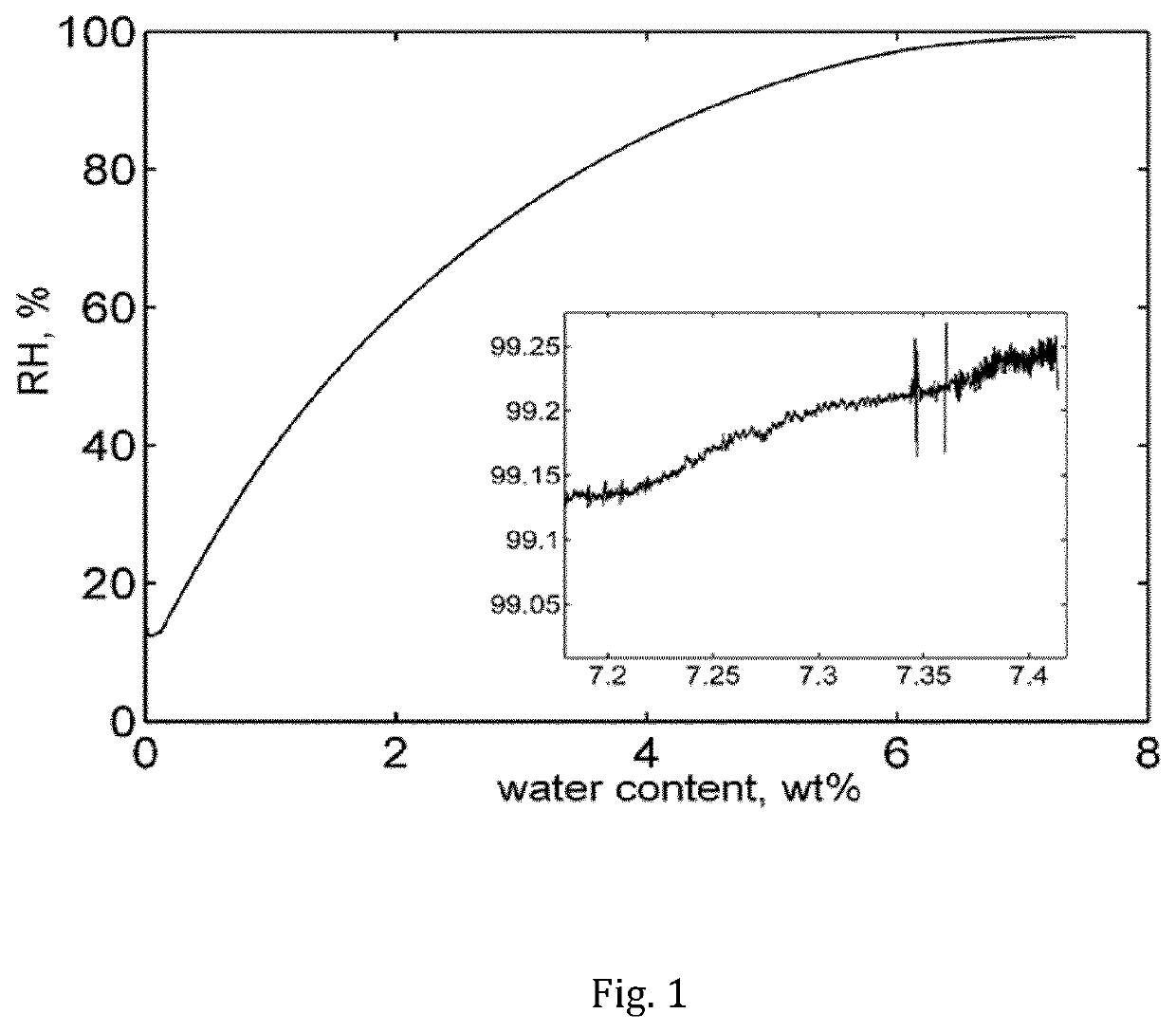
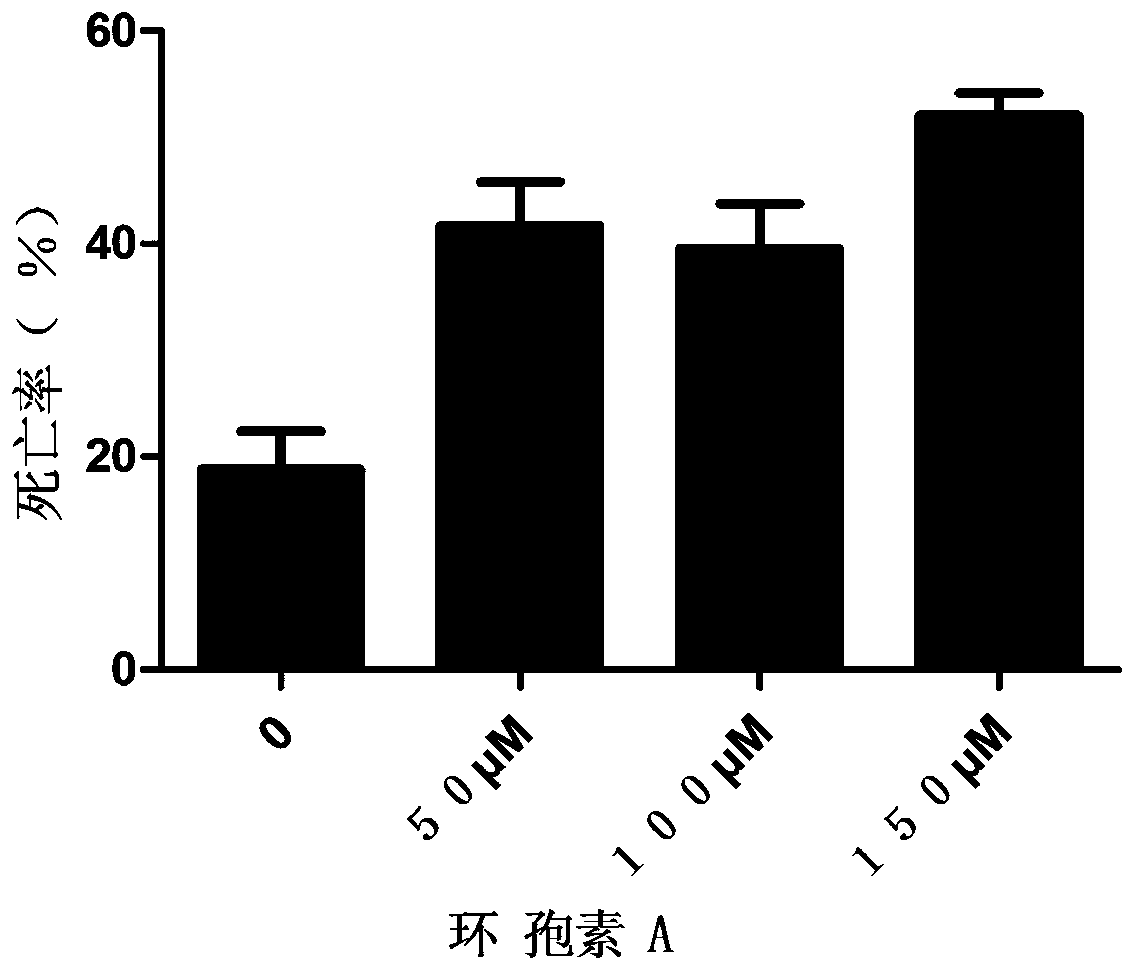
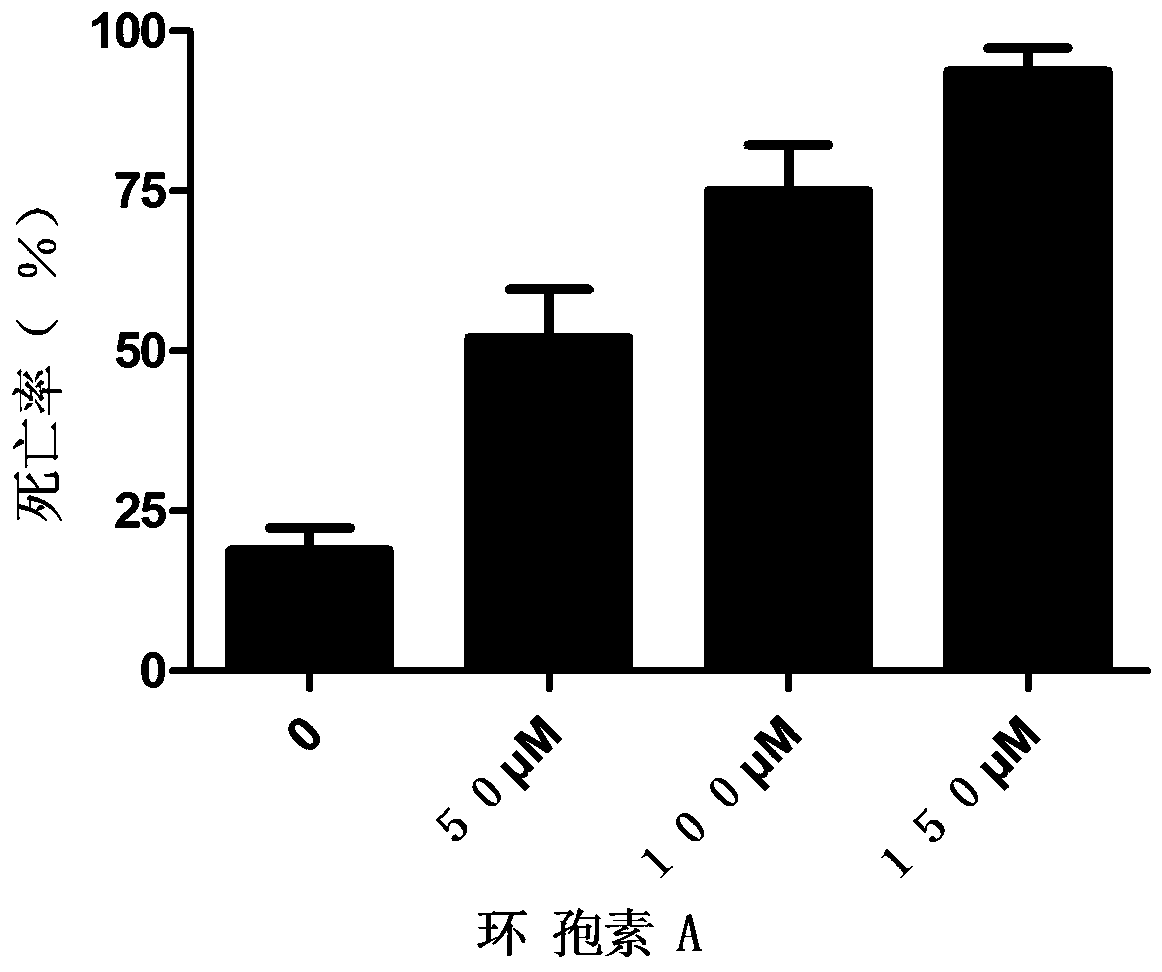
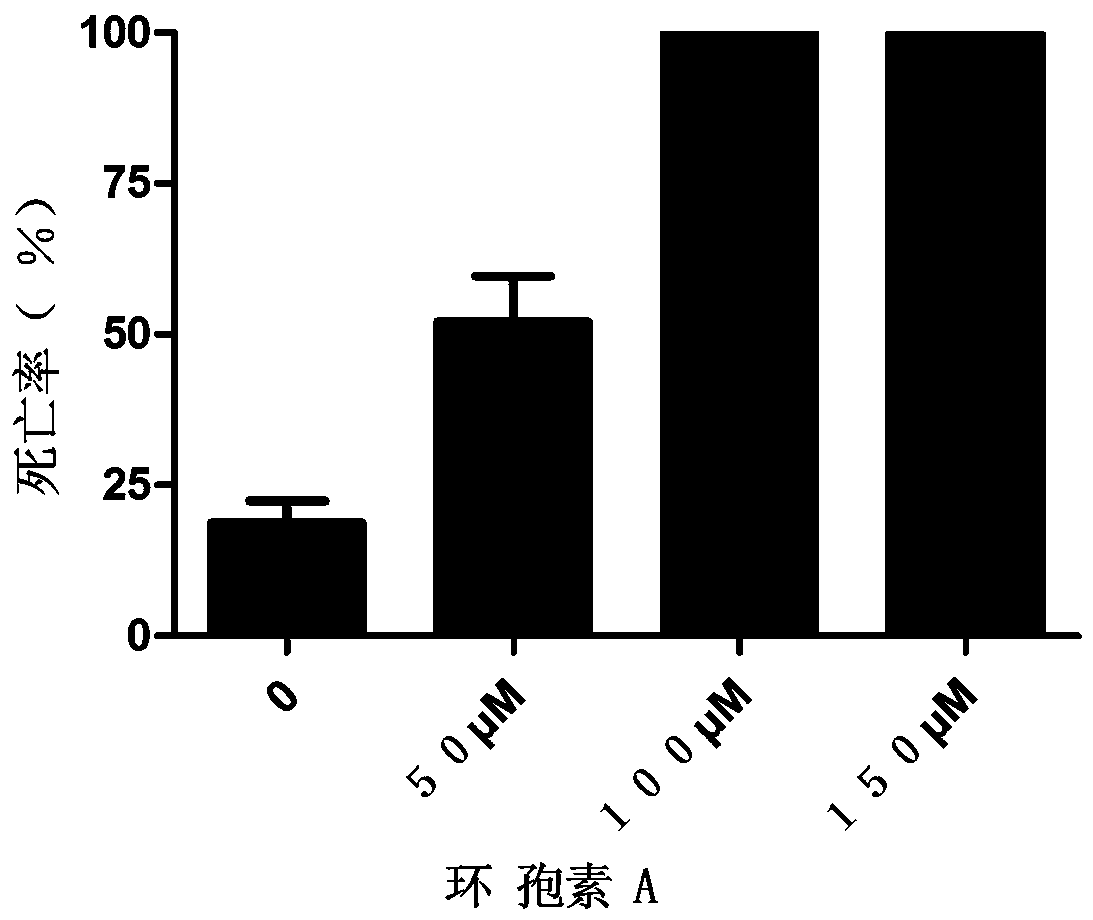
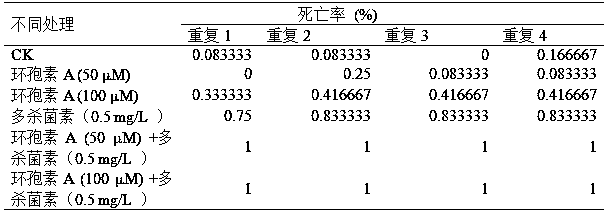
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Ciclosporins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug used to help reduce the risk of rejection of organ and bone marrow transplants by the body
