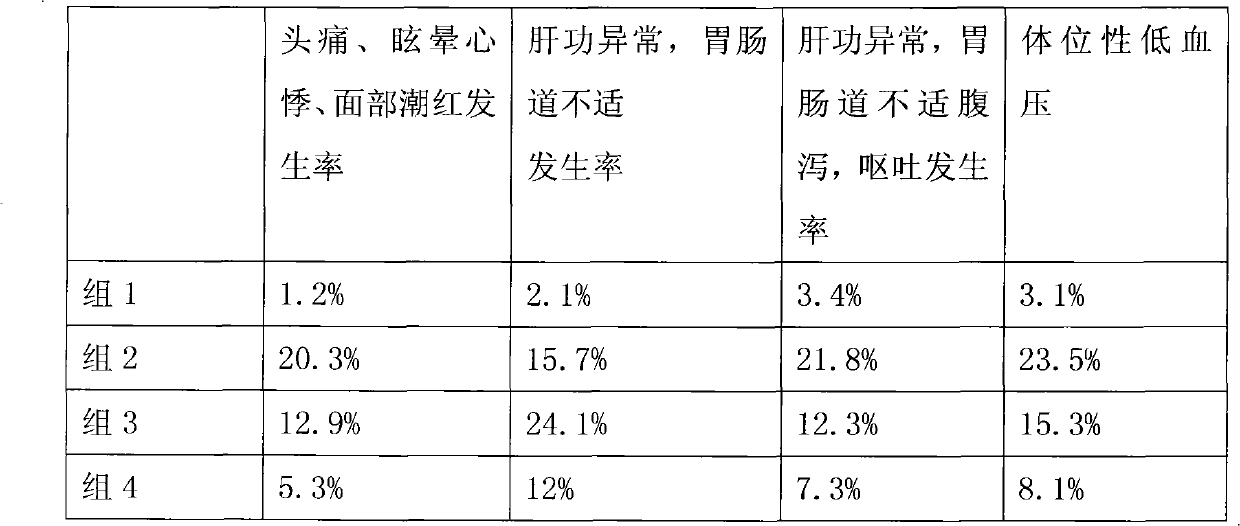
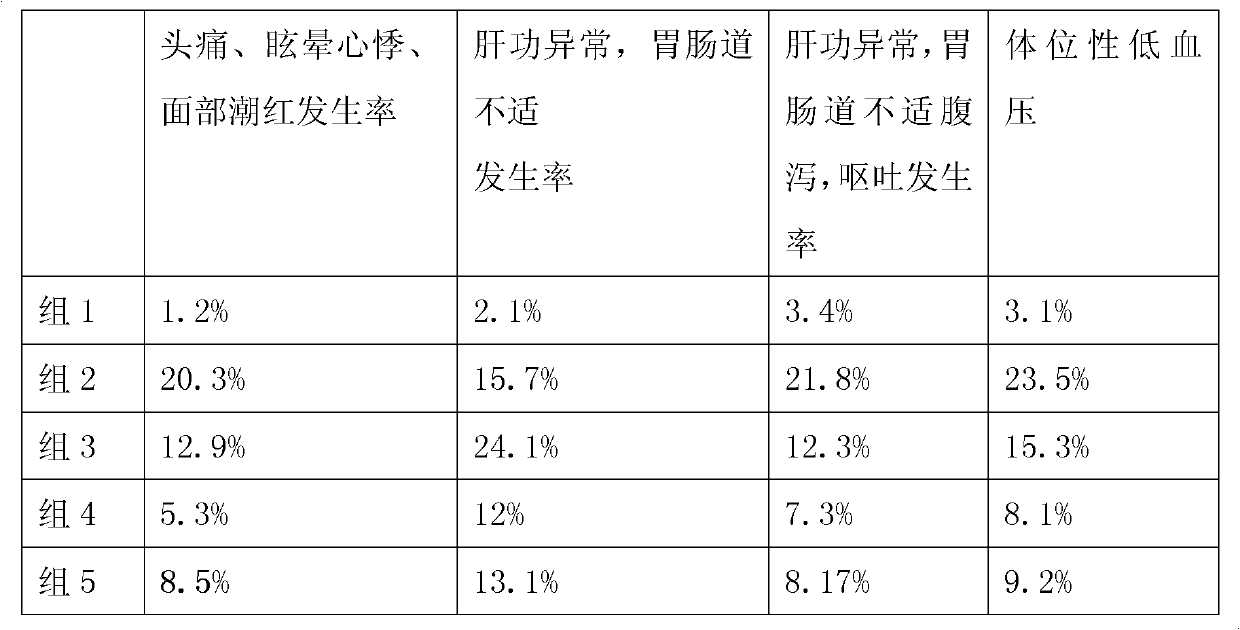
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Isradipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isradipine is used with or without other medications to treat high blood pressure (hypertension).
