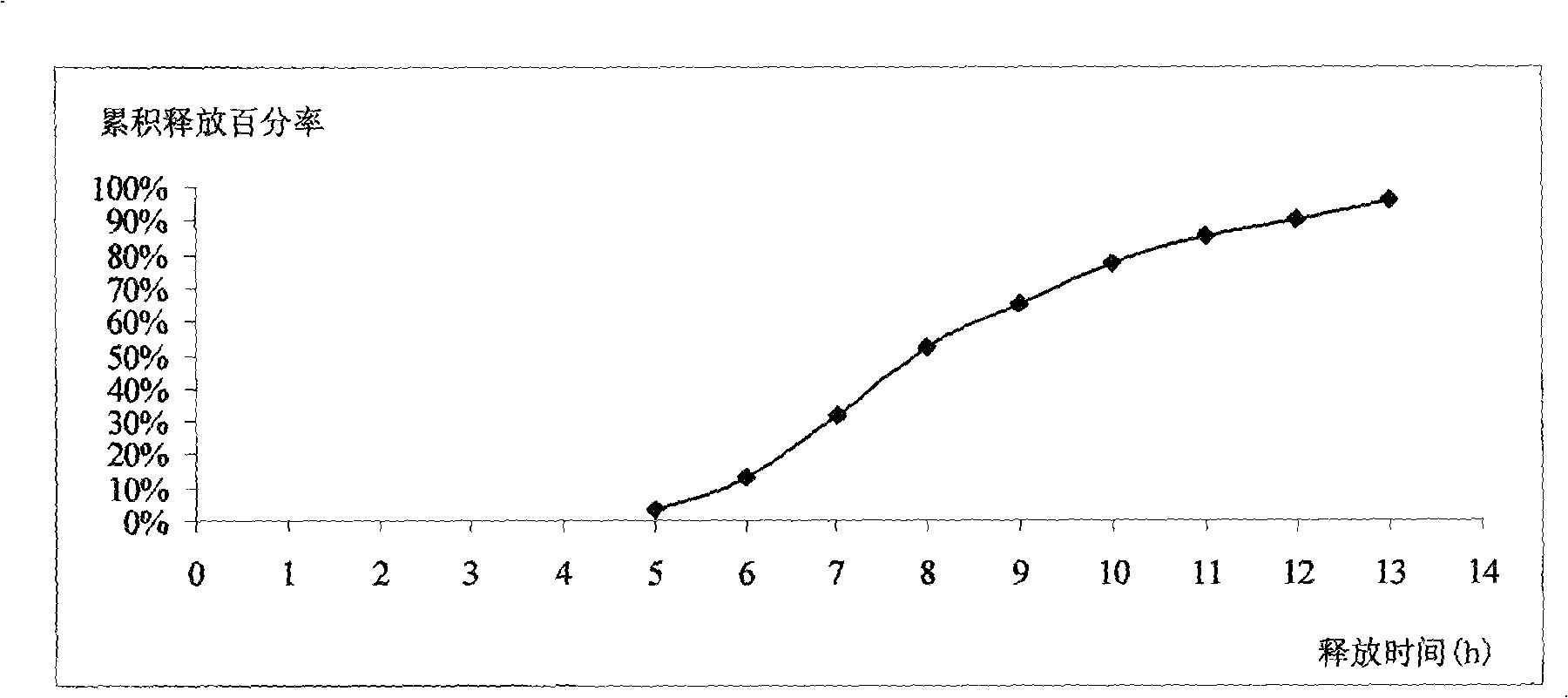
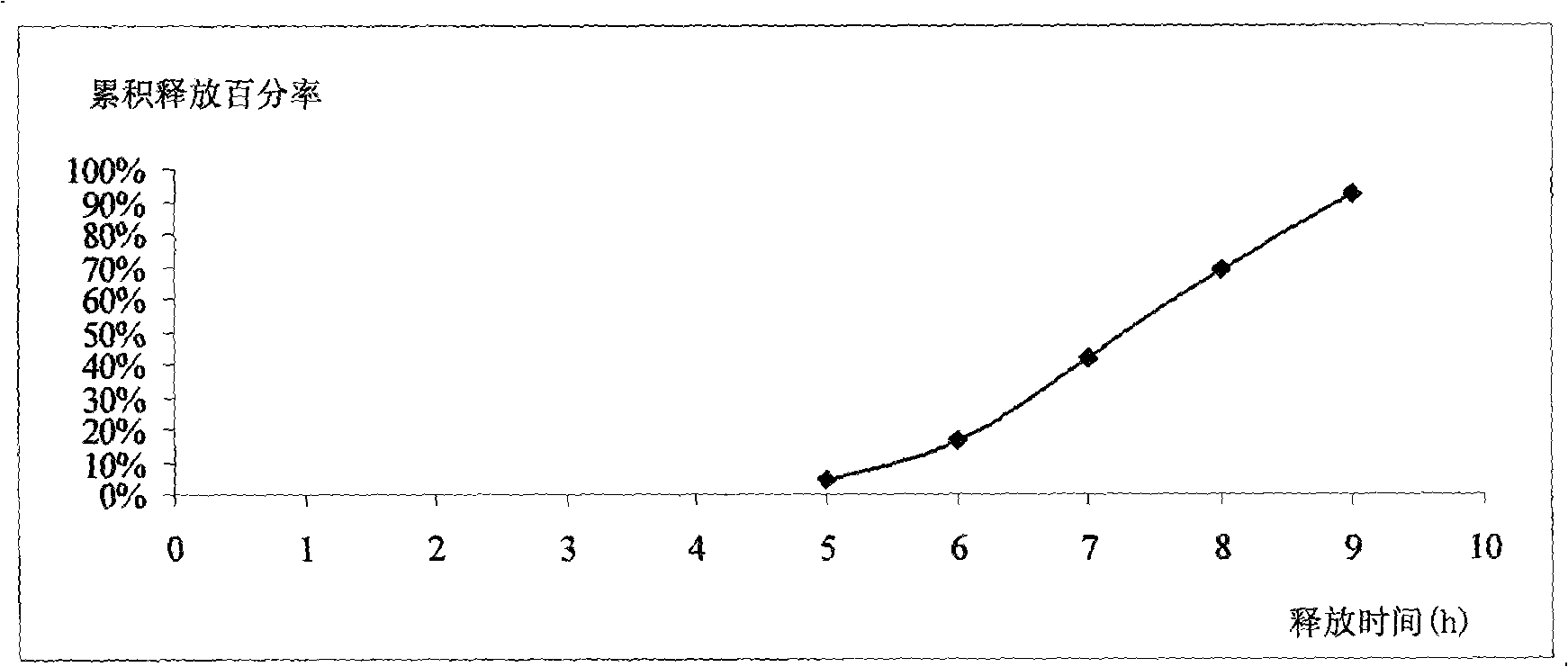
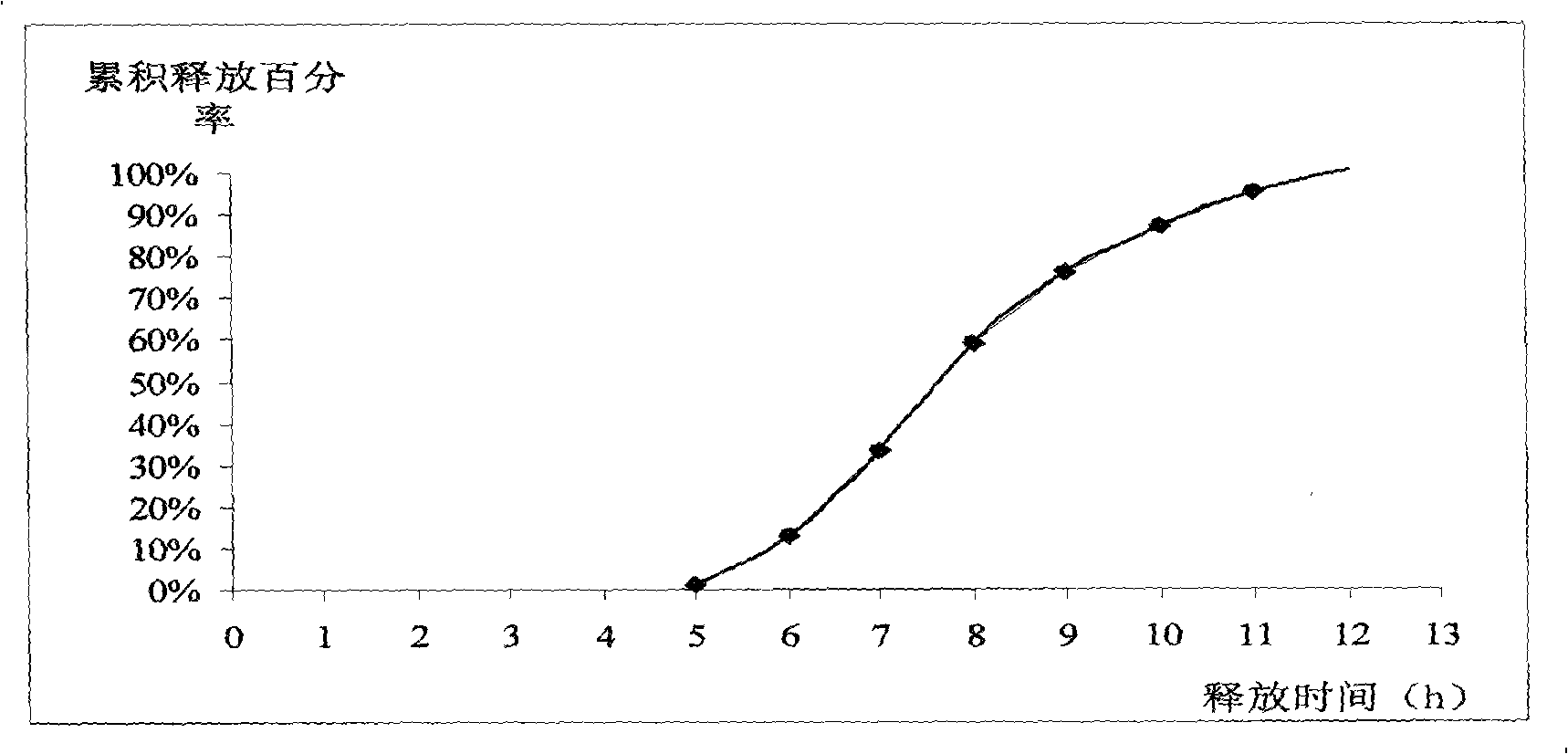
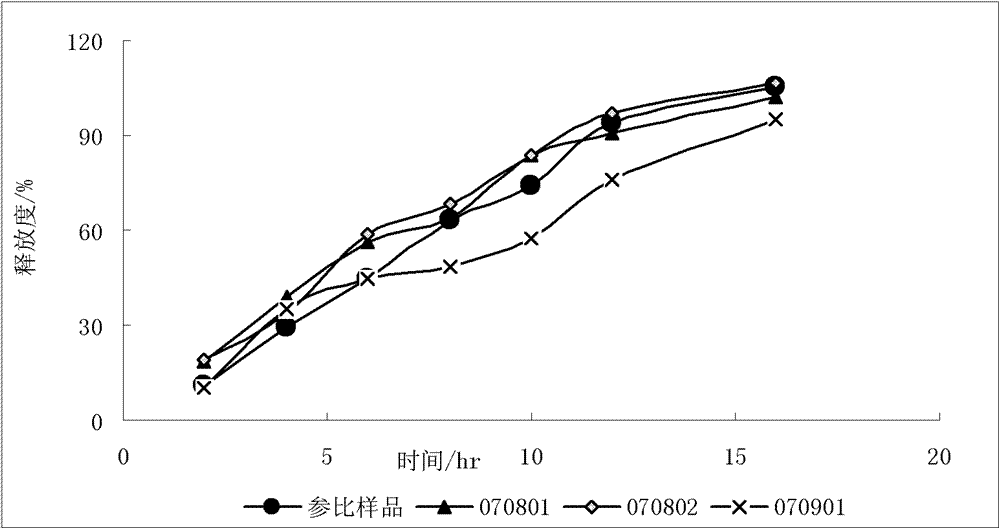
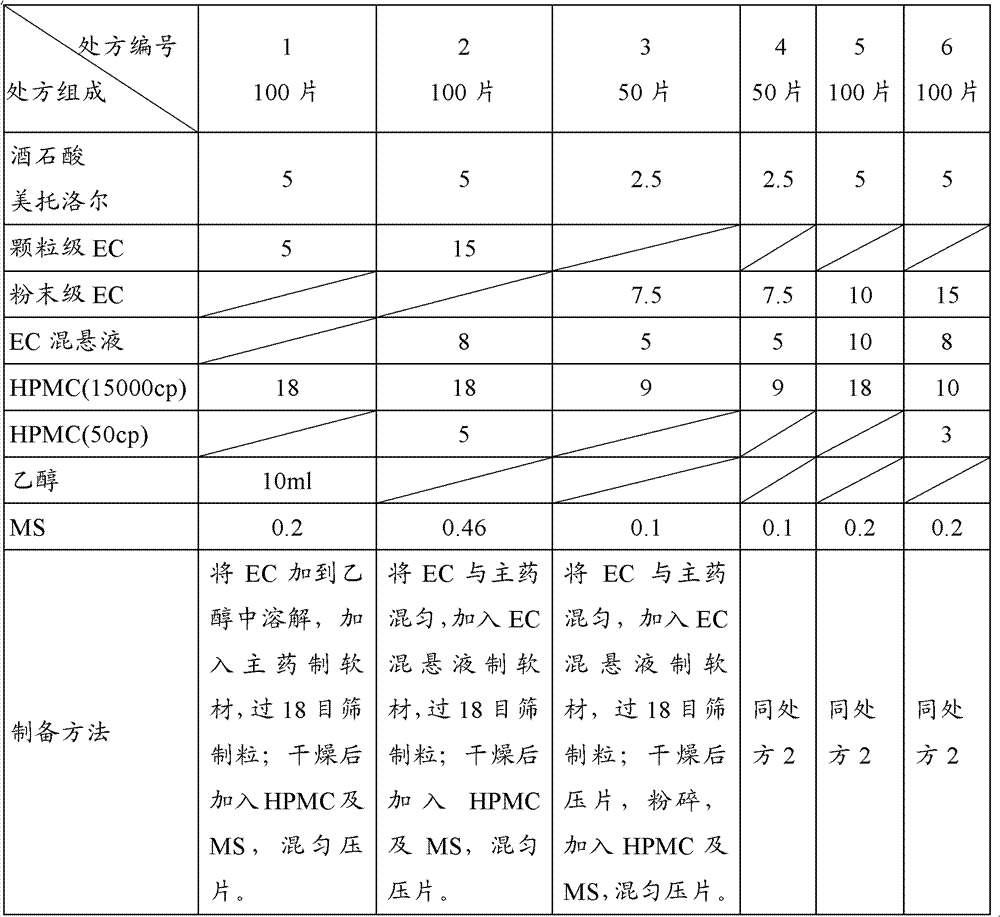
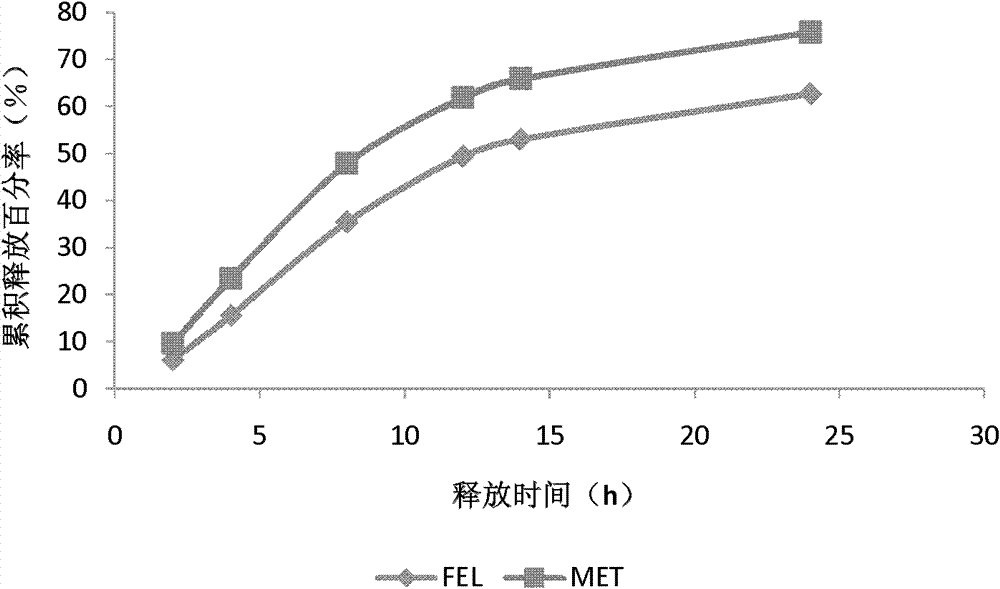
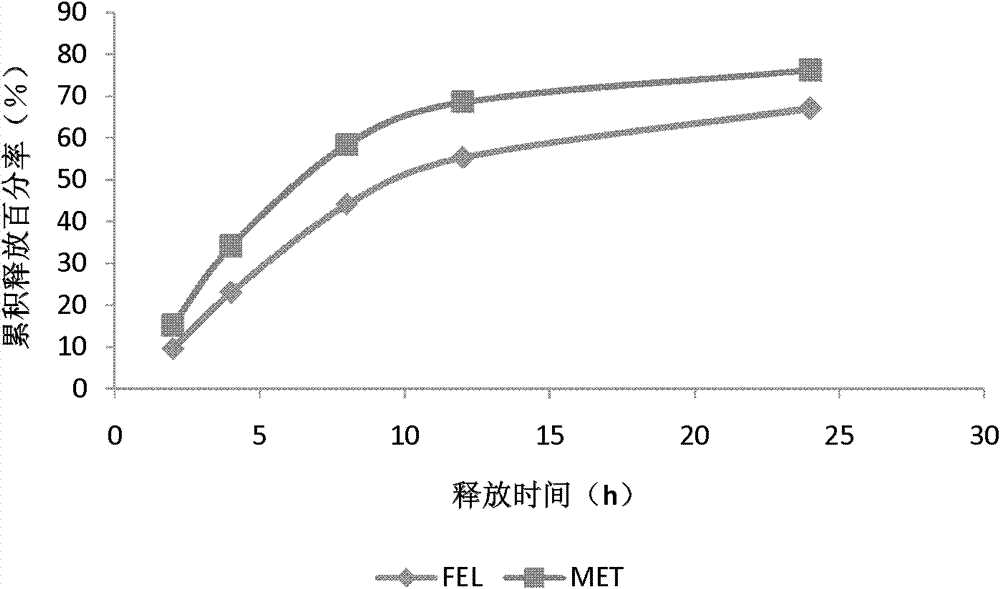
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
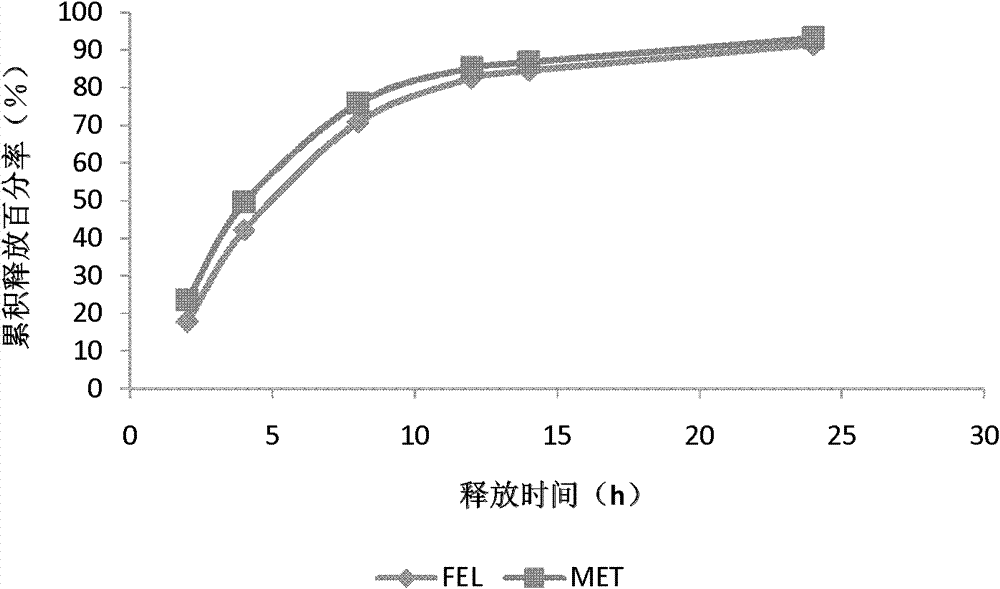
118 results about "Felodipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Felodipine is used to treat high blood pressure (hypertension).
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
