Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
A self-microemulsion, solid technology, applied in the field of medicine, achieves the effects of low production cost, high yield, and reduced liver and kidney toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
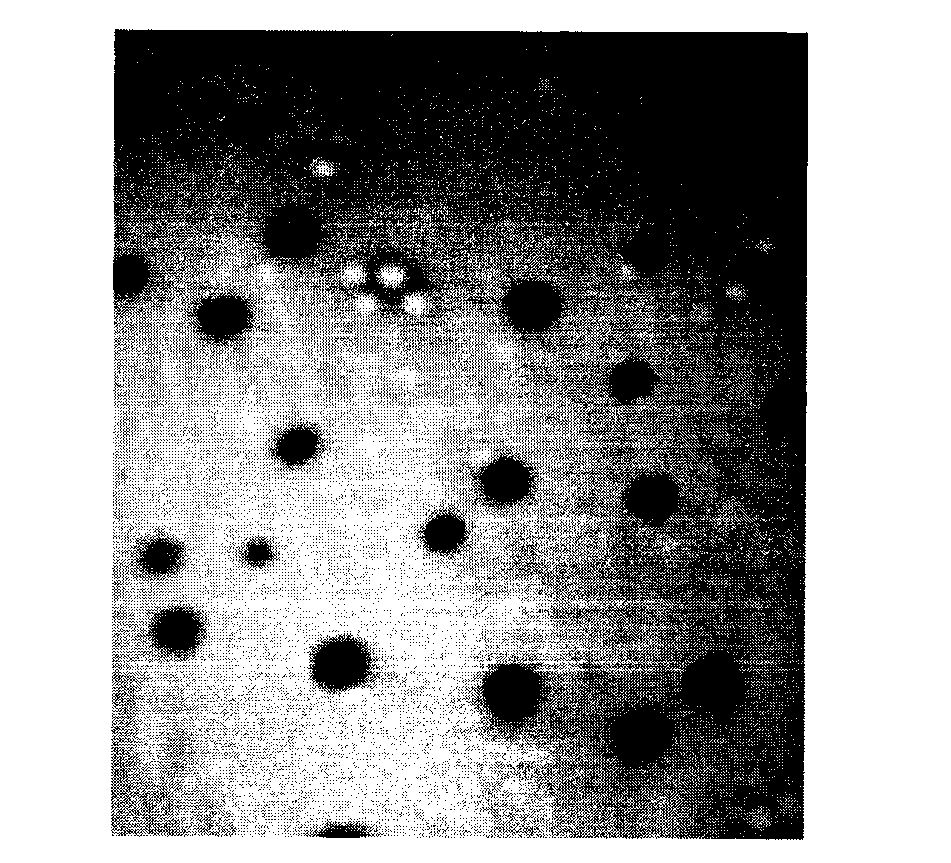
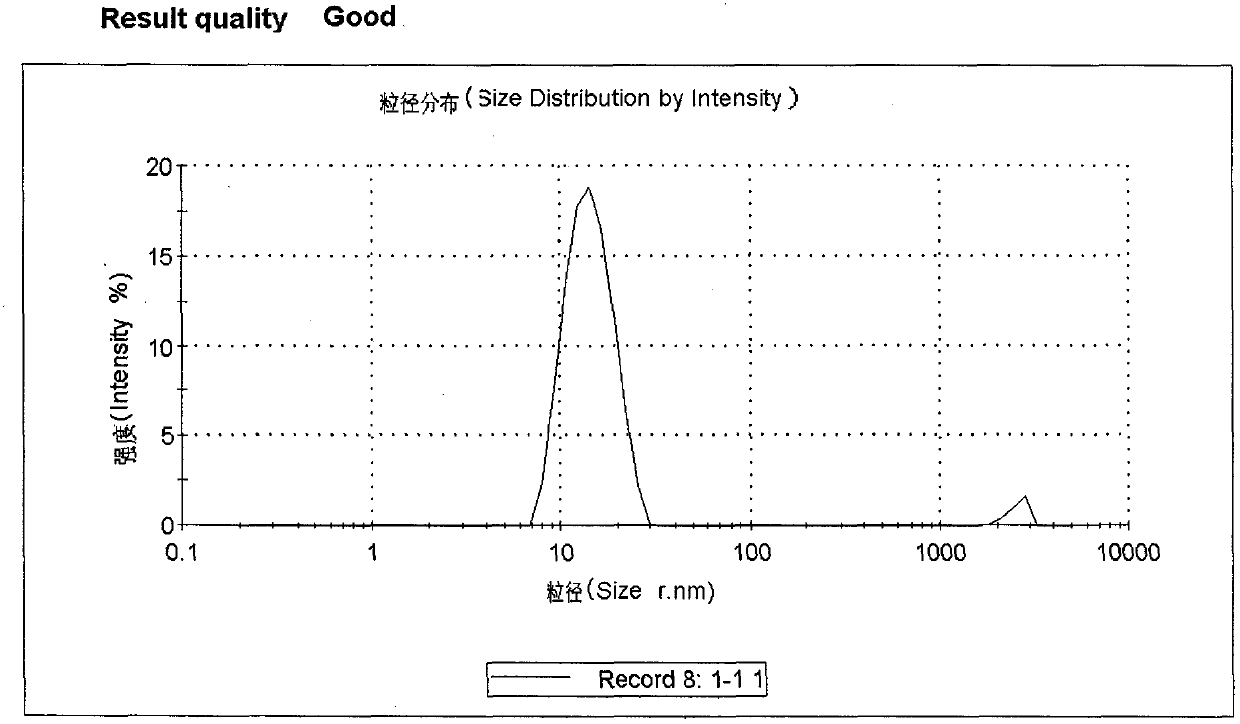
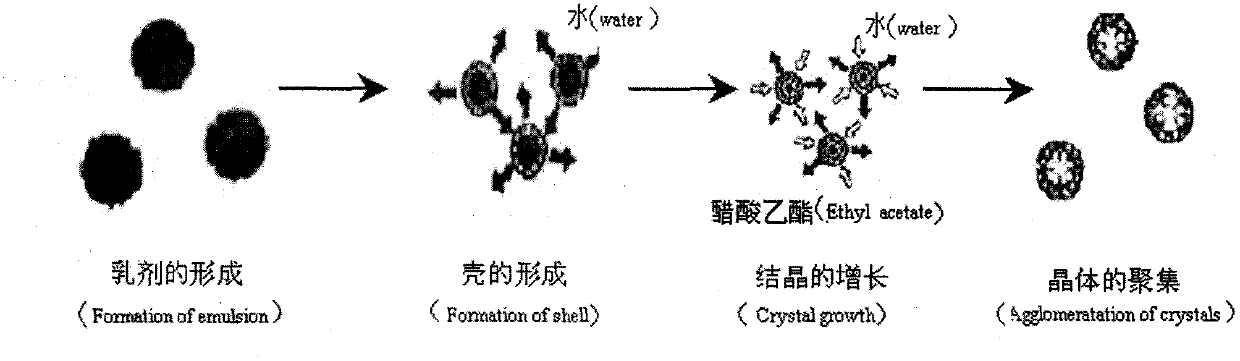
Image
Examples
Embodiment 1
[0034] 【Prescription】
[0035]
[0036] 【Preparation Process】
[0037] Preparation of fenofibrate self-microemulsion: Weigh 1.0g of the insoluble drug according to the prescription, dissolve it in 1.0ml ethanol, and then add the prescription amount of caprylic capric triglyceride, polyoxyethylene hydrogenated sesame oil and propylene glycol in sequence, and stir. The fenofibrate self-microemulsion is obtained evenly.
[0038] Preparation of fenofibrate solid self-microemulsion: weigh 0.5 ethyl cellulose, 0.05g PEG4000 in a small beaker, add 2ml of acetone and 4ml of dichloromethane to completely dissolve or suspend it, add fenofibrate to Microemulsion is placed in a small beaker, add an appropriate amount of micronized silica gel, slowly inject it into a poor solvent containing 0.08% SDS aqueous solution, the temperature is controlled at 25 ℃, the speed is 400 rpm, continuous stirring for 1 hour, add appropriate amount of distilled water, continue to stir for 10 minutes, until Ful...
Embodiment 2
[0041] 【Prescription】
[0042]
[0043] 【Preparation Process】
[0044] Preparation of fenofibrate self-microemulsion: Weigh 2.50g of fenofibrate according to the prescription, dissolve it in 1.0ml ethanol, and add the prescription amount of caprylic capric triglyceride, polyoxyethylene hydrogenated sesame oil and propylene glycol in sequence , Stir evenly to obtain fenofibrate self-microemulsion.