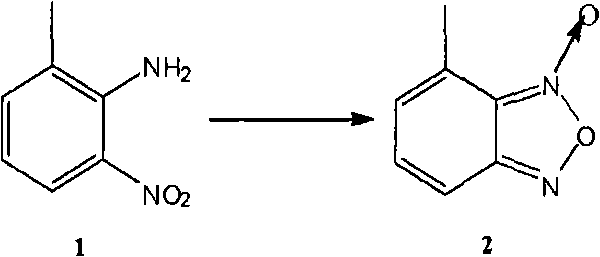
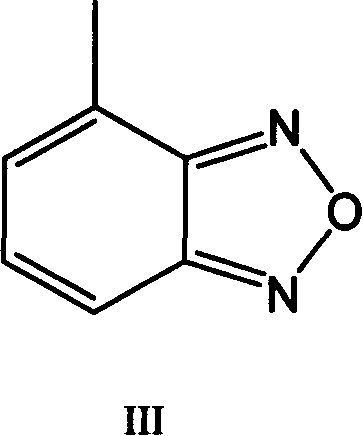
Method for preparing israbipine medicament for treating hypertension
InactiveCN101768153AProcess stabilityImprove product qualityOrganic active ingredientsOrganic chemistryMedicineNitrobenzene
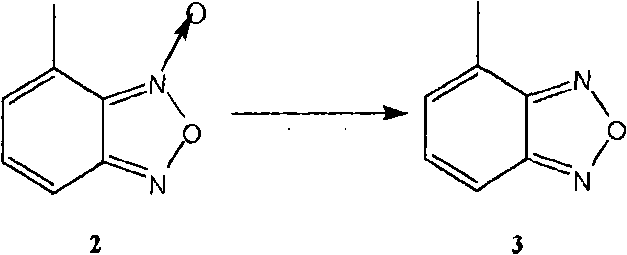
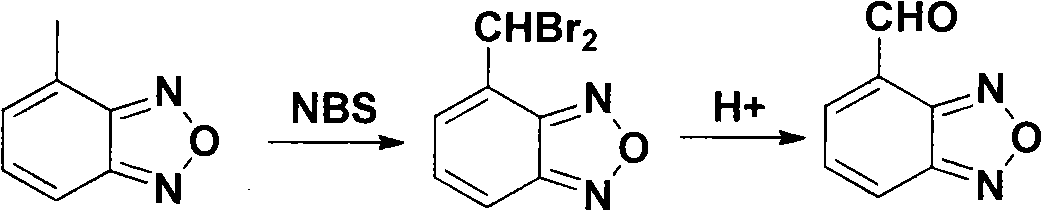
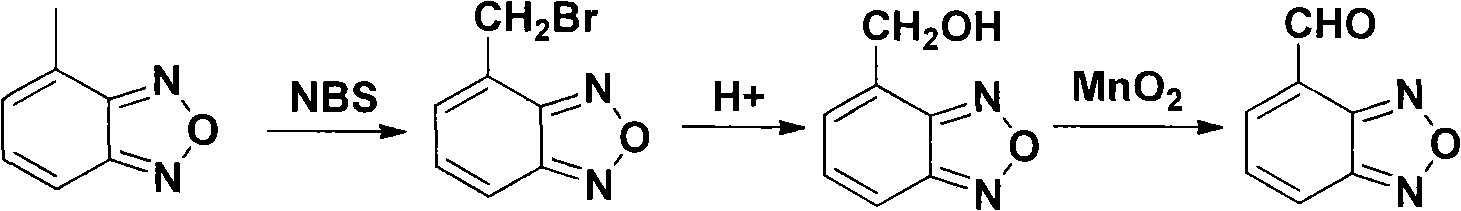
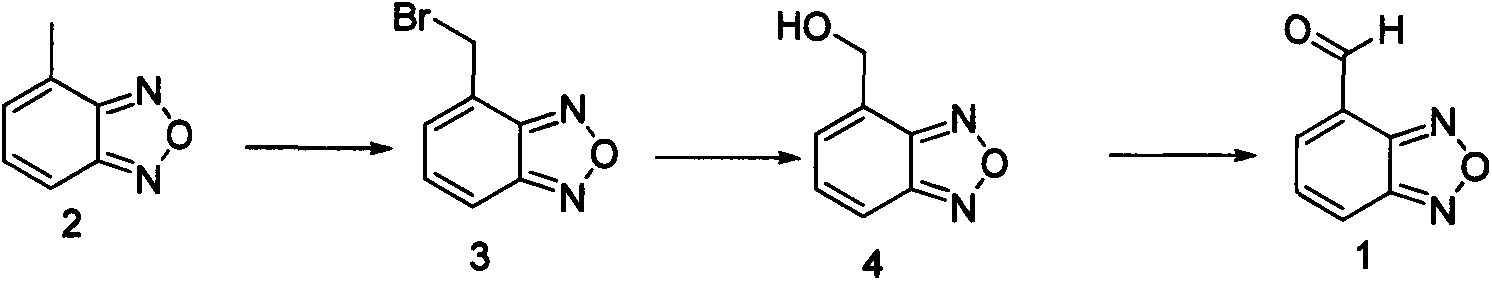
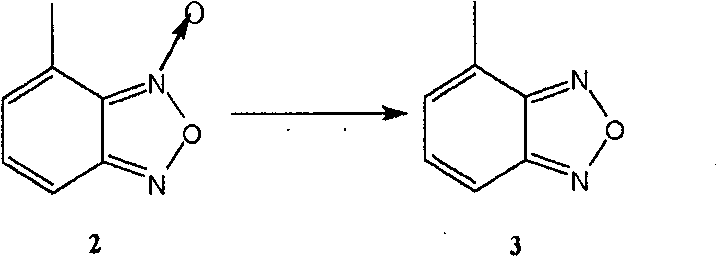
The invention relates to a method for preparing an israbipine medicament for treating hypertension. Particularly the method of the invention comprises the following steps: (a) carrying out oxidative cyclization on 2-amino-3-methyl nitrobenzene to form 4-methyl benzofuroxan oxide; (b) reducing the 4-methyl benzofuroxan oxide to form 4-methyl benzofuroxan; (c) carrying out substitution bromination on the 4-methyl benzofuroxan to form 4-bromomethyl benzofuroxan; (d) carrying out hydrolyzation on the 4-bromomethyl benzofuroxan to form 4-hydroxymethyl benzofuroxan; (e) carrying out oxidation on the 4-hydroxymethyl benzofuroxan to form 4-formaldehyde benzofuroxan; and (f) performing a reaction of the 4-formaldehyde benzofuroxan amd alpha-aminocrotonic acid isopropyl ester to form israbipine. The method of the invention has high yield and simple and convenient operation and can prepare high-purity israbipine.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD +1
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
Method for preparing 4-formoxylbenzofuran
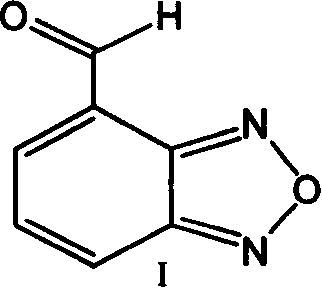
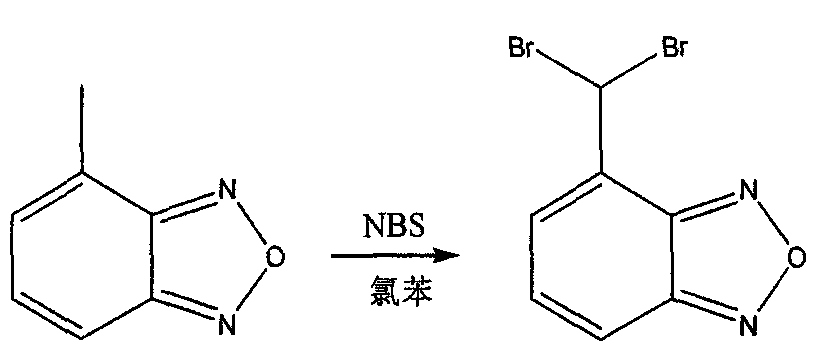
Improved, low-cost, scalable process for the preparation of 4-formylbenzofuran starting from 4-methylbenzofuran, wherein the novel 4-dibromobenzofuran is obtained as an intermediate . According to the method of the invention, 4-methylbenzofuran is brominated to obtain novel 4-dibromobenzofuran, which is then hydrolyzed to obtain 4-formylbenzofuran. 4-Formylbenzofura is a key intermediate in the production of isradipine for the treatment of hypertension and angina.