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Novel clyclosporin analogues
Owner:ENANTA PHARM INC
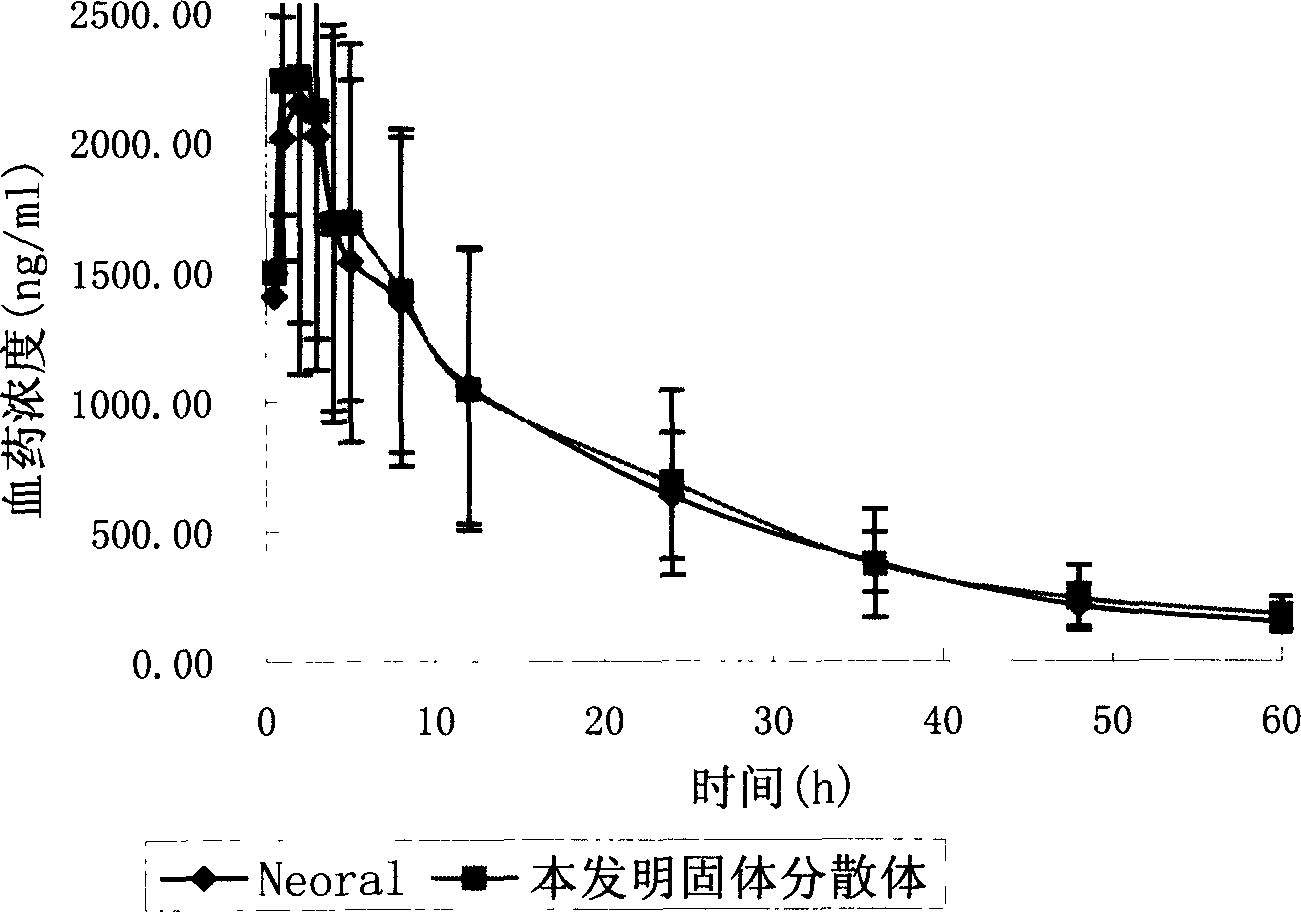
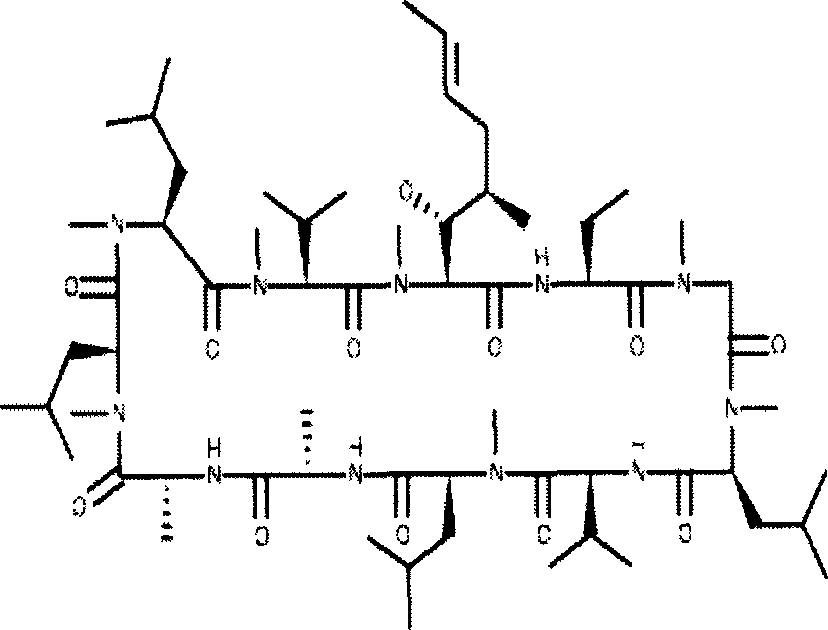
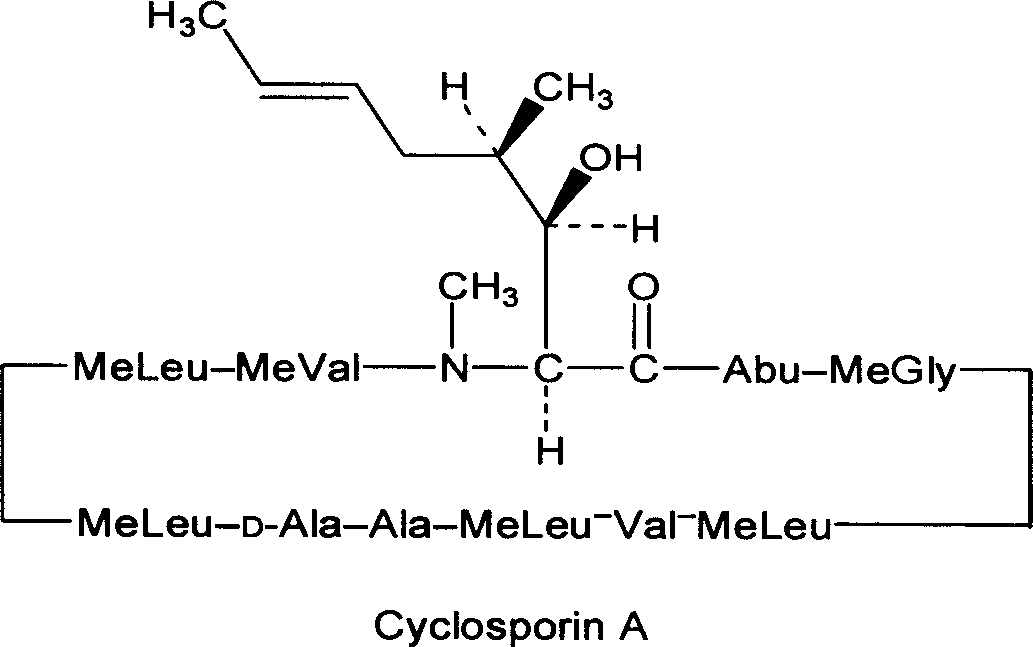
Cyclosporin A dispersion solid and its preparation method
InactiveCN1559606AIncrease dissolution ratePowder deliverySuppositories deliverySolubilityCaplet Dosage Form
A dispersing solid (capsule, tablet, particle, suppository and dripping pill) of cyclosporin A is prepared from cyclosporin A and carrier by solvent method, solvent fusion method, etc. It has high solubility of easy absorption.
Owner:FUDAN UNIV +1
Cyclosporin A semisolid skeleton capsule and its preparation method
A capsule of cyclosporin A and semi-solid skeleton is proportionally prepared from cyclosporin A, oil phase, emulsifier, emulsifying aid, and semi-solid carrier through fusing them except cyclosporin A in water bath at 60 deg.C, stirring, adding cyclosporin A, stirring for full dissolving, cooling and loading it in capsules.