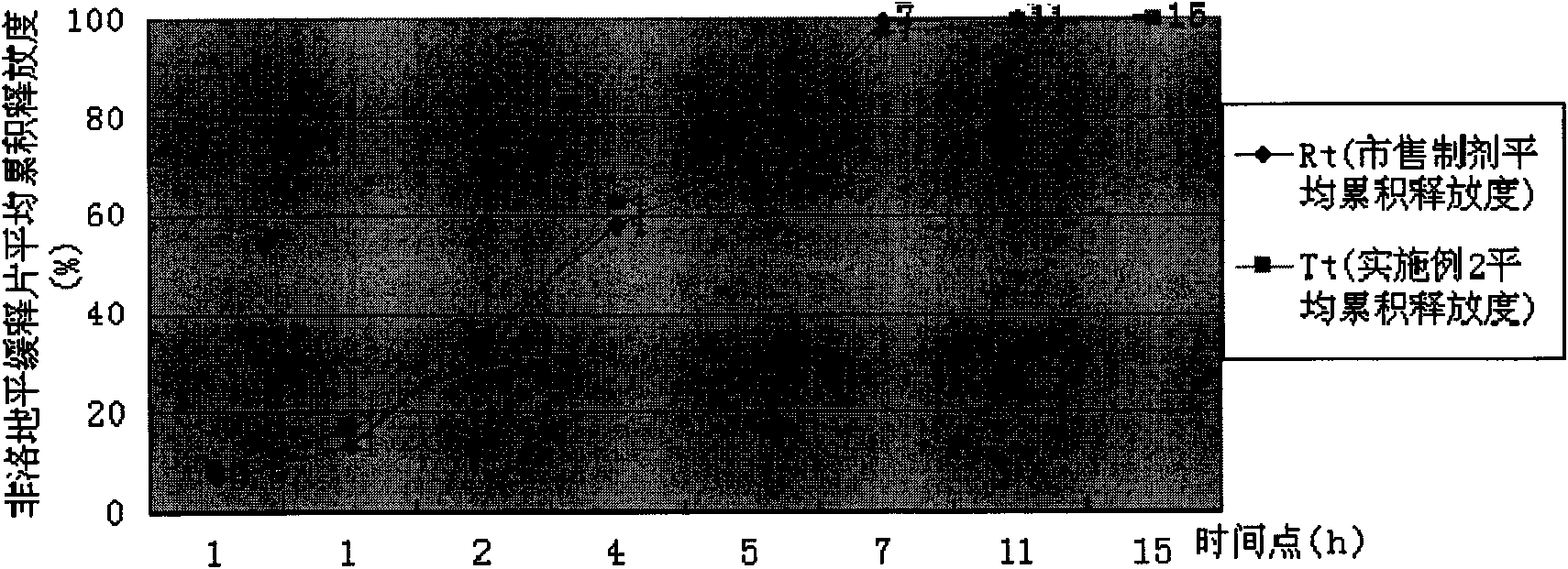
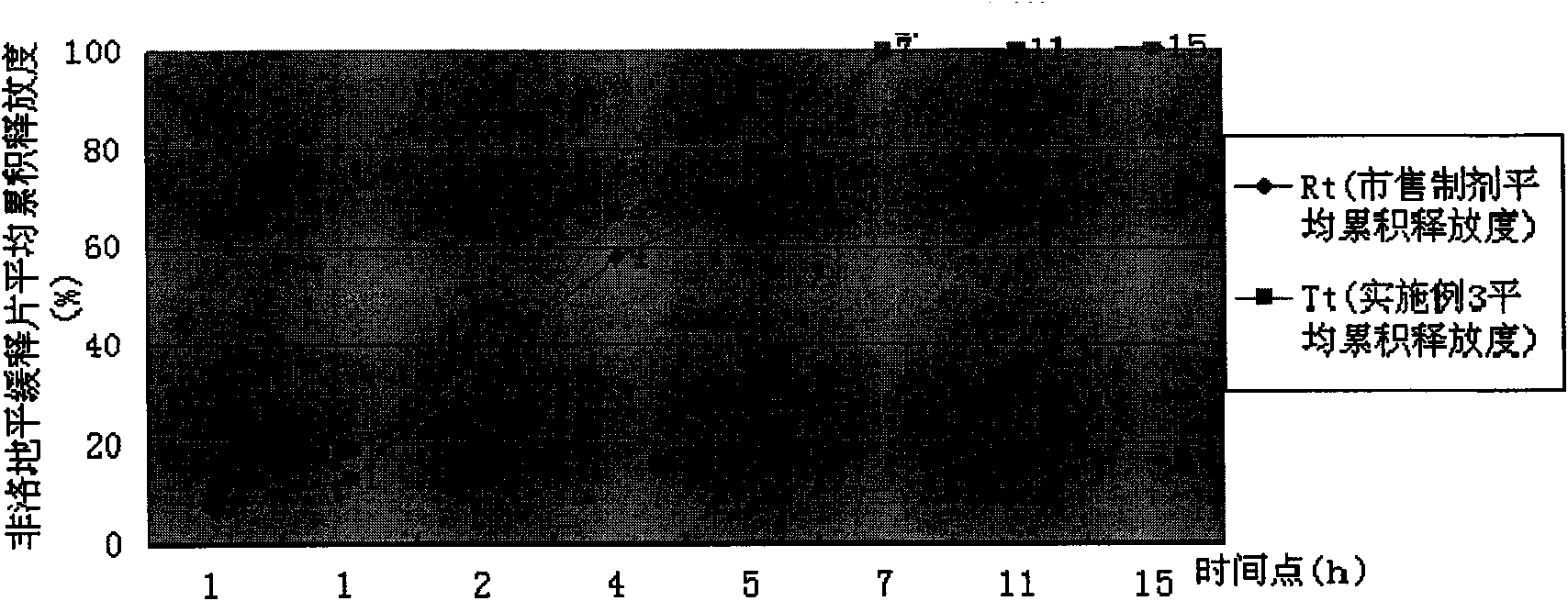
Felodipine controlled-release preparation
InactiveCN1640400AAvoid damageStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveSemipermeable membrane
The present invention discloses a felodipine control-released preparation. It comprises core body and film. Said core body comprises feldodipine with effective therapeutic dose and physiologically-acceptable osmotic pressure regulating agent, osmotic accelerator and adhesive, and its film is made up by using semipermeable membrane material and pore-forming material.