Owner:圣玛精细化工有限责任公司
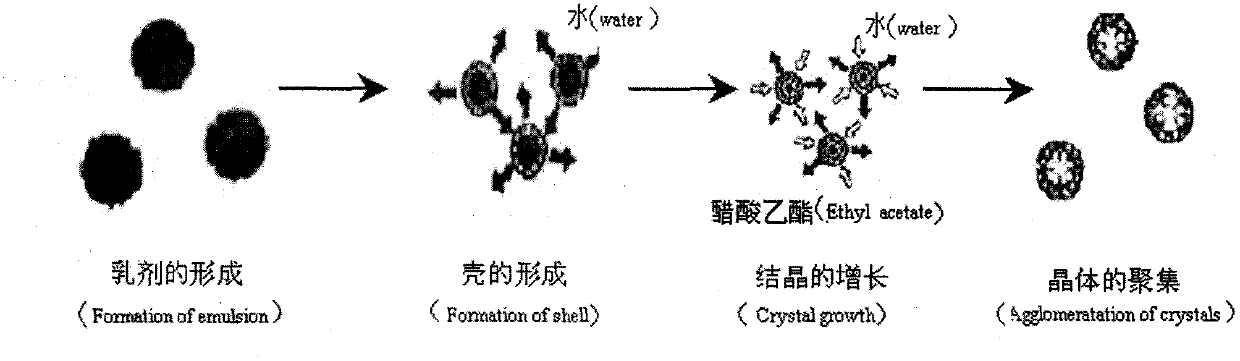
Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
InactiveCN103315960AReduce liver and kidney toxicityAvoid influencePowder deliveryEmulsion deliveryNeogambogic acidCaprylic acid
The invention relates to the medical technology field, and particularly relates to a solid self-microemulsion based on a spherical crystallization technique and a preparation method thereof. The solid self-microemulsion is characterized in that: with the use of the spherical crystallization technique, the solid self-microemulsifying micoparticles are prepared from poorly water soluble drugs in a liquid phase by one step. The solid self-microemulsion with the poorly water soluble drugs comprises the components, by weight: 0.1 to 1.5 g of the poorly water soluble drugs, 4.0 g of a polyoxyethylene hydrogenated castor oil, 2.0 g of capric caprylic triglyceride, 2.0 g of tpropylene glycol, 1.0 ml of ethanol, 4.0 ml of dichloromethane, 0.5 to 1.1 g of ethylcellulose (or Eudragit RS100, RL100), 0.05 g of PEG4000, and 0.5 g of colloidal silicon dioxide. The poorly water soluble drugs include cyclosporine A, fenofibrate, glimepiride, cilnidipine, isradipine, simvastatin, baicalein, neogambogic acid, puerarin, cyclovirobuxine D, silymarin and the like.
Owner:胡容峰
Sustained release delivery of isradipine
Sustained release oral formulations of israpidine, methods of preparing same, and methods of using sustained release oral formulations to provide controlled delivery of israpidine. The sustained release oral formulations include israpidine and a sustained release polymer, and provide substantially zero order release of israpidine over an extended period.
Owner:RELIANT PHARMACEUTICALS INC
Synthesis method for antihypertensive agent isradipine and preparation of isradipine
ActiveCN102846609AHigh yieldEasy to operateOrganic active ingredientsOrganic chemistryAcetoacetatesAcetic acid
The invention relates to a method for synthesizing an antihypertensive agent containing furazan ring, comprising using 4-methylbenzofurazan as raw material, carrying out bromination and Sommelet reaction to prepare important intermediate 4-benzofurazancarboxaldehyde, carrying out condensation, and carrying out Hantzsch reaction to prepare target compound. The target compound can be directly or indirectly added with pharmaceutically-acceptable excipient in conventional process to prepare into clinically-acceptable dosage forms, such as tablet and capsule, to take better curative action.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
A method for preparing isradipine key intermediate 4-formylbenzofura
InactiveCN102276547ASimple processSuitable for industrializationOrganic chemistryAcyl groupHydrolysis
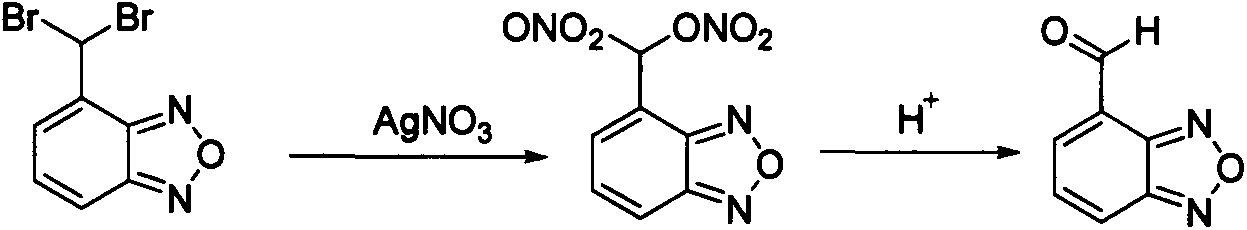
The invention discloses a novel preparation method of an isradipine key intermediate 4-formoxylbenzofuran. According to the invention, 4-(bisbromomethyl) benzofuran reacts in a mixed system of silver nitrate aqueous solution and organic alcoholic solution to generate a nitric acid ester intermediate; the intermediate product, without being separated, can directly and mildly hydrolyze under an acidic condition to obtain the 4-formoxylbenzofuran.