Owner:SHENYANG PHARMA UNIVERSITY
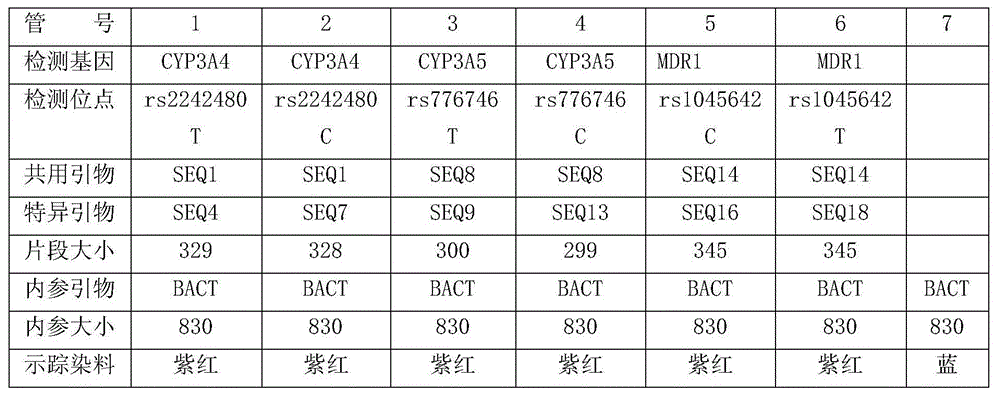
Primers for detecting polymorphism of CYP3A4, CYP3A5 and MDR1 genes with ARMs-PCR (amplification refractory mutation system-polymerase chain reaction) method and prepared kit
InactiveCN104805181AIngenious designEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationMultiplex polymerase chain reactionMutation
The invention discloses primers for detecting polymorphism of CYP3A4, CYP3A5 and MDR1 genes with an ARMs-PCR (amplification refractory mutation system-polymerase chain reaction) method and a prepared kit. Primer sequences are shown in SEQ 1-SEQ 18. The primers can perform typing testing on CYP3A4, CYP3A5 and MDR1 genes of humans quickly and accurately through specific amplification after primer combination. Genetic typing or preparation of the typing kit can be performed according to the primers and the primers are used for detecting metabolic capacity of Chinese people for related drugs such as tacrolimus, cyclosporine A and the like, so that a reference basis can be provided for special doctors who determine optimal drug dosage and individual drug use, and drug use risks are reduced.
Owner:UNION STEMCELL & GENE ENG
Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
InactiveCN103315960AReduce liver and kidney toxicityAvoid influencePowder deliveryEmulsion deliveryNeogambogic acidCaprylic acid
The invention relates to the medical technology field, and particularly relates to a solid self-microemulsion based on a spherical crystallization technique and a preparation method thereof. The solid self-microemulsion is characterized in that: with the use of the spherical crystallization technique, the solid self-microemulsifying micoparticles are prepared from poorly water soluble drugs in a liquid phase by one step. The solid self-microemulsion with the poorly water soluble drugs comprises the components, by weight: 0.1 to 1.5 g of the poorly water soluble drugs, 4.0 g of a polyoxyethylene hydrogenated castor oil, 2.0 g of capric caprylic triglyceride, 2.0 g of tpropylene glycol, 1.0 ml of ethanol, 4.0 ml of dichloromethane, 0.5 to 1.1 g of ethylcellulose (or Eudragit RS100, RL100), 0.05 g of PEG4000, and 0.5 g of colloidal silicon dioxide. The poorly water soluble drugs include cyclosporine A, fenofibrate, glimepiride, cilnidipine, isradipine, simvastatin, baicalein, neogambogic acid, puerarin, cyclovirobuxine D, silymarin and the like.
Owner:胡容峰
Biodegradable drug delivery for hydrophobic compositions
InactiveUS20190160171A1Cyclic peptide ingredientsPharmaceutical non-active ingredientsPolyesterPolymer science
A biodegradable drug delivery compositions comprising a triblock copolymer containing a polyester and a polyethylene glycol and a diblock copolymer containing a polyester and an end-capped polyethylene glycol, as well as at least one pharmaceutically active principle or hydrophobic active principle such as medroxyprogesterone acetate, levonorgestrel, cyclosporine, progesterone or bupivacaine is disclosed.
Owner:MEDINCELL SA
Ophthalmic compositions comprising ciclosporin
The present invention relates to compositions in the form of a clear solution comprising ciclosporin with low residual water content and 1-perfluorobutylpentane. The compositions may be used for topical administration to the eye.
Owner:NOVALIQ GMBH
Cyclosporine A fast release solid dosage forms and preparation thereof
InactiveCN101239048AImprove apparent solubilityGood stabilityInorganic non-active ingredientsCyclic peptide ingredientsSolubilitySide effect
Owner:SHANGHAI JIAO TONG UNIV
Topical cyclosporine-containing formulations and uses thereof
ActiveUS10918694B2Reduced acuityBlurred visionOrganic active ingredientsSenses disorderAlcoholCyclosporins
Provided herein are formulations for topical ophthalmic formulations containing 0.087-0.093 wt % of cyclosporine, and methods of making and using such formulations. In some aspects and embodiments the formulations may include a polyoxyl lipid or fatty acid, and / or a polyalkoxylated alcohol and may include nanomicelles. Also included herein are methods of treating or preventing diseases or conditions, such as ocular diseases or conditions.
Owner:SUN PHARMA INDS
Ophthalmic compositions comprising ciclosporin
The present invention relates to compositions in the form of a clear solution comprising ciclosporin with low residual water content and 1-perfluorobutylpentane. The compositions may be used for topical administration to the eye.
Owner:NOVALIQ GMBH
New drug for preventing and treating mythimna separata and use method thereof
ActiveCN110637821AThe control effect is confirmedHigh insecticidal activityBiocideAnimal repellantsNew medicationsPest control
The invention belongs to the field of toxicological activity of pest control, and particularly relates to a new drug for controlling mythimna separata and a use method of the new drug. The new drug iscyclosporin A, wherein the mythimna separata refers to 2-year-old mythimna separata. The method for preventing and treating the mythimna separata by using the new drug comprises the step of mixing the new drug with an artificial feed to feed the mythimna separata. According to the invention, the cyclosporin A is mixed with the mythimna separata artificial feed, and by setting different doses of the cyclosporin A, the prevention and treatment effect on the mythimna separata is detected after 7 days. The prevention and treatment effect of the cyclosporin A is evaluated, and the prevention and treatment dosage of the cyclosporin A is determined. And a new active functional group is provided for the design of a standby insecticide or new drug for preventing and treating the mythimna separata.