Owner:HEBEI MEDICAL UNIVERSITY +1
Sustained-release preparation containing felodipine and preparation method thereof
ActiveCN101103964AControl releaseSolve the release problemOrganic active ingredientsPharmaceutical delivery mechanismFiller ExcipientAqueous solution
The invention provides a preparation method of a sustained-release preparation containing felodipine. The invention is characterized in that the felodipine and bond are dissolved in ethanol absolute solution or water solution containing more than 60 percent ethanol, then are fixed with matrix sustained-release necessities and filling agent and finally are moistened and granulated by the ethanol absolute solution or the water solution of ethanol. The invention also provides the sustained-release preparation prepared by the method and usage in preparation of medicine for treating hypertension.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Double layer osmotic pump controlled release felodipine medicine composition
The double layer osmotic pump controlled release felodipine medicine composition includes double layer core and coating film. The double layer core contains felodipine and pharmaceutically acceptable excipient; and the coating film is semi-permeating film with or without pore creating agent. The double layer osmotic pump controlled release felodipine medicine composition of the present invention can control the release of medicine in some specific time period, has reduced medicine taking times, smooth in vivo blood medicine concentration and radically raised medicine safety and effectiveness.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
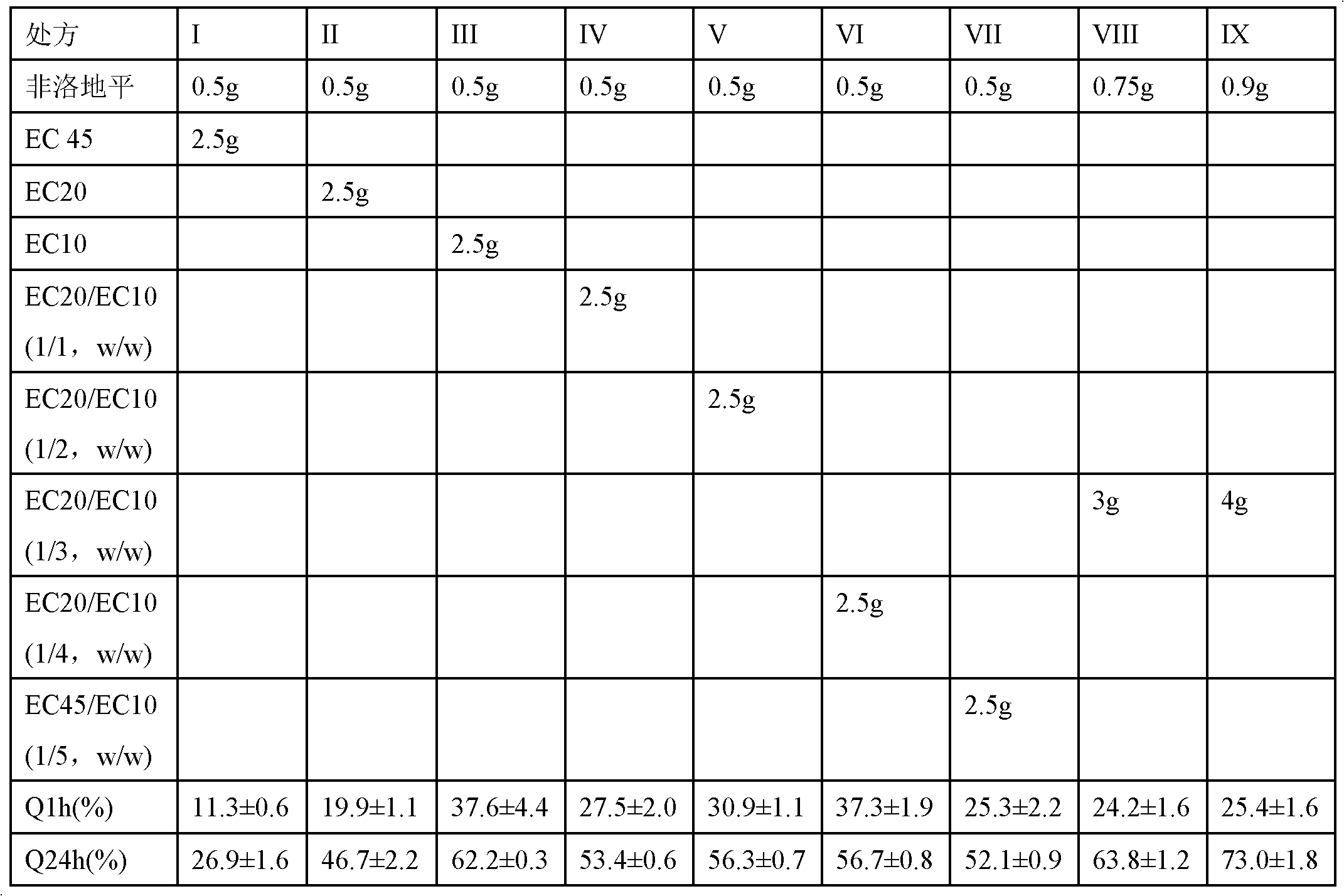
Felodipine pharmaceutical composition based on dry process granulation
InactiveCN102462663AReduce the impact of stabilityAvoid joiningOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventAdhesive
The invention relates to a hypotensor felodipine pharmaceutical composition and a preparation method thereof. The composition comprises felodipine, a dispersing agent, a filling agent, a disintegrating agent, a solubilizing agent, a lubricant and a dry adhesive. The preparation method of the composition comprises the steps of: firstly, micronizing the felodipine with the dispersing agent, and then carrying out dry process granulation to prepare a felodipine pharmaceutical preparation. In the preparation method, the water and organic solvent are not used, the moist heat process and production energy consumption are reduced, and the quality control in the production process is facilitated. The felodipine pharmaceutical preparation prepared with the preparation method provided by the invention is uniform in content and good in dissolution rate.
Owner:YAOPHARMA CO LTD
Preparation method of felodipine sustained release tablets
ActiveCN101843598ASuitable for industrialized mass productionHigh yieldOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletFiller Excipient
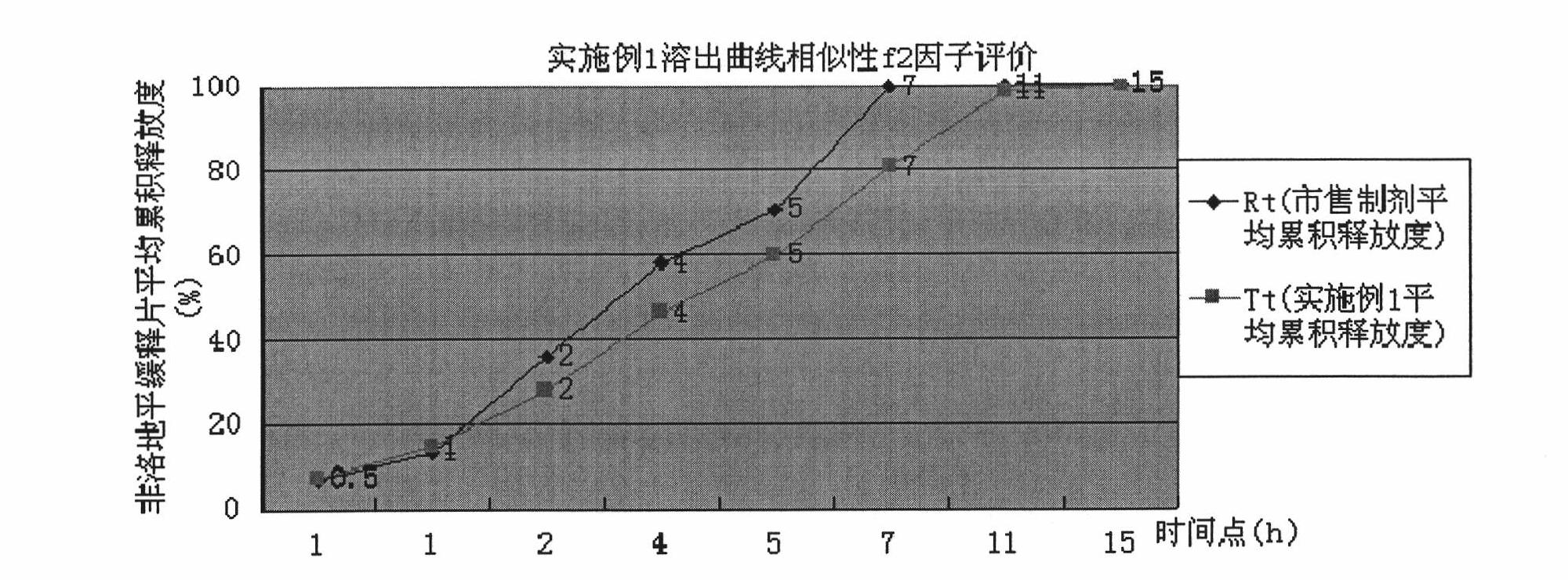
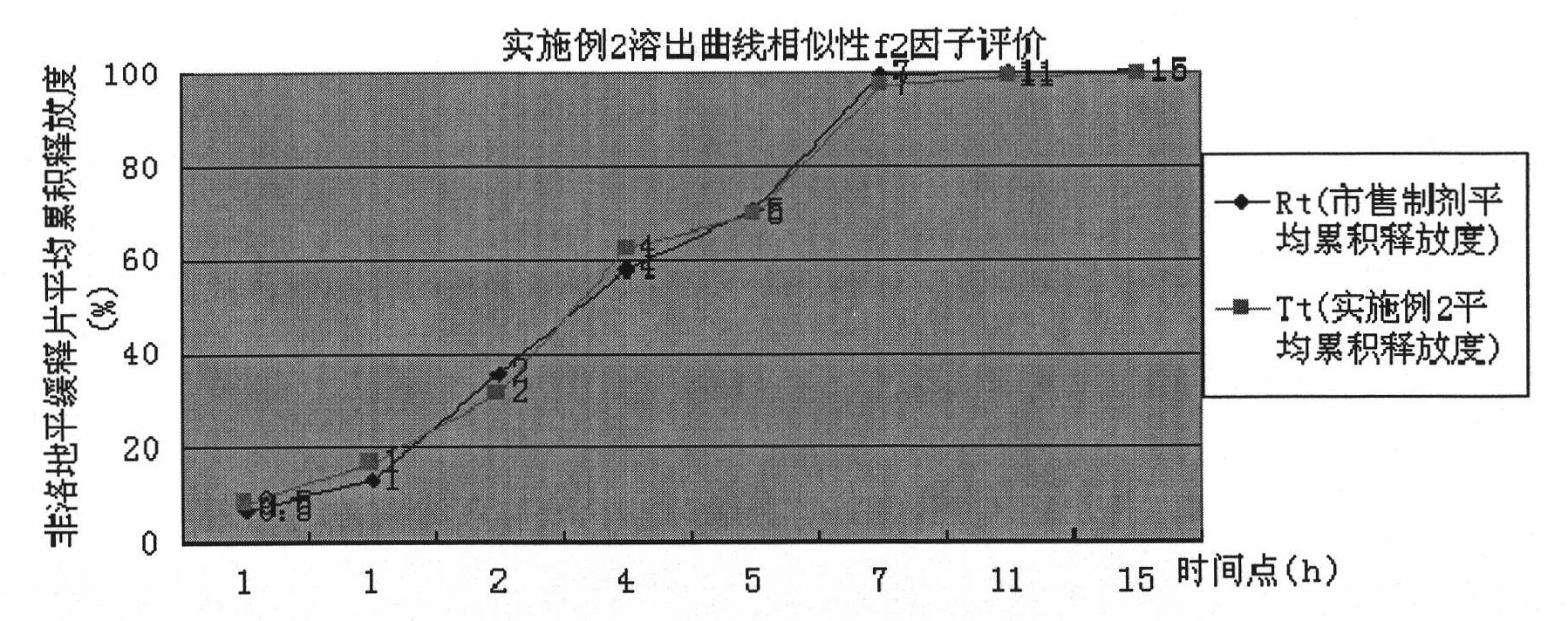
The invention discloses a preparation method of felodipine sustained release tablets. The method is characterized by comprising the following steps of: mixing felodipine accounting for 1-10 percent of the total component weight, a part of hydroxypropyl methylcellulose accounting for 20-55 percent of the total component weight and a filler; and wetting by using a wetting agent to carry out wet granulation; after wet granules are dried at low temperature, mixing the dried granules with residual hydroxypropyl methylcellulose; adding a lubricating agent and then tabletting to prepare cores containing main drugs; and coating by using a coating material to obtain the felodipine sustained release tablets. The preparation method is easy to control and suitable for industrial scale production, has high product yield and reduces the production cost. Compared with merchant like preparations, the felodipine sustained release tablets obtained by using the preparation method has similar in vitro release curve.