[0045] Preparation of fenofibrate solid self-microemulsion: Weigh 0.5g ethyl cellulose and 0.05g PEG4000 in a small beaker, add 2ml acetone and 4ml chloroform to completely dissolve or suspend it, add the above-mentioned fenofibrate self-microemulsion Milk in a small beaker, add an appropriate amount of micronized silica gel, slowly inject it into a poor solvent containing 0.08% SDS aqueous solution, control the temperature at 25°C, rotate at 400rpm, continue stirring for 1h, add appropriate amount of distilled water, continue stirring for 10min, until aggregate The body particles are n...
Embodiment 3
[0048] 【Prescription】
[0049]
[0050]
[0051] 【Preparation Process】
[0052] Preparation of glimepiride self-microemulsion: Weigh 1.0g glimepiride according to the prescription, dissolve it in 1.0ml ethanol, and add the prescription amount of caprylic acid capric triglyceride, polyoxyethylene hydrogenated sesame oil and propylene glycol in sequence. Stir evenly to obtain glimepiride self-microemulsion.
[0053] Preparation of glimepiride solid self-microemulsion: weigh 0.5mg ethyl cellulose and 0.05g PEG4000 into a small beaker, add 2ml acetone and 4ml dichloromethane to completely dissolve or suspend it, add the above glimepiride Add 1.0g of self-microemulsion in a small beaker, add appropriate amount of micronized silica gel, slowly inject it into a poor solvent containing 1% PVA aqueous solution, control the temperature at 25°C, rotate at 400rpm, continue stirring for 1h, add appropriate amount of distilled water, and continue stirring 10min, until the aggregate particles do...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com