Owner:HENAN AGRICULTURAL UNIVERSITY
Whole blood type freeze-dried powder immunosuppressant quality control substance as well as preparation method and application thereof
The invention discloses a whole blood type freeze-dried powder immunosuppressant quality control substance as well as a preparation method and application thereof. The substance is a freeze-dried product mainly prepared from human whole blood serving as a matrix and an immunosuppressant. The immunosuppressant comprises one or more of tacrolimus, sirolimus, everolimus and cyclosporin A, or furthercomprises glucocorticoid immunosuppressants: one or more of hydrocortisone, cortisone, prednisolone, prednison and mycophenolic acid ester immunosuppressants for blood detection. The substance has theadvantages that the uniformity and the stability are good, low-temperature storage at -70 DEG C is not needed, and the substance can be used as a calibration product of immunosuppressant kits of different methodologies and can also be used as a quality control product for clinically monitoring an immunosuppressant detection system.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Ciclosporin A proliposome, pharmaceutical composition and preparation method thereof
ActiveCN102008713AImprove stabilityHigh dissolution ratePowder deliverySolution deliveryLipid formationDispersity
The invention discloses a ciclosporin A proliposome for oral administration, a pharmaceutical composition and a preparation method thereof. The ciclosporin A proliposome comprises the following pharmaceutical active ingredients and non-active ingredients by weight percent: 1-25% of ciclosporin A, 2-40% of lecithin, 0.5-12.5% of cholesterol, 21-96.45% of polyol and 0.05-1.50% of vitamin E. The proliposome is prepared from the ciclosporin A, a lipid membrane material, a hydrophilic matrix and the like through the ultrasonic dispersion method and the reverse phase evaporation method, and a liposome is formed spontaneously after the dispersion in water. The proliposome can improve the dispersity and the oral bioavailability of ciclosporin A which is an insoluble drug, lead the stability to be good and be more applicable to industrial production in comparison with the ordinary liposome.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
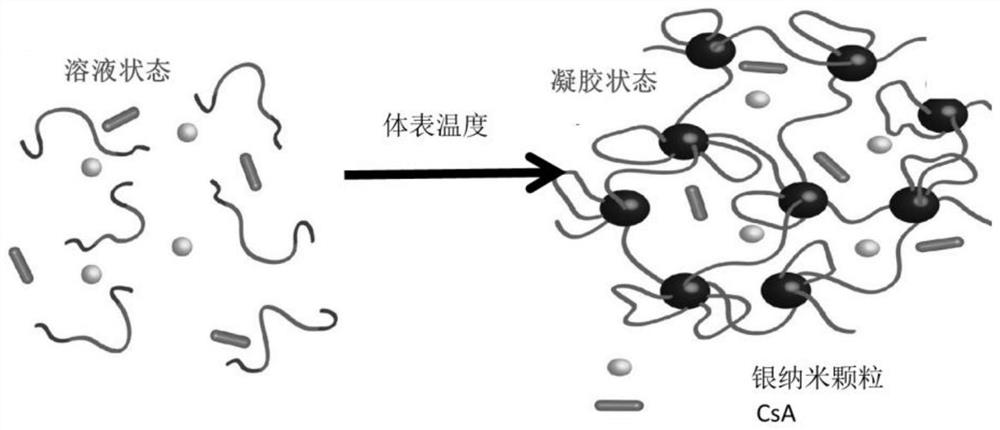
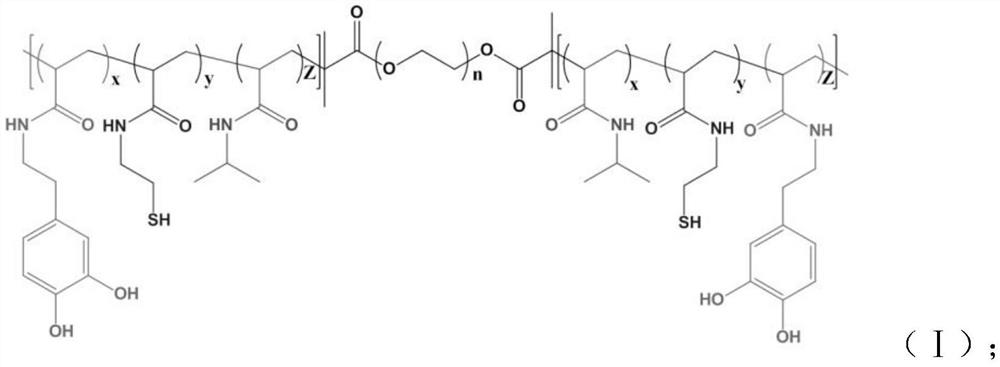
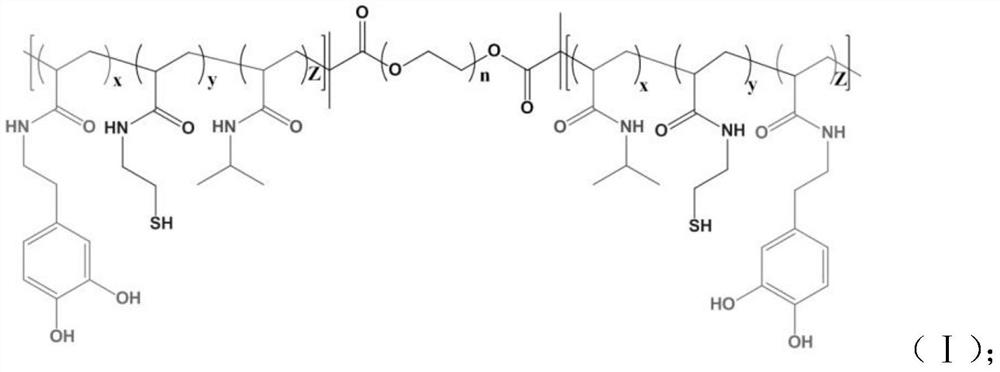
Hydrogel with excellent antibacterial performance for burn wound healing and preparation method of hydrogel
InactiveCN111643720AGood temperature responseEasy to store and transportBandagesComposite materialCiclosporins