Owner:CHANGZHOU PHARMA FACTORY
Composition containing dihydropyridine calcium channel blocker and preparation method thereof
InactiveCN101548973APlay a sustained release roleLower blood pressure blood pressurePill deliveryHeterocyclic compound active ingredientsAngiotensin-converting enzymeAdditive ingredient
The invention discloses a composition containing a dihydropyridine calcium channel blocker and a preparation method thereof and relates to a sustained-release preparation of felodipine, which comprises felodipine which is dihydropyridine calcium channel blocker and function as an active ingredient, enalapril which is angiotensin converting enzyme inhibitor, as well as a slow release material, a filling agent and a lubricating agent which function as auxiliary materials; wherein the slow release material contains hydroxypropyl methylcellulose. As the enalapril which is angiotensin converting enzyme inhibitor is added in coating solution, the curative effect of the sustained-release preparation is improved.
Owner:YANGZHOU PHARM CO LTD +1
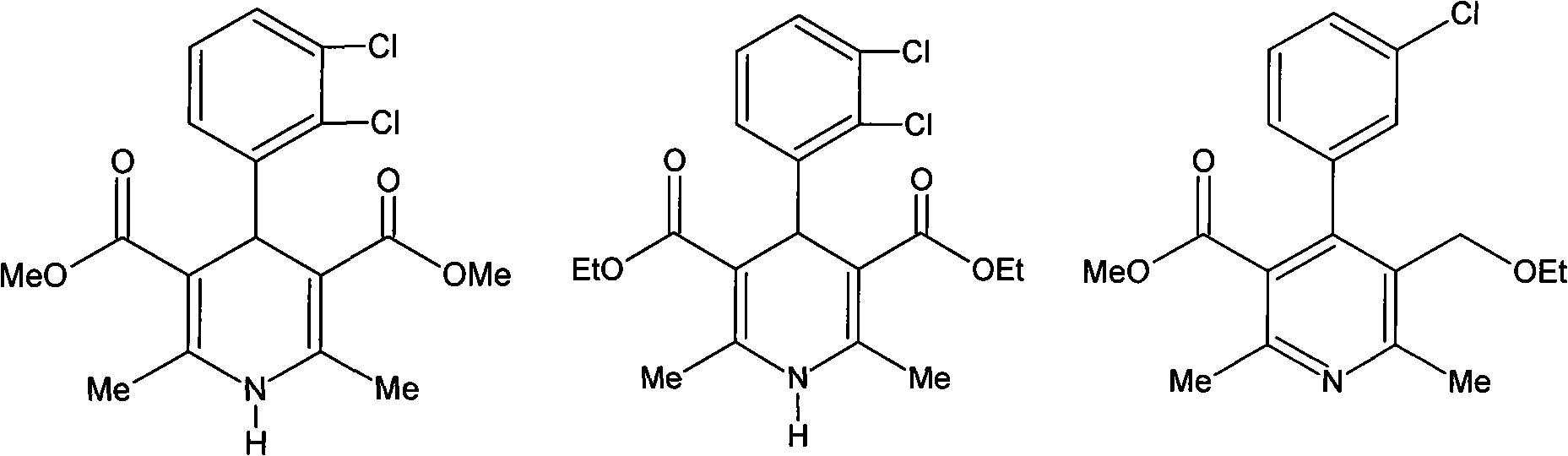
The preparation method of felodipine
InactiveCN102285911AEasy to operatePreparative route steps are short and effectiveOrganic chemistryAcetic acidEthyl ester
The invention discloses a method for preparing felodipine, which belongs to the technical field of felodipine synthesis. The method is characterized by comprising the following steps of: performing a Knoevenagel condensation reaction of 2,3-dichlorobenzaldehyde and methyl acetoacetate which are used as initial raw materials to obtain 2,3-dichlorobenzylidene acetoacetate; and performing a Michael cyclization reaction of 2,3-dichlorobenzylidene acetoacetate and 3-amino ethyl crotonate to obtain the felodipine to obtain the felodipine. The method has the advantages that the preparation route is simple, short and reliable; the preparation period is short; the method is simple and convenient to operate and has high safety and low environmental pollution; residual toxicity of a medicament is low; and the post treatment process has high efficiency.
Owner:SHAOXING UNIVERSITY
Medicinal composition containing felodipine and metoprolol
The invention discloses a medicinal composition containing felodipine and metoprolol, which contains the felodipine and the metoprolol with a grain diameter D90 of between 10 and 200mu m, wherein the felodipine and the metoprolol are double-layer tablets prepared by dispersing in respective pharmaceutically acceptable carriers respectively in the medicinal composition. The composition can be steadily released, has good stability at the same time, and is used for the hypertension which has poor treatment effect by other medicaments.
Owner:BEIJING D VENTUREPHARM TECH DEV
Controlled release preparation and preparation method thereof
InactiveCN101879146AEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsIn vivoPermeation
The invention provides a controlled release preparation and a preparation method thereof. The controller release preparation is characterized in that dual-layer osmotic pump tablet semi-permeable membrane is covered by a drug-containing coating layer which can rapidly release partial drug active constituent of marked dosage; and a dual-layer osmotic pump tablet core is formed by two layers, one layer contains most drug active constituent of the marked dosage and permeation enhancing substances, the other layer contains permeable substances, the tablet core is covered by the semi-permeable membrane containing polymers, and a small hole is arranged on the semi-permeable membrane on the side of a drug-containing active constituent layer. The controlled release preparation solves the problem that the release of the dual-layer osmotic pump tablet in vitro and in vivo is lagged behind for 1-3 hour, and can reduce the incomplete release of the active constituent. The preparation method of the controlled release preparation comprises the following steps that the drug active constituent accounting for large part of the marked content is prepared in the dual-layer tablet and then the rest drug active constituent is covered in the coating layer of the dual-layer tablet. Preferably, felodipine is selected as the drug active constituent.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Slow-release tablets containing felodipine and metoprolo salt, and preparation method thereof
ActiveCN102727460ASimple processOrganic active ingredientsPharmaceutical delivery mechanismFluidized bedMedicine
The present invention provides slow-release tablets containing felodipine and a metoprolo salt, and a preparation method thereof. The slow-release tablets comprise a tablet core, a drug-containing layer, a slow-release coating layer and a film coating layer from the inside to the outside, wherein the tablet core is prepared by tableting a metoprolol salt, an insoluble slow-release framework material, and other pharmaceutically-acceptable excipients, and the drug-containing layer comprises micronized felodipine, a solubilizing agent and a binder. According to the present invention, the process is simple and feasible, the characteristic of 24-hour continuous release is provided, the direct tablet core coating manner is adopted to prepare the slow-release tablet, the production process can be completed in the traditional production workshop, and disadvantage of requirement of the fluidized bed and other equipment in the original process is overcome.
Owner:SHANDONG UNIV +1
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Felodipine sustained-release preparation and preparation method thereof
The invention discloses a felodipine-containing sustained-release preparation, which comprises a tablet core and a thin film, wherein the inside of the tablet core contains an effective curative dose of felodipine and a physiologically acceptable pharmaceutic adjuvant. The sustained-release preparation is characterized by consisting of the felodipine, a sustained-release material, a bulking agent, an adhesive, a coloring agent, a lubricant, a wetting agent and a film-forming material. The sustained-release preparation prepared can control drug release effectively to achieve ideal treatment effect, enable human bodies to obtain stable treated blood concentration and optimize the curative effect dose.
Owner:COSCI MED TECH CO LTD
Medicinal composition comprising quick-release pellets containing Enalapril or Enalapril-acid addition salt and slow-release pellets containing Felodipine
ActiveCN102247366AEffective plasma concentrationSmooth releasePharmaceutical delivery mechanismCardiovascular disorderBlood concentrationPatient compliance
The invention relates to a medicinal composition containing Enalapril or Enalapril-acid addition salt and Felodipine, prepared by putting slow-release pellets containing Felodipine and quick-release pellets containing Enalapril or Enalapril-acid addition salt in capsules. Each dosage unit of the medicinal composition contains 2.5-25mg of Enalapril maleate and 2.5-25mg of Felodipine. The medicinal composition can stabilize blood pressure quickly, and keep effective blood concentration for 24 hours, with increasing patient compliance. The medicinal composition is administered only once a day.
Owner:白云山威灵药业有限公司
Felodipine controlled release formulation and preparation method thereof
ActiveCN101292962AOvercome the inconvenience of taking medicineOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical drugBrain vessel
The invention provides a felodipine controlled release preparation for delaying the release and the preparation method thereof. The controlled release preparation can delay the release for 4 to 6 hours and then continue to release for some time to increase the duration time of a drug. Patients can take the drug before sleeping, the effective dosage of the drug is released continuously beginning from next morning, so that the effect of the drug and the occurrence of a disease appear at the same rhythm. As a result, the patients of high blood pressure can safely pass the high occurrence time of cardiovascular events, which overcomes the inconvenience of common preparations cause by taking the drug early in the morning for the 'Morning Blood Pressure Surge' of high blood pressure.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Felodipine sustained-release tablet and preparation method thereof
ActiveCN102188401AEffective solubilizationGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletHigh energy
The invention relates to a felodipine sustained-release tablet and a preparation method thereof. The sustained-release tablet contains an effective amount of felodipine, polyvinylpyrrolidone K30 or polyvinylpyrrolidone K25, wherein the ratio of the polyvinylpyrrolidone K30 or polyvinylpyrrolidone K25 to the felodipine is 3 to 12 : 1, sustained-release materials and the like. The preparation method of the felodipine sustained-release tablet comprises the following steps: adding a solubiliser into an organic good solvent of felodipine for dissolution, and then adding the felodipine for completedissolution of the solubiliser; adding a prepared solution into a adsorbent mixture, and performing sieving and granulating; and then carrying out drying under a normal pressure to obtain sustained-release dry particles containing solubilized felodipine, and then carrying out granulating, tabletting, and coating to obtain a felodipine sustained-release tablet. According to the invention, the felodipine is solubilized, which enables the curative effect to be enhanced. Besides, a traditional hard-soluble medicament sustained-release tablet has complicated preparation process with high energy consumption, and is not easy to produce and uncontrollable in quality; however, the above problems are solved by the preparation method provided in the invention. Therefore, the simple, low-energy, and high-efficient industrialized production is allowed according to the invention.