Owner:SHANDONG INST OF PHARMA IND
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
Owner:SLA PHARMA AG
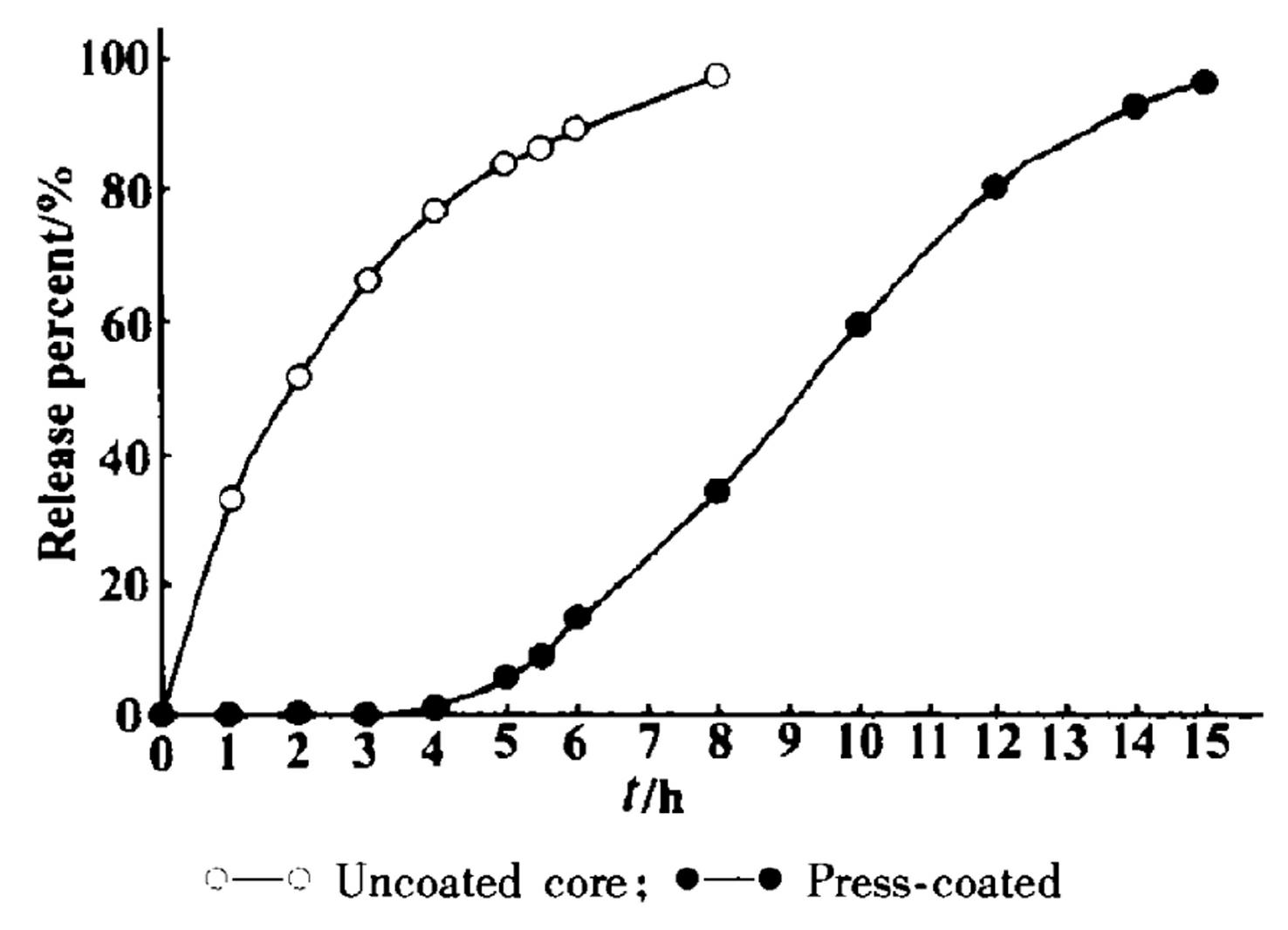
Isradipine controlled-release tablet and preparation method and application thereof
ActiveCN102688215ASolve the problem of chrono-dependent dosingOrganic active ingredientsMetabolism disorderRelease timeControlled Release Tablet
The invention discloses an isradipine controlled-release tablet. The controlled-release tablet consists of an isradipine-containing controlled-release tablet core and a controlled-release coat. The invention provides an isradipine controlled-release tablet capable of delaying the release time, and solves the problem of obvious time dependence of the drug administration for cardiovascular diseases; and meanwhile, phosphatidylcholine is added to the tablet, thus the controlled-release tablet has a certain treatment effect on diabetic nephropathy.
Owner:CHONGQING CONQUER PHARML
Specific steady-state R-type CA2+ channel blockers and use thereof
InactiveUS7109175B1Decrease proliferationRelieve side effectBiocideCarbohydrate active ingredientsMandevilla illustrisChannel blocker
The present invention relates to Ca2+ channel blockers and more particularly to the R-type Ca2+ channel blockers. More specifically, the invention relates to Ca2+ channel blockers activity of Mandevilla velutina and Mandevilla illustris. The present invention further concerns saponin-like compounds isolated from Mandevilla species. The present invention also relates to the treatment of several pathologies that involve the nifedipine-insensitive but isradipine sensitive steady-state R-type Ca2+ channel and the use of steady-state R-type Ca2+ channel blockers in the treatment of these pathologies.