The invention provides hydrogel with excellent antibacterial performance for burn wound healing and a preparation method of the hydrogel. The hydrogel is an ABA type triblock reticular copolymer formed by polymerizing a polyethylene glycol monomer and an N-isopropylacrylamide monomer, wherein the copolymer comprises a mussel bionic tissue bonding group and a nanoparticle anchoring group; the hydrogel is loaded with silver nanoparticles and zinc nanoparticles, and cyclosporine A is carried on the surface and the interior of the hydrogel. The hydrogel material prepared by the invention has the characteristics of high strain, biocompatibility, adhesiveness, heat sensitivity, injectability, microbial resistance and the like, and can promote the healing speed of full-thickness burn wounds. Meanwhile, the antibacterial activity is excellent.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Myoblast treatment of diseased or weakened organs
InactiveUS20070009499A1Increase blood flowImprove bindingBiocidePeptide/protein ingredientsHeterograftsWeakness
Bioengineering the regenerative heart or other body organ in need of greater muscle mass or improved blood perfusion provides a novel treatment for organ weakness or failure. In the case of cardiac failure treatment, on May 14, 2002, a 55-year-old man suffering ischemic myocardial infarction received 25 injections carrying 465 million cGMP-produced pure myoblasts into his myocardium after coronary artery bypass grafting. Three myogenesis mechanisms were elucidated with 17 human / porcine xenografts using cyclosporine as immunosuppressant. Some myoblasts developed to become cardiomyocytes. Others transferred their nuclei into host cardiomyocytes through natural cell fusion. As yet others formed skeletal myofibers with satellite cells. De novo production of contractile filaments augmented heart contractility. Human myoblasts transduced with VEGF165 gene produced six times more capillaries in porcine myocardium than placebo. Xenograft rejection was not observed for up to 20 weeks despite cyclosporine discontinuation at 6 weeks.
Owner:LAW PETER
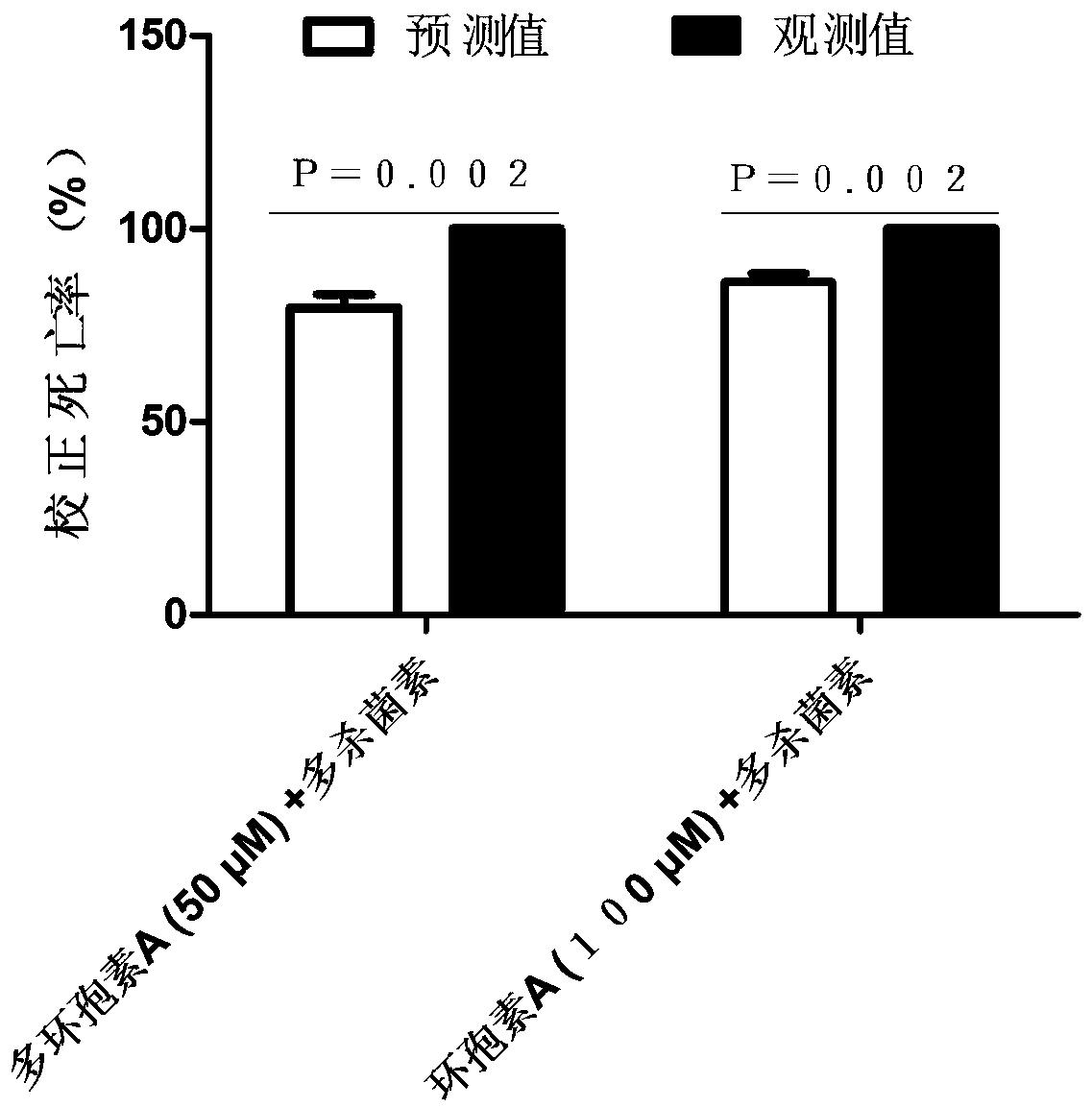
Drug synergist for preventing and treating mythimna separata and using method thereof
ActiveCN110731340ASignificant effectImprove the effect of prevention and controlBiocideAnimal repellantsSpinosadVermin
The invention belongs to the field of toxicological activity of pest control, and particularly relates to a drug synergist for preventing and treating mythimna separata and a using method of the drugsynergist. The drug synergist for preventing and treating mythimna separate is cyclosporin A, wherein a medicine is spinosad, wherein the mythimna separata is a two-year-old larva. The method for preventing and treating the mythimna separata by utilizing the drug synergist comprises the following steps: mixing the spinosad with a certain concentration with cyclosporin A, then mixing with an artificial feed of the mythimna separata again, and then feeding. The cyclosporin A disclosed by the invention has a remarkable effect of preventing and treating mythimna separata by the spinosad, and can be used as a synergist for preventing and treating mythimna separata by the spinosad so as to improve the prevention and treatment effect of agricultural pests by the spinosad.