Owner:山东威高药业有限公司
Felodipine slow-release microspheres and preparation method thereof
ActiveCN102579362AImprove bioavailabilityMeets the requirements for felodipine extended-release tabletsOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseBlood concentration
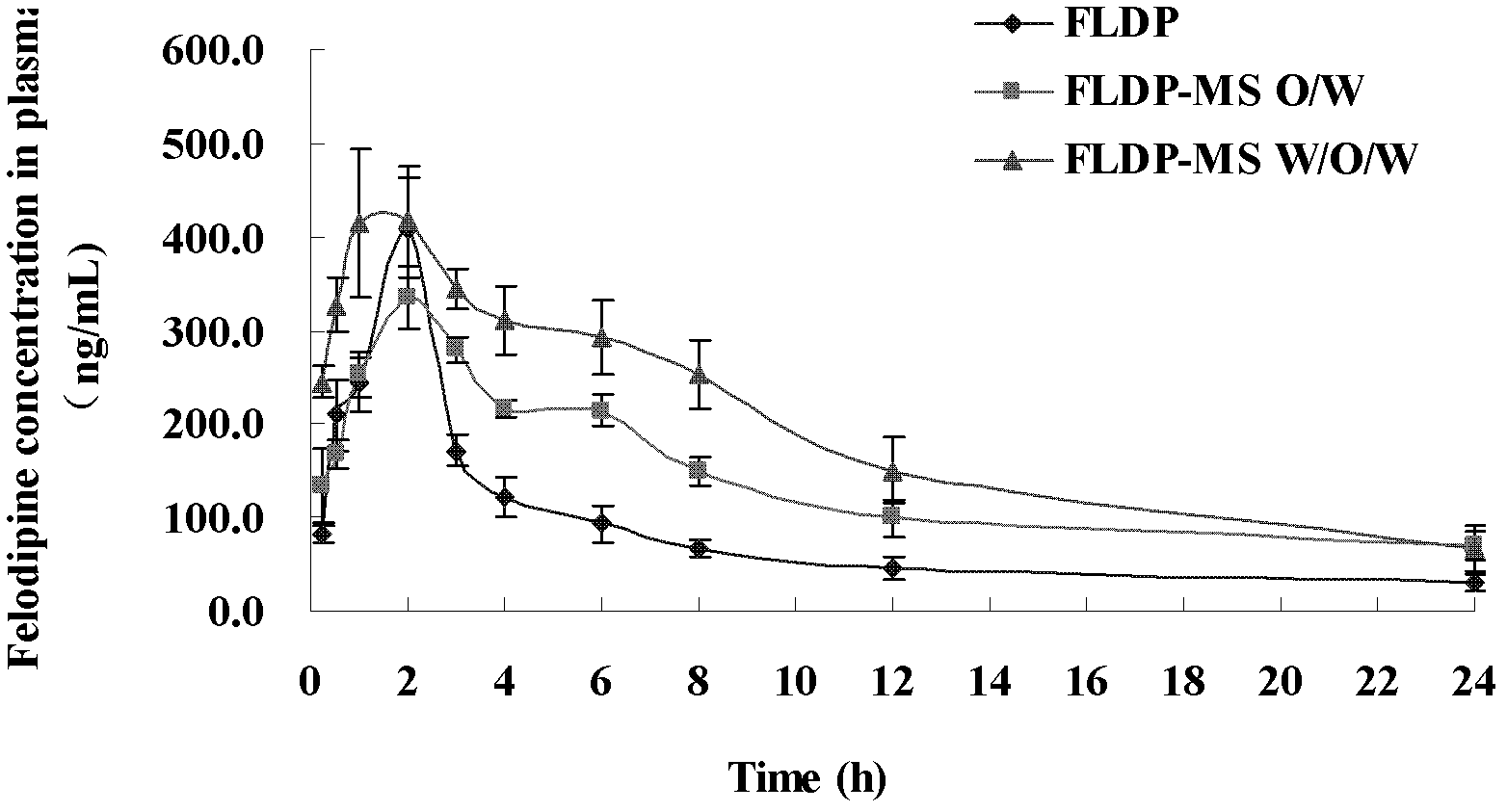
The invention discloses felodipine slow-release microspheres which consist of felodipine, ethylene cellulose and a drug release regulator and are prepared by adopting a W1 / O / W2 emulsification method, and the mass percentages of the felodipine, the ethylene cellulose and the drug release regulator are respectively 1-50, 36-99 and 0-44. The felodipine slow-release microspheres prepared in the invention adopt an in-vitro release condition of felodipine slow-release tablets in the United States Pharmacopeia (USP) and can slowly release drugs in 24 hours in vitro drug release, and compared with a felodipine dug suspension and felodipine slow-release microspheres prepared by an O / W method, the blood concentration and the oral bioavailability in vivo by adopting the felodipine slow-release microspheres prepared in the invention released are improved.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of antihypertensive drug felodipine
ActiveCN101613314AHigh yieldHigh purityOrganic active ingredientsOrganic chemistryAlcoholDihydropyridine
The invention discloses a preparation method of felodipine. The method is mainly characterized by adding strong acid cation exchange resin to alcoholic solution of methyl 2-(2,3-dichlorobenzylidine)acetoacetate and ethyl-3-aminocrotonate as a catalyst, which shortens reaction time by more than 1 / 4 and improve yield of cyclization reaction by 6-8%. A crude product felodipine prepared by the preparation method has increased refining yield and product purity. The method is also applicable to preparing other 4-sustituted-1,4-dihydropyridine antihypertensive drugs with chemical structure and pharmaceutical and clinical actions similar to felodipine.
Owner:HEFEI LIFEON PHARMA
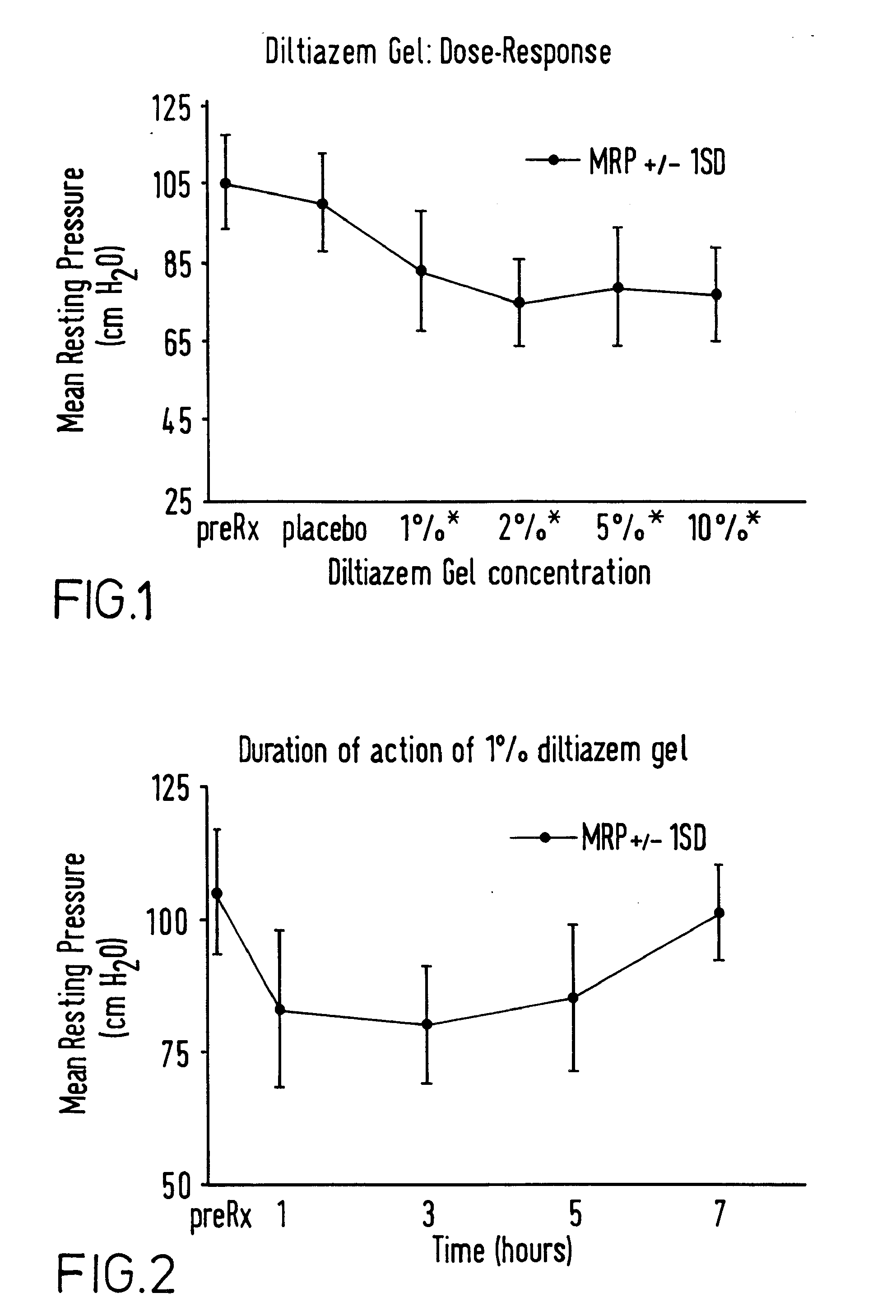
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
A method and composition are provided for the treatment of an anorectal disorder and for controlling the pain associated therewith. The method comprises administering to a subject in need of such treatment therapeutically effective amounts of a calcium channel blocker either alone or together with a nitric oxide donor. Amlodipine, anipamil, barnidipine, benidipine, bepridil, darodipine, diltiazem, efonidipine, felodipine, isradipine, lacidipine, lercanidipine, lidoflazine, manidipine, mepirodipine, nicardipine, nifedipine, niludipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, perhexiline, tiapamil, verapamil and pharmaceutically acceptable salts thereof, are suitable calcium channel blockers.
Owner:SLA PHARMA AG
Felodipine sustained-release tablet and method for controlling sustained-release of Felodipine sustained-release tablet
ActiveCN101574324BReduce reworkReduce manufacturing costOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a Felodipine sustained-release tablet and a method for controlling the sustained-release of the Felodipine sustained-release tablet. The Felodipine sustained-release tablet comprises Felodipine and HPMC high polymer material, the Felodipine accounts for 2.0-7.0% of the total sum of the granules or the powder, wherein, the viscosity range of the granules or the powder of theprepared Felodipine sustained-release tablet is respectively 2000-2400 centipoise and 6840-7240centipoise. The Felodipine sustained-release tablet has quality conforming to the standard and similar release property with the preparation of the same type sold in markets, and the quality control is carried out before production, thus reducing re-doing, saving production cost, reducing energy consumption and improving productivity.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Felodipine ramipril compound sustained-release preparation and preparation method thereof
InactiveCN102600451AUniform absorption rateSmall differences in individual bioavailabilityOrganic active ingredientsDipeptide ingredientsSustained release pelletsSide effect
The invention discloses a compound sustained-release preparation containing felodipine and ramipril and a preparation method of the compound sustained-release preparation, wherein the compound sustained-release preparation is prepared by mixing felodipine sustained-release pellets and ramipril immediate-release pellets according to a specific dose proportion. The felodipine sustained-release pellets can sustainably release for 12 hours, and can be taken once a day, so as to keep the antihypertensive effect for a long time, the ramipril immediate-release pellets can be quickly absorbed so as to achieve the antihypertensive effect and treat the plasma concentration, due to the combination of the two kinds of pellets, the antihypertensive effect can be greatly enhanced, the toxic and side effect of the medicine can be reduced, the administration frequency can be reduced, sustainably stable antihypertensive effect can be achieved in a human body, the patient compliance can be enhanced, the compound sustained-release preparation consists of hundreds and thousands of pellets in uniform particle sizes, so that the damage of individual pellets will not cause burst release of the whole preparation, and compared with the sustained release tablets, the compound sustained-release preparation disclosed by the invention has the advantages of higher safety, lower irritation to gastrointestinal tract, more stable plasma concentration and capability of effectively improving the clinical effect.