Owner:UNIV DE SHERBROOKE
Ointment for treating high blood pressure and preparation method thereof
InactiveCN101947222AFast absorptionImprove bioavailabilityOrganic active ingredientsAerosol deliverySide effectMedicine
The invention relates to a western medicament ointment for treating high blood pressure and preparation method thereof, wherein the ointment contains 10 to 60 weight parts of reactive compounds including isradipine. The preparation method comprises the following steps of: 1) mixing base materials in percentage by weight, heating the mixture at 60 to 90 DEG C and stirring to dissolve the mixture; 2) grinding and sieving the reactive compounds, then adding the reactive compounds into the mixture obtained by the step 1) and stirring for 15 to 25 minutes, and lowering the temperature to 20 to 60 DEG C to obtain finished products. The ointment can be absorbed by human body through skin, and the synergy of active compounds can greatly improve the bioavailability of the ointment; moreover, the ointment has a great degree of security and a few side effects and avoids the symptoms including headache, vertigo, cardiopalmus and face flush basically.
Owner:北京水圣木科技有限责任公司
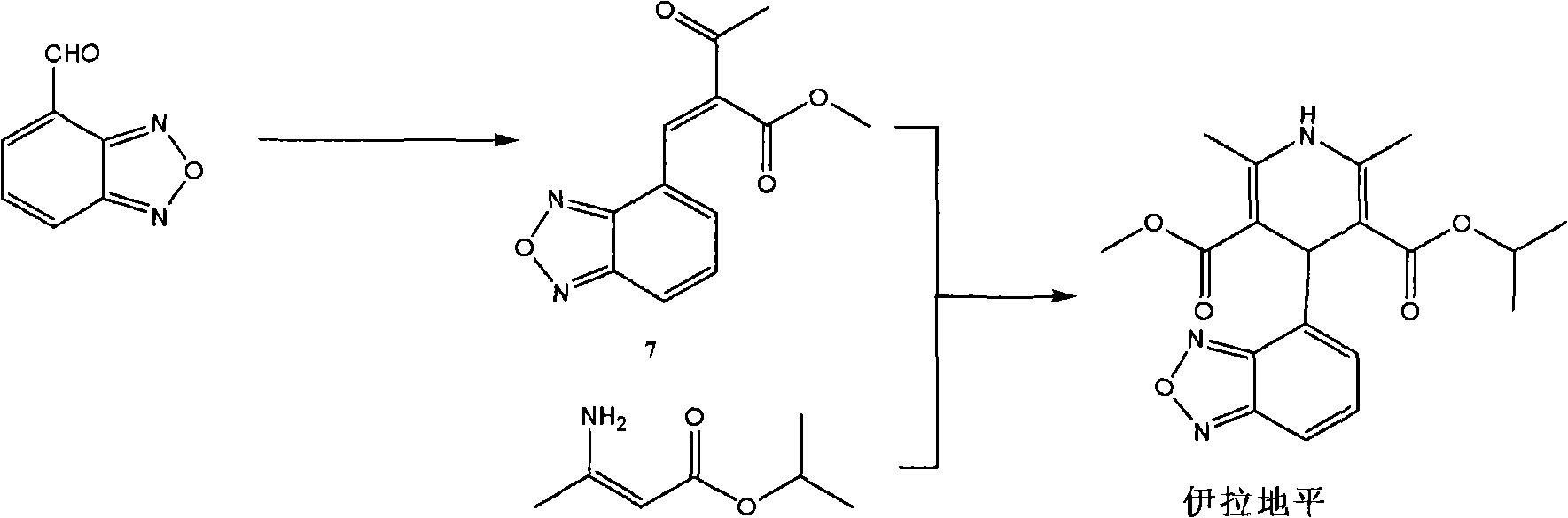
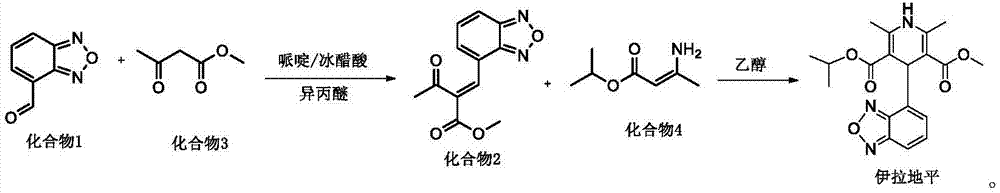
Process for the manufacture of Isradipine
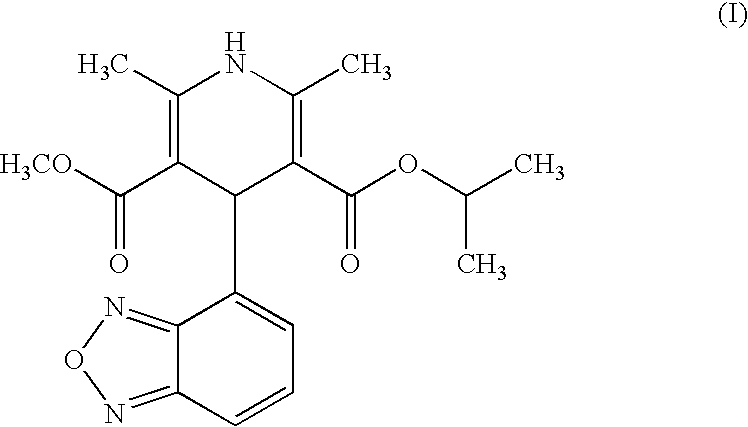
The present invention relates to an improved method for the manufacture of Isradipine, 4-(4-Benzofurazanyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid methyl 1-methylethyl ester. Which, involves reacting 2,1,3-benzoxadiazole-4-carboxaldehyde with methyl acetoacetate in the presence of acetic acid and piperidine in diisopropyl ether. To obtain the product 2-acetyl-3-benzofurazan-4-yl-acrylic acid methyl ester which is then reacted with isopropyl-β-aminocrotonate in ethanol at 25 to 35° C. to obtain the product.
