Isradipine pharmaceutical composition with improved bioavailability
A technology of isradipine and its composition, which is applied in the field of isradipine pharmaceutical preparations, can solve the problems of poor solubility, low bioavailability of active ingredients, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
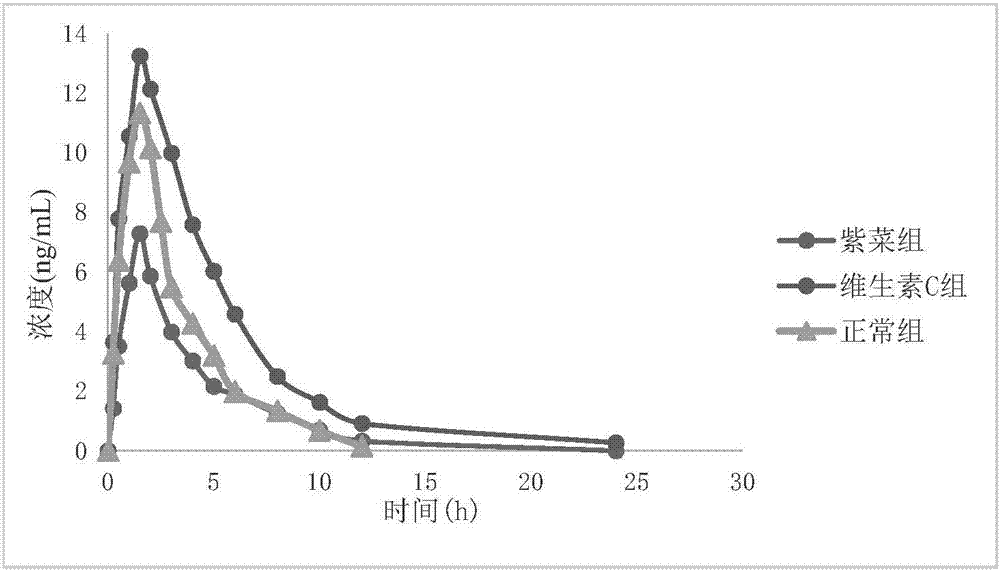
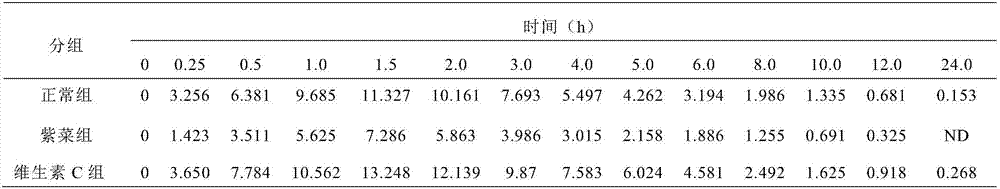
[0030] Twelve healthy Beagle dogs were selected and randomly divided into three groups, 4 dogs in each group, which were fed with normal group, seaweed (containing Fe3+ ions) group and vitamin C group respectively. During the test period, Beagle dogs were fasted overnight without water (feeding deionized water) for at least 12 hours, collected venous blood, separated plasma, and used it as a 0-hour blood drug concentration sample. Then began to feed according to the experimental grouping. The dogs in the normal group were not fed food, the dogs in the laver group were fed with 100ml of laver soup, and the dogs in the vitamin C group were fed with 0.1g of vitamin C tablets (1 tablet, 0.1g / tablet). After 30 minutes, each dog was fed with isradipine tablet 5 mg (2 tablets, 2.5 mg / tablet). After administration, blood samples were collected at 0.25h, 0.5h, 1h, 1.5h, 2h, 3h, 4h, 5h, 6h, 8h, 10h, 12h, and 24h, placed in anticoagulant tubes containing EDTA, and centrifuged at 3000r / mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com