Method for preparing high-purity isradipine
An isradipine and high-purity technology, which is applied in the field of chemical pharmacy, can solve the problems of cumbersome post-treatment of isopropyl acetoacetate, and achieve the effects of convenient purification and treatment of finished products, high yield and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
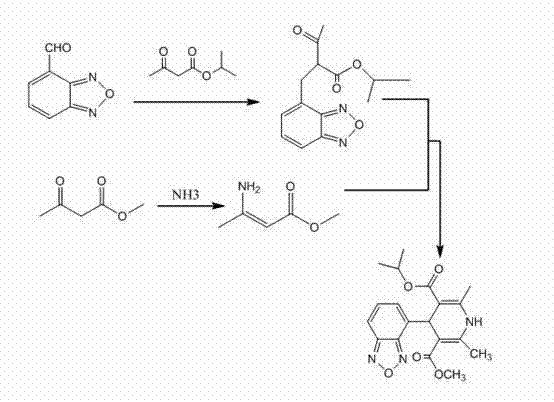
[0048] Example 1: Preparation of 2-acetyl-3-benzofurazan-4-yl-isopropyl acrylate (compound 2)
[0049] Add 22.2g (0.15mol) of isopropyl acetoacetate and 10ml of acetic anhydride into the reaction flask, cool in an ice-salt bath to below 5°C, add 1ml of concentrated sulfuric acid dropwise, and slowly add 2,1,3-benzo Oxadiazole-4-carboxaldehyde 14.8g (0.1mol), after the addition is complete, remove the ice-salt bath, stir and dissolve the solid, continue to keep warm for 3.5 hours, and monitor the reaction to 2,1,3-benzoxadiol Terminate the reaction when the remaining amount of azole-4-carboxaldehyde is less than 1%, add 20ml of isopropanol and stir evenly, cool, stir in an ice bath for 1 hour, filter, wash the filter cake with a small amount of isopropanol, then wash with a large amount of water, and dry to obtain 23.9g yellow crystals, yield 86.6%, HPLC Purity (sum of cis and trans isomer purity) ≥ 99.0%.
Embodiment 2
[0050] Example 2: Preparation of methyl 3-aminocrotonate (compound 3)
[0051] Add 55ml of methyl acetoacetate into a three-necked round-bottomed flask, lower the temperature to below 0°C, keep warm and feed ammonia gas to stir the reaction for 2 hours, monitor by TLC until the reaction is complete, let it stand overnight below -10°C, filter it with suction, and dry it under reduced pressure 53.6g of solid was obtained, melting point: 82-83°C, yield 93.2%.
Embodiment 4
[0055] Embodiment 4: the preparation of isradipine
[0056]Add 11g of methyl 3-aminocrotonate and 60ml of isopropanol into the reaction flask, add 23.9g of 2-acetyl-3-benzofurazan-4-yl-isopropylacrylate under stirring, and stir in the dark for 20 hour, the reaction solution was concentrated to dryness, added ethyl acetate 100ml, water 20ml, separated after stirring, the organic phase was dried with anhydrous sodium sulfate, filtered, and the ethyl acetate was reclaimed to dryness to obtain 31g of isradipine crude product. Add 40ml of isopropanol, heat to dissolve, filter while hot, let the filtrate stand for crystallization below 5°C for 16 hours, filter, wash the filter cake with a small amount of frozen isopropanol and dry it in vacuum at 40°C to obtain yellow isradipine 25.7g, yield 80% (HPLC purity 99.4%, homologue impurity content less than 0.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com