Owner:SHASUN CHEM & DRUGS LTD
Method for preparing high-purity isradipine
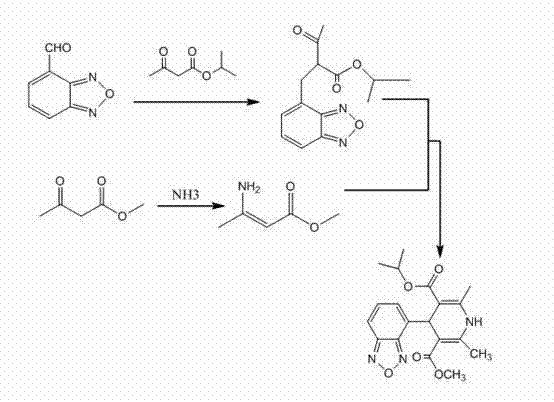
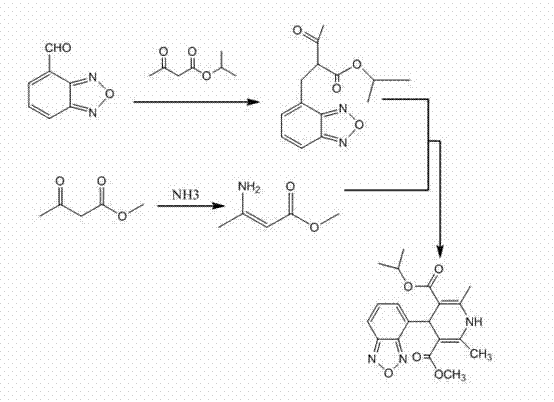
The invention discloses a method for preparing high-purity isradipine. The method includes dehydrating and condensating 4-formaldehyde benzofuroxan in atent solvent to form 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate; subjecting methyl acetoacetate to ammonolysis to form 3-amino crotonic acid methyl ester; subjecting 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate and 3-amino crotonic acid methyl ester to reaction to form isradipine. The method for preparing high-purity isradipine is simple in operation process, convenient to operate, high in yield and capable of preparing high-purity isradipine.
Owner:SICHUAN BAILI PHARM CO LTD
Granule for treating hypertension
InactiveCN102580090AProtectiveImprove bioavailabilityOrganic active ingredientsGranular deliveryMedicinal herbsSide effect
The invention relates to a granule for treating hypertension. The granule is characterized by comprising a calcium channel blocker, a beta receptor inhibitor, Chinese medicinal herbs and a medicinal carrier, and is prepared from the following components in percentage by weight: 0.1-60 percent of isradipine, 3-15 percent of labetalol, 10-20 percent of yellowmouth dutchmanspipe root and 7-70 percent of the medicinal carrier. The granule has the advantages of high safety, high synergistic effect among raw material components, high bioavailability, and capability of effectively reducing the side effects of separate azelnidipine on the health of a patient.
Owner:北京水圣木科技有限责任公司
Isradipine rapidly disintegrating oral tablet and preparation method thereof
InactiveCN105168159AEasy to carryImprove medication complianceOrganic active ingredientsPill deliveryMedicineSURFACTANT BLEND
The invention discloses an isradipine rapidly disintegrating oral tablet and a preparation method thereof, belonging to the field of preparation of an isradipine rapidly disintegrating oral tablet. The invention discloses firstly an isradipine rapidly disintegrating oral tablet; the isradipine rapidly disintegrating oral tablet consists of the following components: isradipine, a framework material, a suspending aid, a surfactant, a flavoring agent and a pH regulating agent, wherein the parts of the various components by weight are as follows: 2.5-10 parts of isradipine, 60-95 parts of framework material, 1-10 parts of suspending aid, 0.1-5 parts of surfactant, 0.1-5 parts of flavoring agent and 0.1-2 parts of pH regulating agent. The isradipine rapidly disintegrating oral tablet disclosed by the invention is good in appearance, low in friability, good in taste, short in dissolving time limit, rapid to take effect, convenient to take and good in medication compliance.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Isradipine composition medicine, capsule preparation, and preparation method and application of capsule preparation
ActiveCN103830237AHigh activityOrganic active ingredientsPharmaceutical non-active ingredientsActive componentFerulic acid
The invention discloses an isradipine composition medicine, aiming at bringing the medicine pair function of isradipine and ferulic acid in medicine combination into play. The medicine comprises active components, namely, isradipine and ferulic acid, in a weight ratio of (5-10): (2-6); the medicine pair function of isradipine and ferulic acid in medicine combination is brought into play; good activity is shown in treating atherosclerosis, and the activity is superior to that of independent medication of isradipine and ferulic acid.
Owner:CHONGQING CONQUER PHARML
Treatment for dopaminergic disorders
ActiveUS9463186B2Delay progressBypass side effectOrganic active ingredientsDihydropyridineNitric Oxide Synthase Inhibitors
Owner:NORTHWESTERN UNIV
Preparation method of pharmaceutical composition capable of increasing dissolution rate of indissolvable drug isradipine
InactiveCN104784181ALarge specific surface areaImprove wettabilityOrganic active ingredientsPharmaceutical non-active ingredientsDrugMicronization
The invention relates to a pharmaceutical composition capable of increasing a dissolution rate of an indissolvable drug isradipine. The pharmaceutical composition can effectively increase the dissolution rate of isradipine. An isradipine solid preparation, such as a tablet, is prepared by a solid dispersion technology and a micronization technology and prepared from a pharmaceutically acceptable diluent or carrier, so that the indissolvable isradipine can be released quickly from the preparation. The product is stable in quality, high in dissolution rate, beneficial to increase absorption of isradipine in a human body, capable of effectively improving the bioavailability, and suitable for industrial production.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Method for synthesizing isradipine medicament midbody 4-formyl benzo furazan
The invention relates to a method for synthesizing an isradipine medicament midbody 4-formyl benzo furazan. The method comprises a step of preparing the 4-formyl benzo furazan by using reagents of 4-methyl benzo furazan, an oxidant selenium dioxide and the like. The method has the characteristics of low cost, high yield, short production period and the like. In addition, the reaction condition of the method is easy to realize and control, and the industrial production is facilitated.