Owner:广州科的信医药技术有限公司
Medicine composite for treating and relieving high blood pressure
The invention discloses a medicine composite for treating and relieving high blood pressure and a preparation method thereof. The composite comprises 10-320mg of telmisartan and 1-20mg of felodipine slow-release preparation. The composite is used for treating patients with moderate and acute high blood pressure and patients whose blood pressure can not be adequately controlled after the high blood pressure is treated by an angiotensin II receptor antagonist or a calcium antagonist.
Owner:广西方略集团崇左制药有限公司
Compound blood pressure reducing prepn containing angiotonin converzyme inhibitor, calcium ion agonist and Estazolam
InactiveCN1526398AGood curative effectLittle side effectsOrganic active ingredientsPill deliveryCaptoprilSide effect
The present invention provides one new kind of compound blood pressure reducing preparation containing angiotonin converzyme inhibitor, calcium ion agonist, Estazolam and pharmaceutically acceptable carrier. The angiotonin converzyme inhibitor is selected from Enalapril, Ramipril, Benalapril, Lisinopril, Acertil, etc. as well as their mixture; and the calcium ion agonist is selected from Nitrendpine, Amlodipine Besylate, Nifedipine, Felodipine, etc. as well as their mixture. The present invention utilizes the synergistic effect between different medicines to raise the blood pressure lowering effect, reduce side effect and improve the compliance of patient.
Owner:杜晓锋
Felodipine solid dispersion (SD) and preparation method thereof
InactiveCN103006568ASimple preparation processEasy to preparePowder deliveryOrganic active ingredientsOrganic solventHot melt
The invention discloses a felodipine drug solid dispersion (SD) and a preparation method thereof. The felodipine SD is prepared by taking the indissolvable drug felodipine as the active ingredient, mixing the active drug with a polymer carrier uniformly in a certain proportion and then carrying out hot-melt extrusion. The SD and the preparation method have the beneficial effects that the dissolution rate of felodipine can be effectively increased; and meanwhile, the preparation process is simple and the problems of solvent residues and the like caused by addition of organic solvents are avoided.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Metoprolol Tartaric Acid and Felodipine slow-release double-layer tablet and preparation method thereof
ActiveCN102188423BPress evenlyNo risk of breakageOrganic active ingredientsPill deliveryAdditive ingredientCurative effect
