Isradipine controlled-release tablet and preparation method and application thereof
A technology of isradipine and controlled-release tablets, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery to achieve the effect of delaying the release time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 isradipine controlled release tablet
[0021] The prescription for the controlled-release tablet core is: isradipine 20mg, phosphatidylcholine 40mg, HPMC K15M 40mg, EC 100cp30mg, PVP K3020mg; the prescription for the controlled-release coating is: HPMC K15M 37.5mg, EC 0.01Pa·s 112.5mg, PVP K3060mg, MCC 90mg. Mix isradipine, phosphatidylcholine, ethyl cellulose, hydroxypropyl methyl cellulose and polyvinylpyrrolidine evenly, wet granulate, dry, add magnesium stearate and press into tablets to make a controlled-release tablet core; The hydroxypropyl methylcellulose, ethylcellulose, polyvinylpyrrolidone and microcrystalline cellulose in the controlled-release coating raw materials are wet-granulated, dried, and mixed with magnesium stearate to obtain a coating mixture; Half of the mixture is put into the die, the controlled-release tablet core is placed in the center of the die, and the other half of the coating mixture is put into the tabl...
Embodiment 2
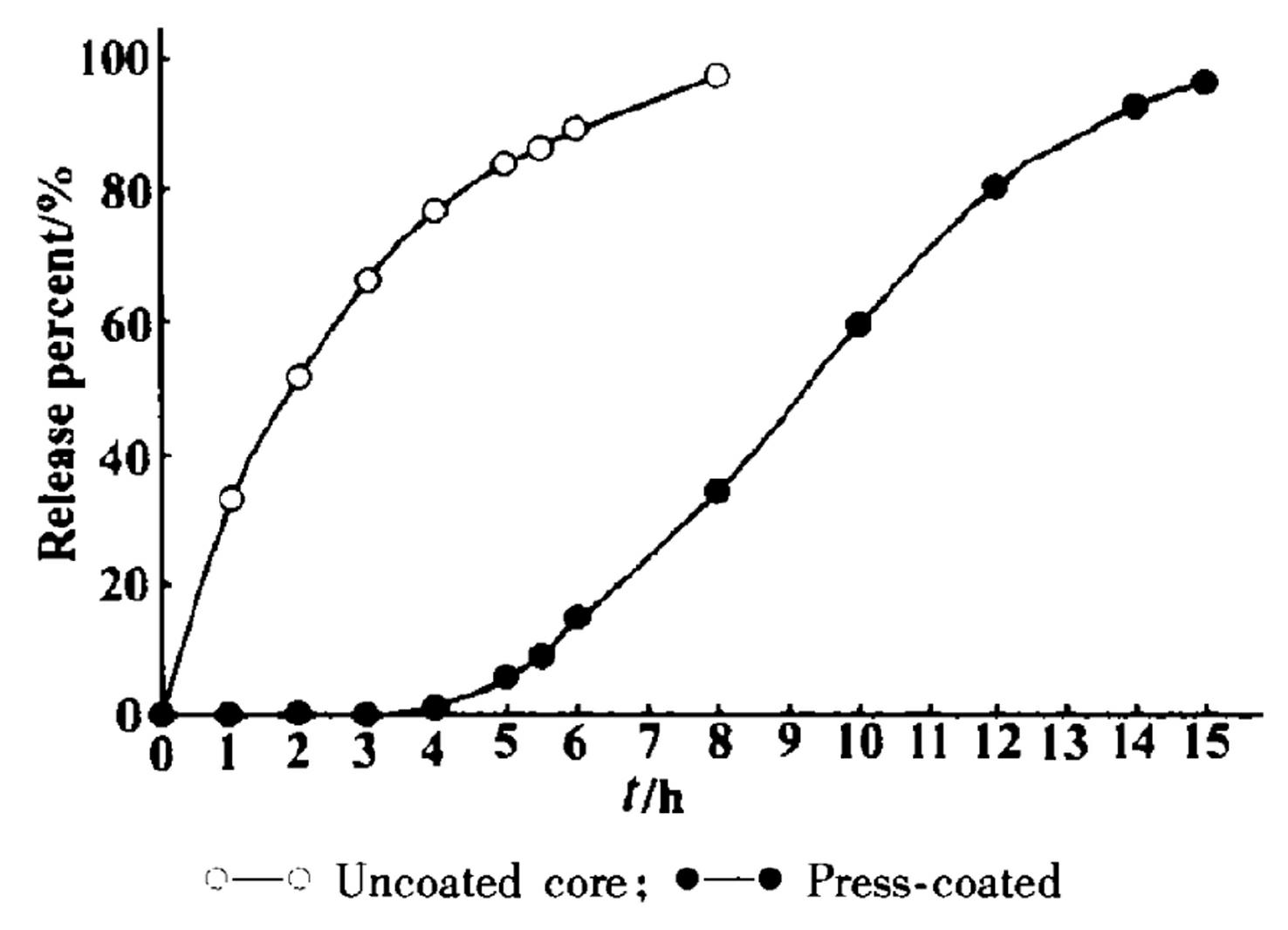
[0022] The in vitro release comparison of embodiment 2 isradipine controlled-release tablet and controlled-release tablet core
[0023] Get the isradipine controlled-release tablet and controlled-release tablet core that embodiment provides, the device that passes through Chinese Pharmacopoeia 2010 edition dissolution test method first method, with distilled water 900mL as dissolution medium, rotating speed is 10Or min -1 , the temperature is (37±0.5) ℃, operate according to the law, take 5mL of the solution at 1, 2, 3, 4, 5, 6, 8, 10, 12 and 14h, filter, and add 5mL of water immediately. Part of the sample solution was diluted with distilled water, and then the ultraviolet absorbance was measured at 236nm, and the drug release percentage at different time points was calculated by the standard curve.
[0024] The most critical index in delayed-release controlled-release tablets is the release time lag (T lag ), the drug release time lag can be determined by the following meth...
Embodiment 3
[0028] The influence of embodiment 3 different controlled-release coatings on the release behavior of isradipine controlled-release tablets
[0029] The controlled-release tablet core prescription of the isradipine controlled-release tablet provided in 1 was fixed, and the influence of the type, specification, dosage and compression pressure of the polymer in the controlled-release coating on the drug release behavior was separately investigated.
[0030] The dosage of hydroxypropyl methylcellulose (HPMC): according to the preparation method of isradipine controlled-release tablets, the coating layers containing 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, and 20% HPMCK4M were prepared respectively. Ladipine controlled-release tablets and determination of drug release curve;
[0031] Hydroxypropylmethylcellulose (HPMC) viscosity: according to the preparation method of isradipine controlled-release tablets, HPMC (E50, K4M, K15M, K100M) with different viscosities were used to prepare israd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com