Owner:江苏倍达医药科技有限公司
Method for preparing isradipine capsule
InactiveCN105030726AImprove bioavailabilityImprove in vitro dissolutionOrganic active ingredientsCapsule deliveryPolymer scienceClinical efficacy
The invention discloses a method for preparing an isradipine capsule. The method comprises the following steps: dissolving an isradipine raw material according to the amount of a prescription in prescribed ethanol, and adding povidone K3 to stir and dissolve; putting prescribed pregelatinized starch and microcrystalline cellulose in a fluidized bed, starting a fan, regulating the air volume to excellent fluidizing, setting the air inlet temperature to be 35-40 DEG C, spraying the solution prepared in the step A when the material temperature rises to 25 DEG C to granulate; and finishing the prepared particles through a 30-mesh screen, adding magnesium stearate, uniformly mixing, and filling capsules with the particles. According to the method, the raw materials and the auxiliaries are used for preparing solid dispersion by adopting a spray drying mode, are prepared to form fine particles by one step, and are used for filling capsules, so that the dissolution in vitro of the capsule can be effectively increased, the production cost can be reduced, the bioavailability of the capsule can be improved, and a better clinical curative effect can be achieved.
Owner:SICHUAN BAILI PHARM CO LTD
Method for preparing high-purity isradipine
The invention discloses a method for preparing high-purity isradipine. The method includes dehydrating and condensating 4-formaldehyde benzofuroxan in atent solvent to form 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate; subjecting methyl acetoacetate to ammonolysis to form 3-amino crotonic acid methyl ester; subjecting 2-acetyl-3-benzofuroxan-4-base-isopropyl acrylate and 3-amino crotonic acid methyl ester to reaction to form isradipine. The method for preparing high-purity isradipine is simple in operation process, convenient to operate, high in yield and capable of preparing high-purity isradipine.
Owner:SICHUAN BAILI PHARM CO LTD
Method for preparing isradipine impurity III
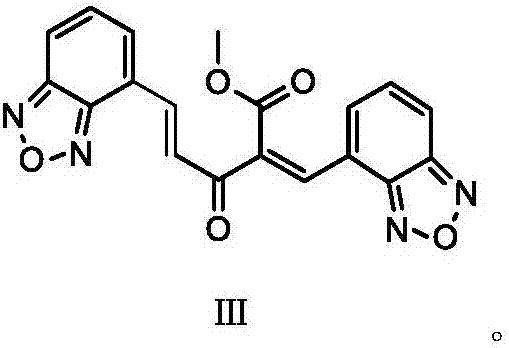
The invention discloses a method for preparing an isradipine impurity III. The method is characterized by comprising the following steps: taking benzodioxazole-4-formaldehyde and 2-acetyl-3-benzofuroxan-4-yl-methyl acrylate as raw materials, carrying out a condensation reaction under weakly alkaline and organic conditions, and separating, thereby obtaining the isradipine impurity III, wherein the equation is as shown in the specification. The isradipine impurity III is simple in preparation process, short in route and readily available in raw materials and does not need to be subjected to column purification, and the obtained product is high in purity (HPLC (High Performance Liquid Chromatography) is more than or equal to 98%) and can be directly used for quality study of isradipine I key intermediate 2-acetyl-3-benzofuroxan-4-yl-methyl acrylate.
Owner:CHONGQING CONQUER PHARML
Isradipine pharmaceutical composition with improved bioavailability
InactiveCN107308159APromote oxidationImprove oxidation capacityOrganic active ingredientsCardiovascular disorderAntioxidantBULK ACTIVE INGREDIENT
The invention discloses an isradipine pharmaceutical composition with improved bioavailability. The invention firstly provides a method for improving the bioavailability of an isradipine medicament, which comprises the step: using an effective amount of isradipine medicament in combination with an antioxidant or a metal ion complexing agent. The invention still further provides a pharmaceutical composition with improved bioavailability and taking the isradipine medicament as an active ingredient, and the pharmaceutical composition contains antioxidants. The antioxidants comprise, but not limited to, free radical absorbers, metal ion chelators, oxygen scavengers, peroxide decomposers or enzyme antioxidants.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Treatment for dopaminergic disorders
ActiveUS20140309260A1Delay disease progressionSide effectBiocideAnimal repellantsOxidative enzymePharmacology
The present invention provides systems, compositions and methods for treatment of dopaminergic disorders (e.g., Parkinson's disease) using the combination of a first compound that inhibits a voltage-gated calcium channel of the type Cav1.3 (e.g., a dihydropyridine such as isradipine), and a second compound that is monoamine oxidase inhibitor and / or is a nitric oxide synthase inhibitor (e.g., rasagiline or derivative thereof).
Owner:NORTHWESTERN UNIV
Isradipine controlled-release tablet and preparation method and application thereof
ActiveCN102688215BSolve the problem of chrono-dependent dosingOrganic active ingredientsMetabolism disorderNephrosisPhospholipid
The invention discloses an isradipine controlled-release tablet. The controlled-release tablet consists of an isradipine-containing controlled-release tablet core and a controlled-release coat. The invention provides an isradipine controlled-release tablet capable of delaying the release time, and solves the problem of obvious time dependence of the drug administration for cardiovascular diseases; and meanwhile, phosphatidylcholine is added to the tablet, thus the controlled-release tablet has a certain treatment effect on diabetic nephropathy.
Owner:CHONGQING CONQUER PHARML
Method for preparing isradipine impurities I
InactiveCN107151234ASimple preparation processRaw materials are easy to getOrganic chemistryMethyl acrylateImpurity
The invention discloses a method for preparing isradipine impurities I. The method is characterized by comprising steps of carrying out deacetylation reaction on 2-acetyl-3-benzofuroxan-4-base-methyl acrylate under alkaline organic conditions; carrying out separation to obtain the isradipine impurities I. The 2-acetyl-3-benzofuroxan-4-base-methyl acrylate is used as a raw material. A reaction formula is shown. The method has the advantages that processes for preparing the isradipine impurities I are simple, the raw material is easily available, the isradipine impurities I which are products are high in purity [the HPLC (high-performance liquid chromatography) is higher than or equal to 98%], and the method can be directly used for quality research on isradipine key intermediates (compounds 2).