The invention provides a Metoprolol Tartaric Acid and Felodipine slow-release double-layer tablet and its preparation method. The slow-release double-layer tablet comprises a Metoprolol Tartaric Acid slow-release layer containing, by weight, 50 parts of Metoprolol Tartaric Acid, 100-150 parts of an ethyl cellulose powder, 24-30 parts of ethyl cellulose in the form of an ethyl cellulose aqueous suspension and 130-230 parts of hydroxypropyl methyl cellulose; and a Felodipine slow-release layer containing 5 parts of Felodipine and 60 parts of hydroxypropyl methyl cellulose. By adjusting the ingredients and their ratio in the Metoprolol Tartaric Acid slow-release layer and the Felodipine slow-release layer, the release speeds of medicament layers can cooperate with each other and the slow-release effect is more reliable, thus reaching a good curative effect; in addition, it is ensured that the medicament layers can be smoothly compacted to form a double-layer tablet, and the hidden trouble of damaged coating particles can be avoided. The preparation method provided by the invention mainly employs the double-layer tablet compacting technology with simple equipment, can save cost and is suitable for industrial production.
Owner:BEIJING RED SUN PHARMA
Single layer osmotic pump controlled release preparation containing metoprolol and felodipine
ActiveCN102784143ASimple manufacturing processOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical formulationFilm coating
The present invention belongs to the field of pharmaceutical preparations, and relates to a single layer osmotic pump controlled release preparation containing metoprolol and felodipine. Specifically the controlled release preparation comprises a tablet core and a semipermeable film coating, wherein the tablet core comprises the following components, by weight, 50-100 parts of metoprolol or a pharmaceutically acceptable salt thereof, 5-10 parts of felodipine, 3-10 parts of hydroxy propyl methyl cellulose, 5-25 parts of povidone, and 5-25 parts of sodium carboxymethyl cellulose. The present invention further relates to a preparation method and a use of the preparation. With the controlled release preparation of the present invention, synchronous and constant speed release of metoprolol and felodipine can be achieved. Compared with preparation of double layer osmotic pump tablets, the preparation method of the present invention has a characteristic of substantial simplification of the preparation process, wherein the method comprises that metoprolol is encapsulated in a pill core, and then conjointly preparing into tablets with felodipine.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