Owner:CHONGQING CONQUER PHARML
A kind of isradipine combination medicine, capsule, preparation method and application thereof
ActiveCN103830237BHigh activityOrganic active ingredientsPharmaceutical non-active ingredientsActive componentFerulic acid
The invention discloses an isradipine composition medicine, aiming at bringing the medicine pair function of isradipine and ferulic acid in medicine combination into play. The medicine comprises active components, namely, isradipine and ferulic acid, in a weight ratio of (5-10): (2-6); the medicine pair function of isradipine and ferulic acid in medicine combination is brought into play; good activity is shown in treating atherosclerosis, and the activity is superior to that of independent medication of isradipine and ferulic acid.
Owner:CHONGQING CONQUER PHARML
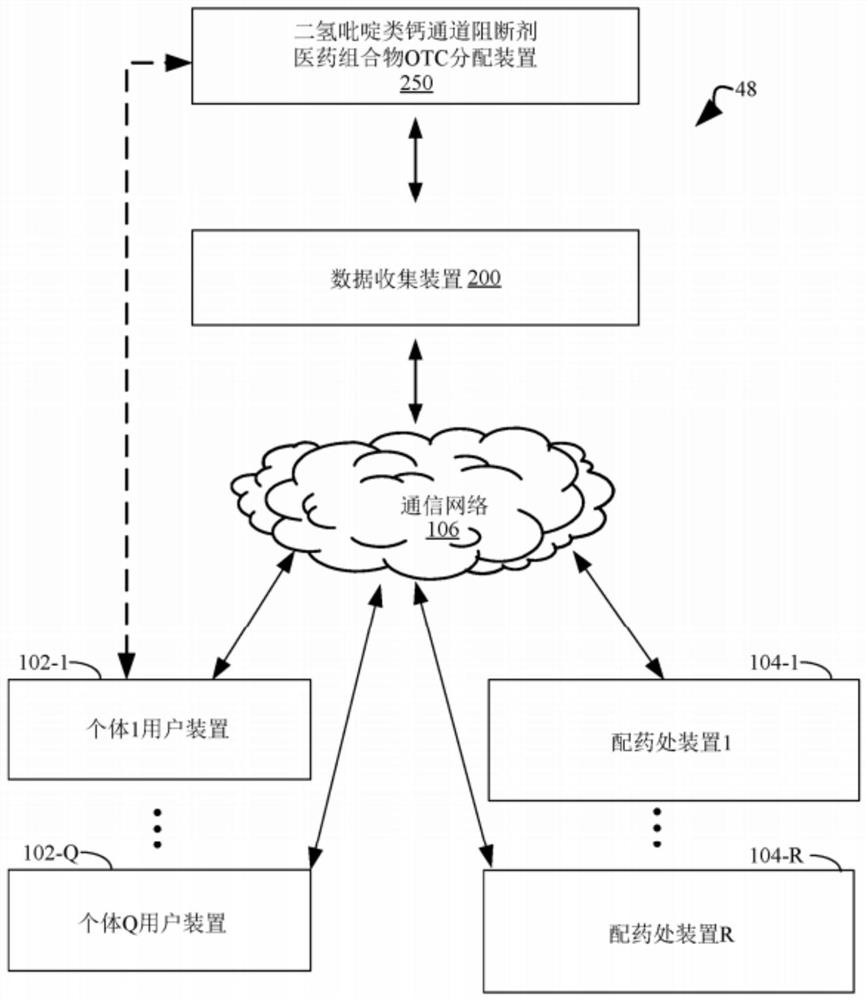
Methods for lowering blood pressure with a dihydropyridine-type calcium channel blocker pharmaceutical composition
PendingCN112955965AOrganic active ingredientsDrug and medicationsDihydropyridineCalcium channel blocker
A method is provided for lowering blood pressure in a subject in need thereof by administering a dihydropyridine-type calcium channel blocker pharmaceutical composition to a subject qualified for over-the-counter access to the dihydropyridine-type calcium channel blocker pharmaceutical composition. In some embodiments, the dihydropyridine-type calcium channel blocker pharmaceutical composition includes isradipine, nifedipine, or nisoldipine. In some embodiments, the dihydropyridine-type calcium channel blocker pharmaceutical composition includes 3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2- chlorophenyl)-6-methyl-l,4-dihydropyridine-3,5-dicarboxylate or a pharmaceutically acceptable salt thereof.
Owner:ASTRAZENCA UK LTD
Isradipine controlled release tablet and preparation method thereof
ActiveCN110327306ASolve product quality problems caused by moisture absorptionAvoid product qualityOrganic active ingredientsInorganic non-active ingredientsCellulose acetatePermeation
The invention provides an isradipine controlled release tablet and a preparation method thereof. The tablet includes a double-layer tablet consisting of a medicine layer and a booster layer, a controlled release semipermeable membrane, drug releasing pores and a moisture-proof coating. The medicine layer includes the main medicine that is isradipine and a carrier, and the carrier mainly includes vinylpyrrolidone and / or vinylpyrrolidone copolymer. The booster layer mainly includes a substance promoting permeation, an insoluble polymer, an osmotic pressure promoter, and the like. The controlledrelease semipermeable membrane mainly includes cellulose acetate, a plasticizer and a pore-foaming agent. Through application of the polymer, the isradipine after being orally taken is released at a controlled speed so that the purpose of maintaining 24 hours of medicine release only by one time of medicine taking each day is achieved, the blood medicine concentration is stabilized, and clinical treatment effects of the medicine are improved.
Owner:DEZHOU DEYAO PHARMA
Method for preparing drug isradipine for treating hypertension
InactiveCN101768153BProcess stabilityImprove product qualityOrganic active ingredientsOrganic chemistryBenzeneNitrobenzene
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com