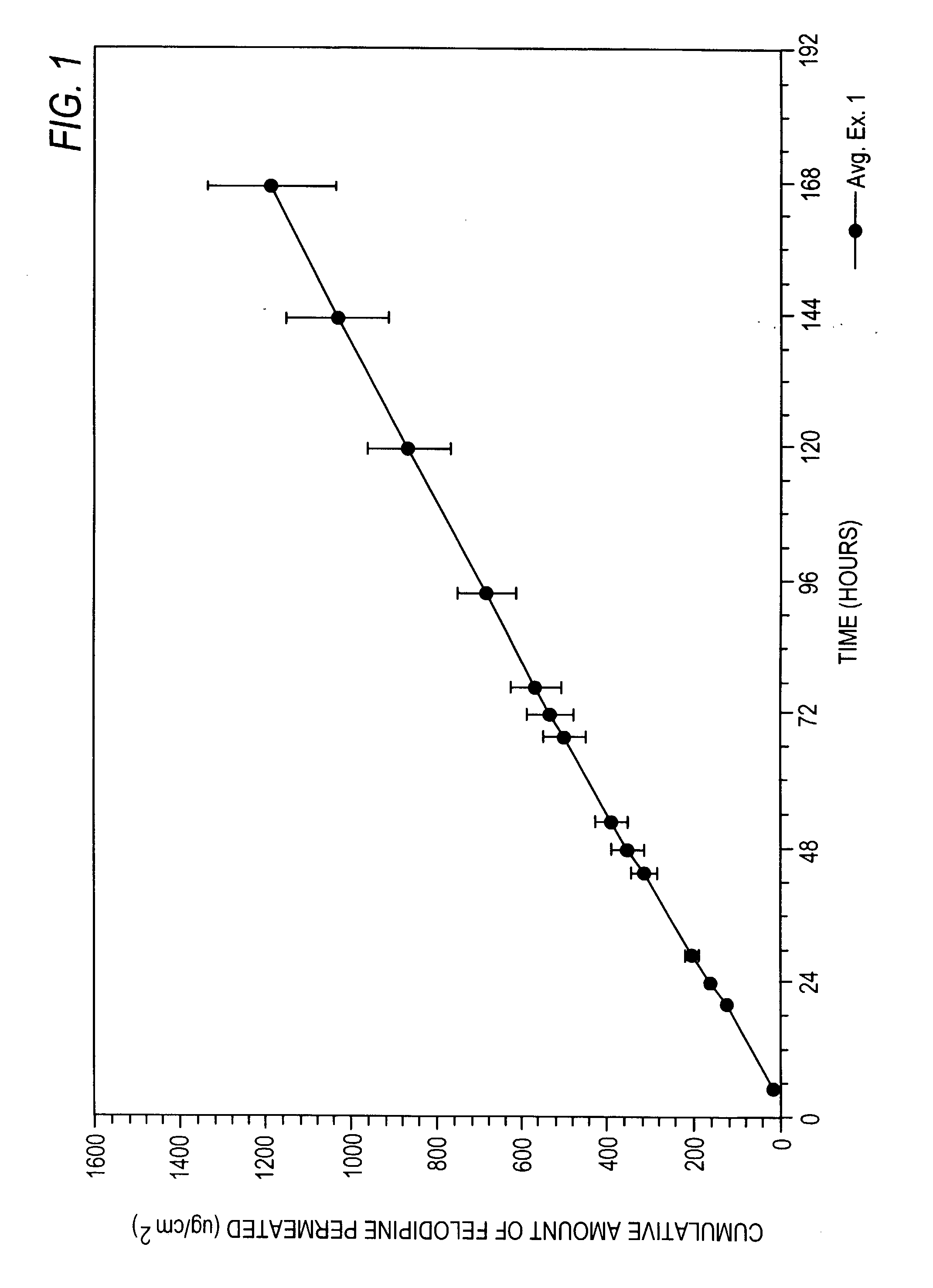
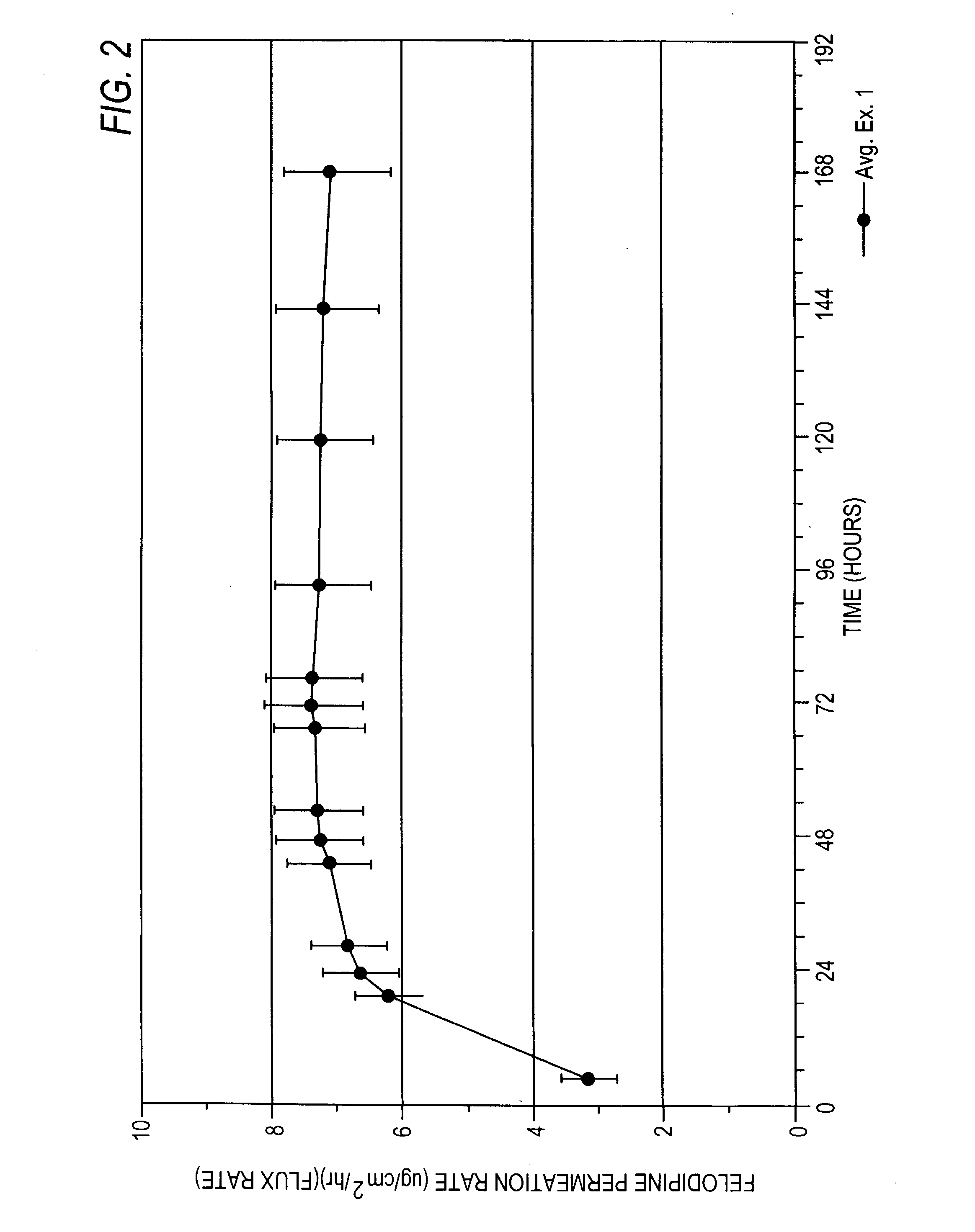
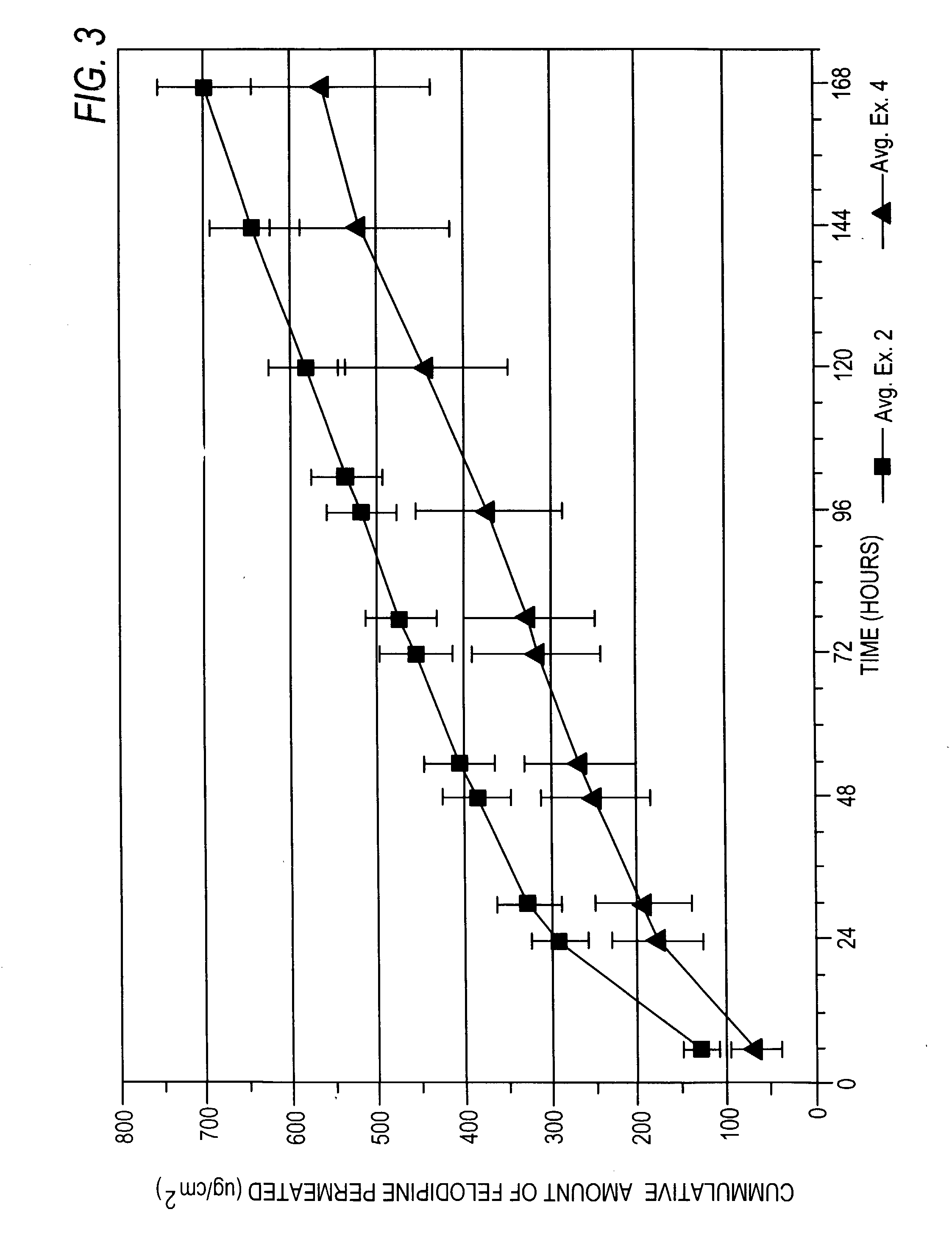
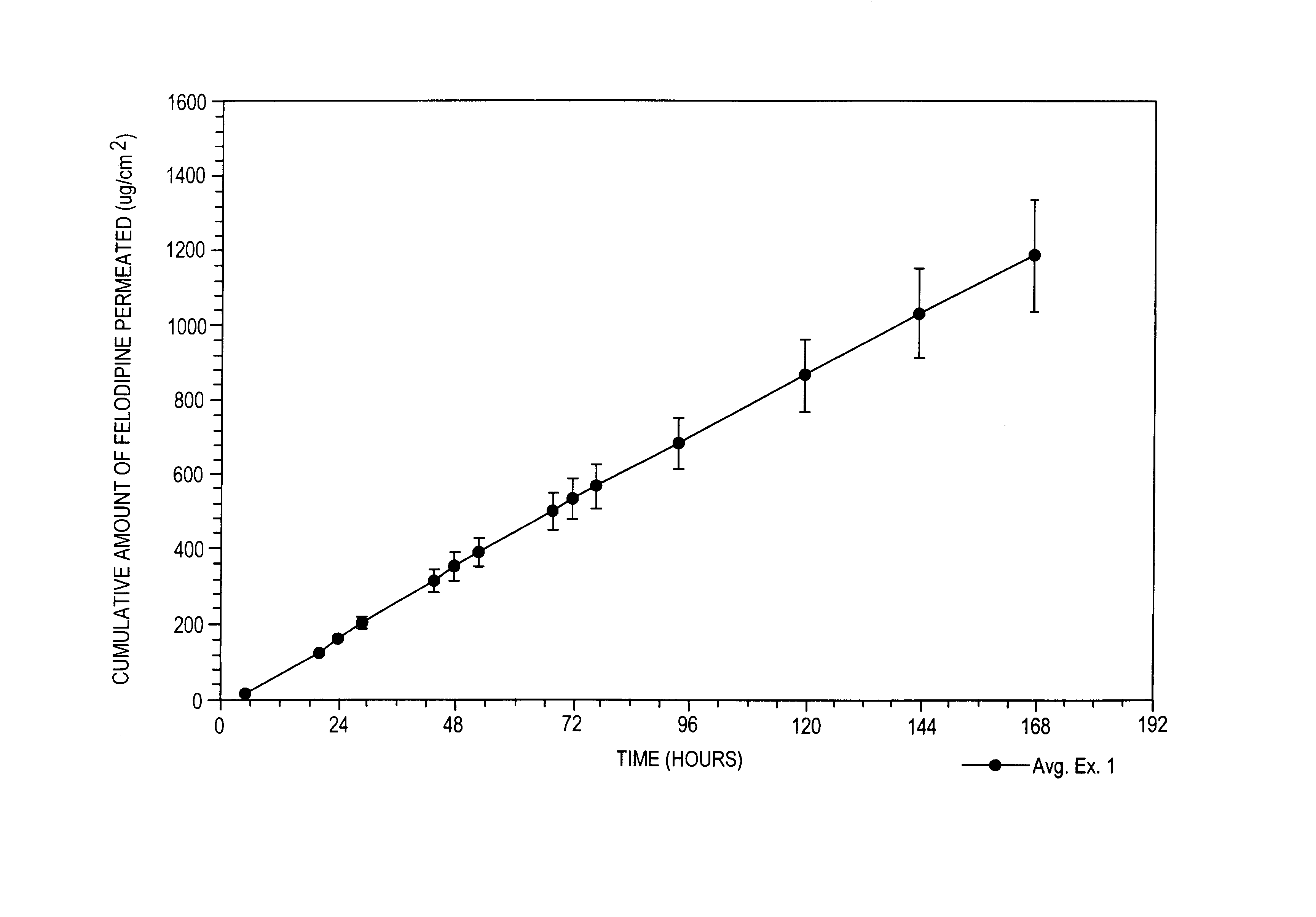
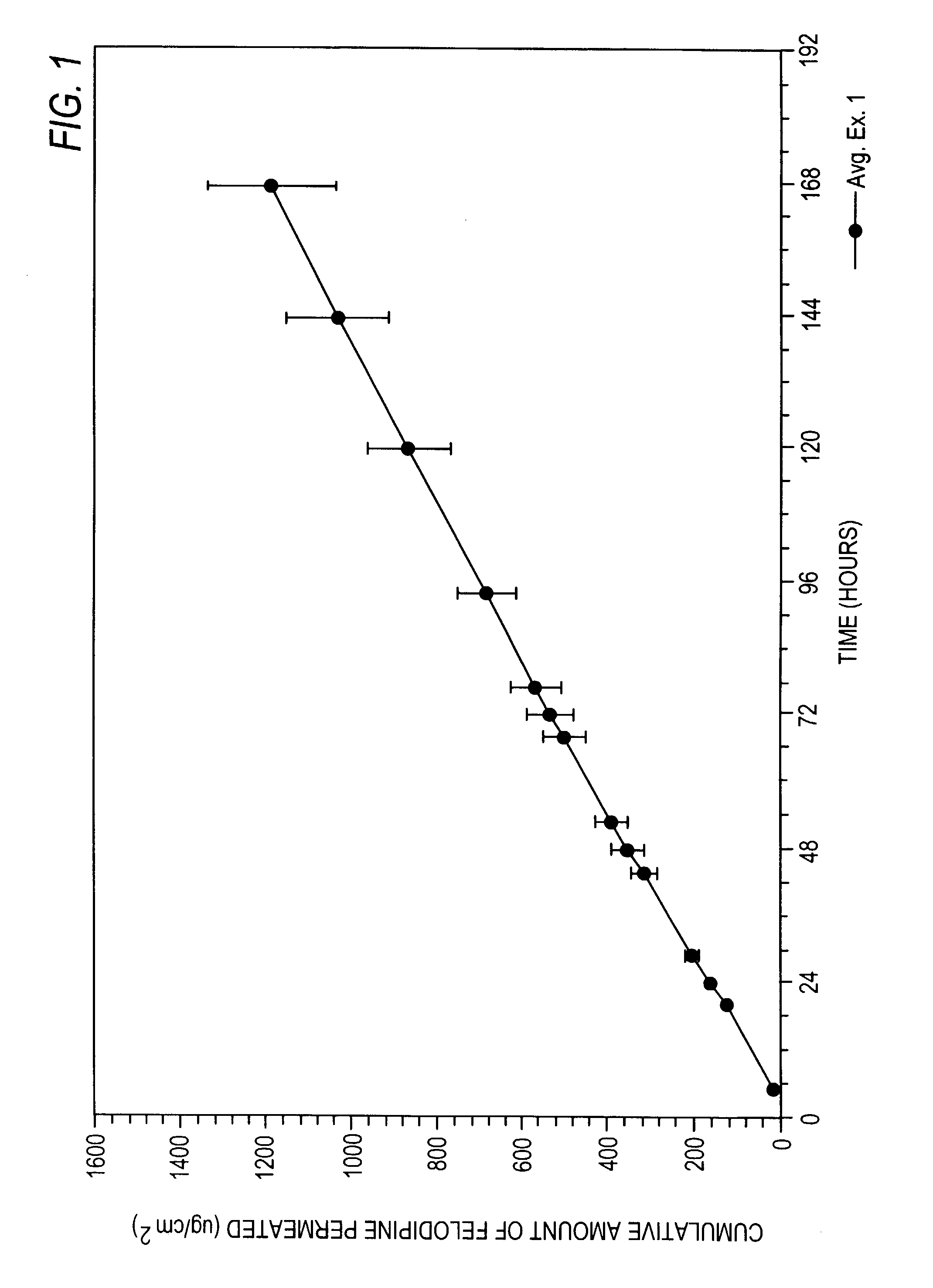
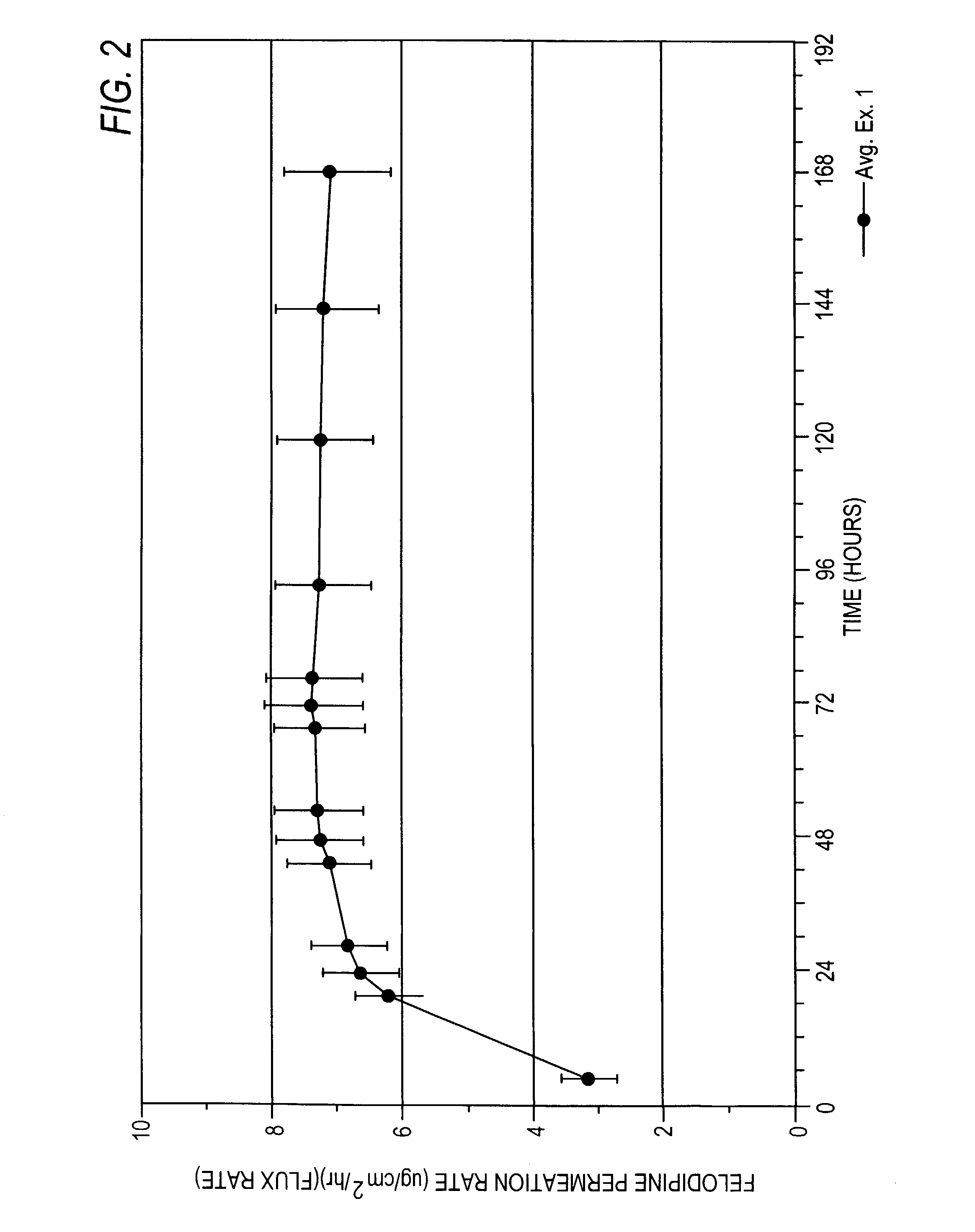
Felodipine transdermal device and methods
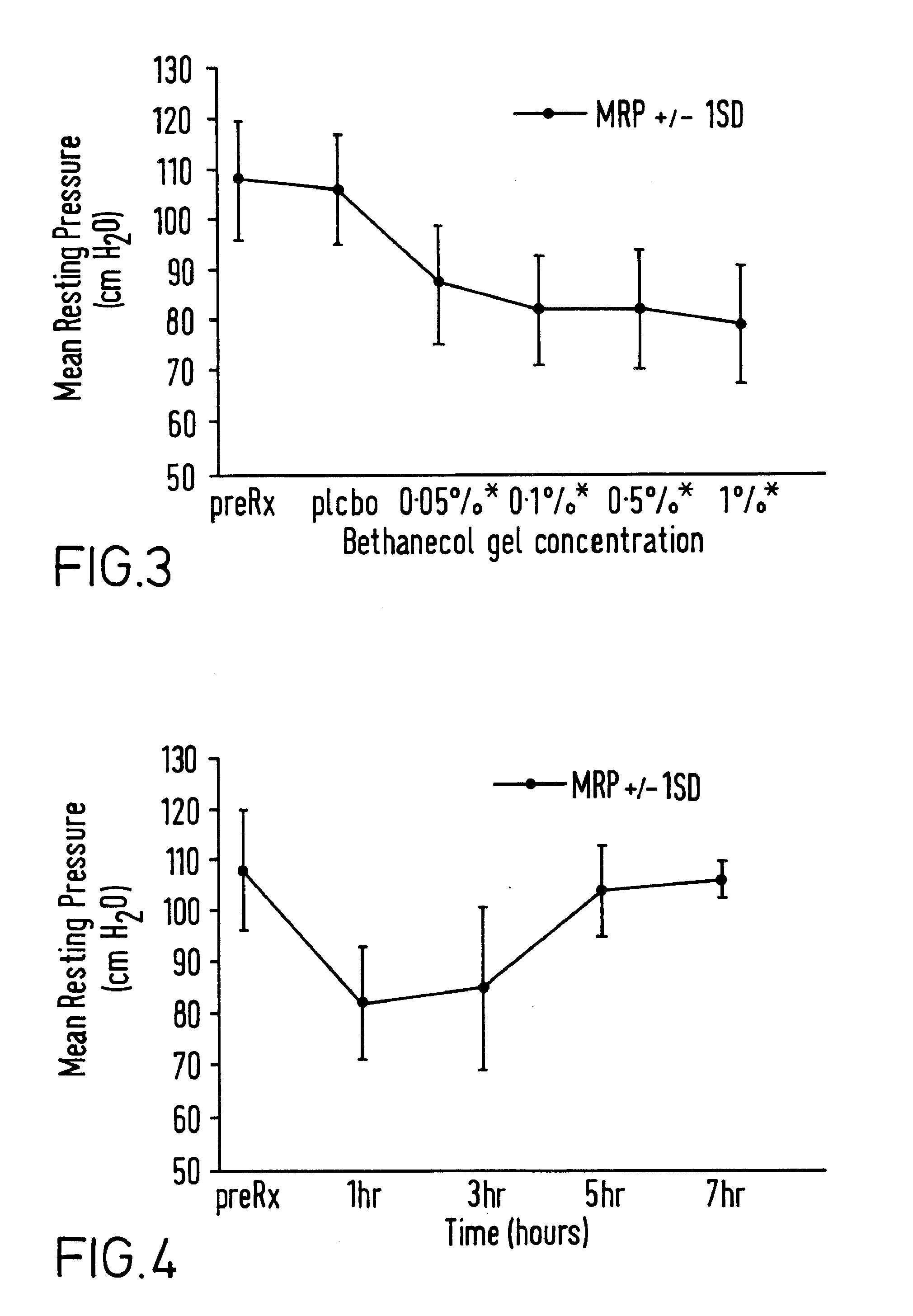
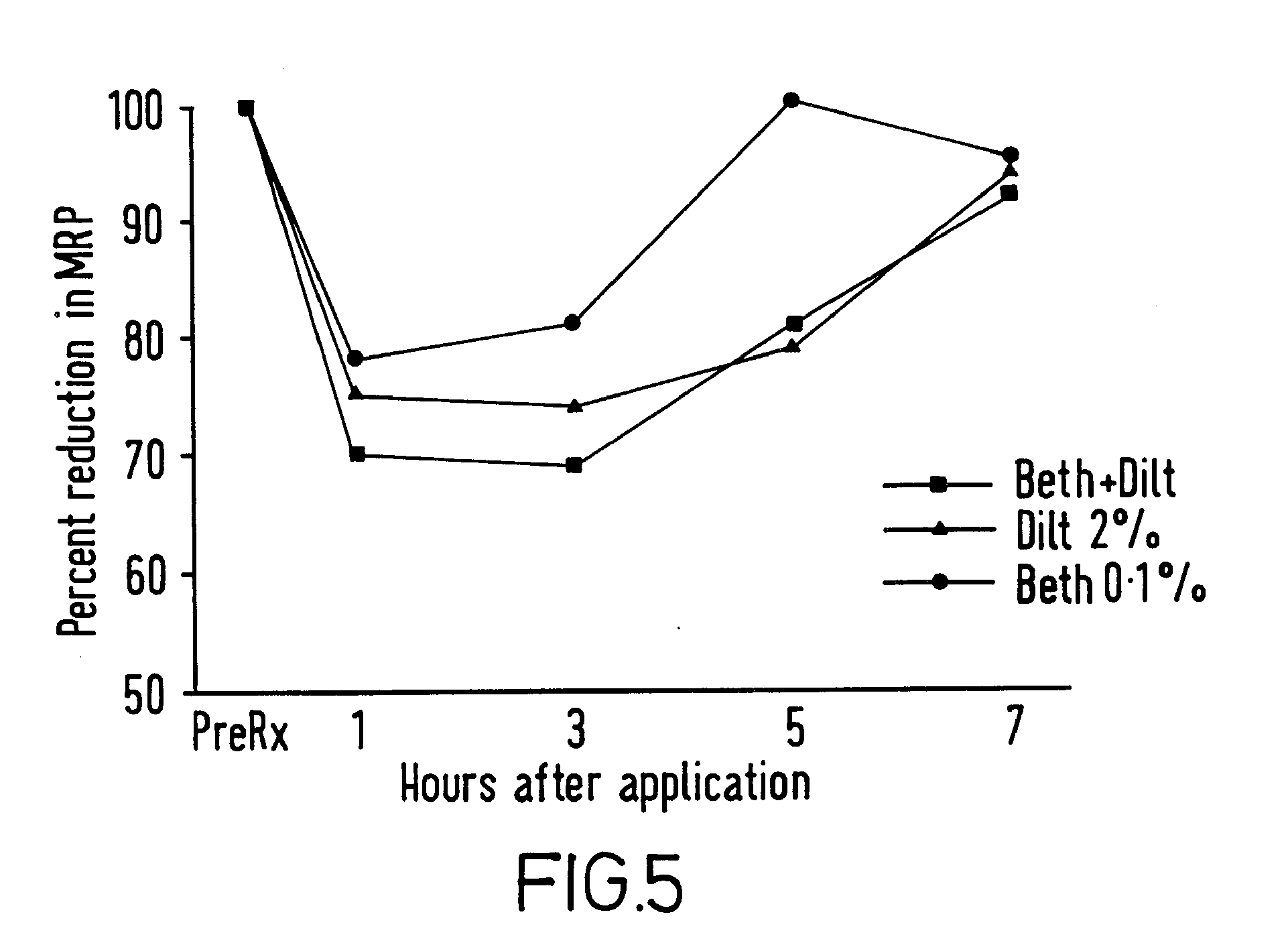
InactiveUS20030072791A1Reduce morbidityEliminate side effectsOrganic active ingredientsAdhesive dressingsPharmacologyFelodipine
A method of effectively treating hypertension in humans is achieved by administering felodipine via a transdermal formulation. Preferably, the transdermal formulation is applied to the skin of the patient and maintained in contact with the skin for at least about 24 hours days, and preferably for about 3 to about 8 days.
Owner:PURDUE PHARMA LP
Felodipine transdermal device and methods
InactiveUS7018649B2Prolonged and effective managementEliminate side effectsOrganic active ingredientsAdhesive dressingsSkin exposurePharmacology
A method of effectively treating hypertension in humans is achieved by administering felodipine via a transdermal formulation. Preferably, the transdermal formulation is applied to the skin of the patient and maintained in contact with the skin for at least about 24 hours days, and preferably for about 3 to about 8 days.
Owner:PURDUE PHARMA LP
Felodipine sustained-release tablet and method for controlling sustained-release of Felodipine sustained-release tablet
ActiveCN101574324AReduce reworkReduce manufacturing costOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a Felodipine sustained-release tablet and a method for controlling the sustained-release of the Felodipine sustained-release tablet. The Felodipine sustained-release tablet comprises Felodipine and HPMC high polymer material, the Felodipine accounts for 2.0-7.0% of the total sum of the granules or the powder, wherein, the viscosity range of the granules or the powder of the prepared Felodipine sustained-release tablet is respectively 2000-2400 centipoise and 6840-7240centipoise. The Felodipine sustained-release tablet has quality conforming to the standard and similar release property with the preparation of the same type sold in markets, and the quality control is carried out before production, thus reducing re-doing, saving production cost, reducing energy consumption and improving productivity.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com