Owner:HENAN AGRICULTURAL UNIVERSITY
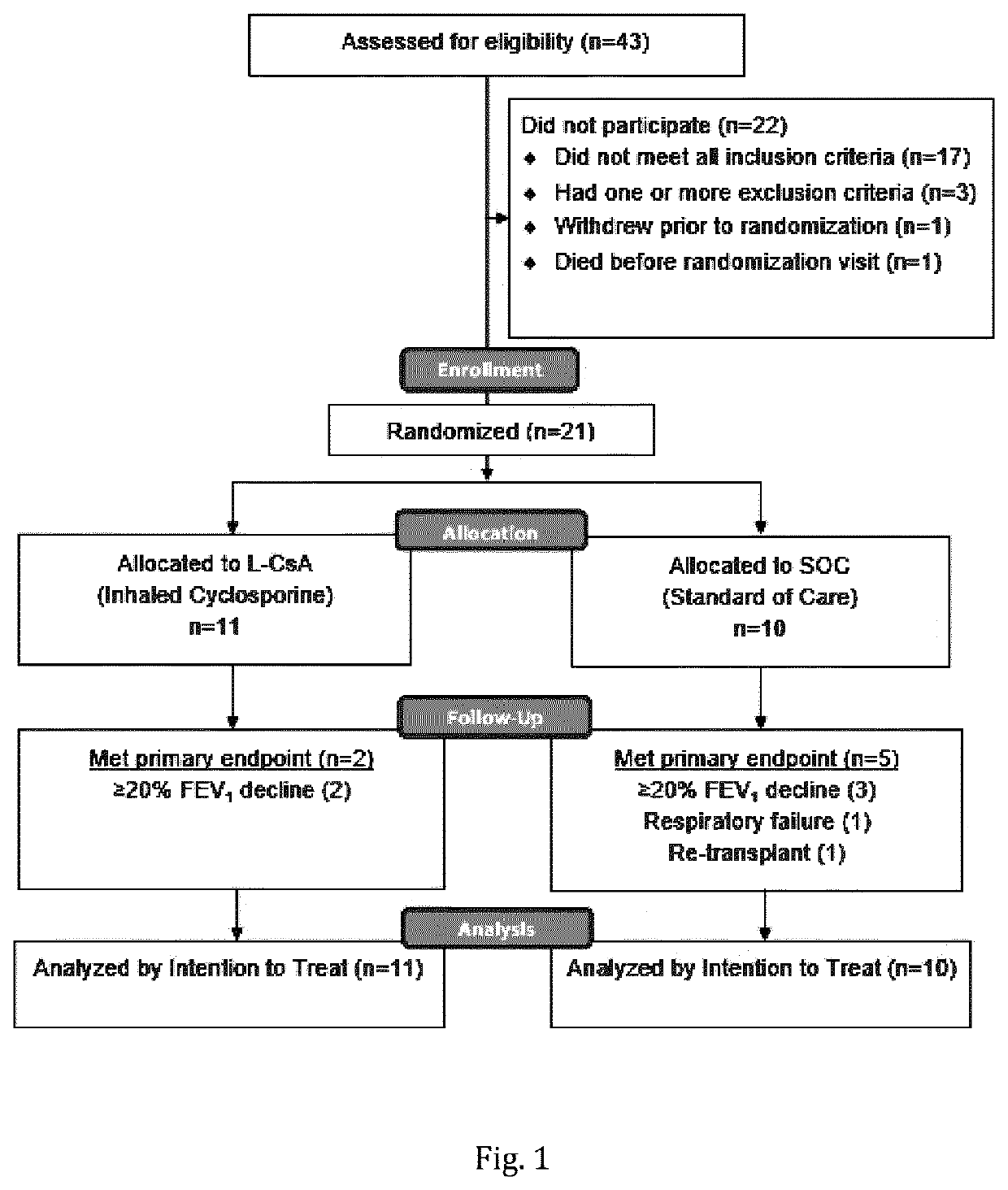
Cyclosporine formulations for use in the treatment of bronchiolitis obliterans syndrome (BOS)
The present invention relates to a composition comprising cyclosporine A (CsA) for use in the prevention of bronchiolitis obliterans syndrome (BOS) in a double lung transplanted patient, or for the treatment of BOS or for the prevention or delay of the progression of BOS in a double lung transplanted patient being diagnosed with BOS, wherein the composition is administered to said patient by inhalation of said composition in aerosolized form comprising a therapeutically effective dose of cyclosporine A.
Owner:BREATH THERAPEUTICS GMBH
Cyclosporins A emulsion preparations for eyes and preparation thereof
InactiveCN101244035ANon-irritatingImprove comfortSenses disorderCyclic peptide ingredientsCelluloseCyclosporins
The invention provides a cyclosporine A emulsion preparation for eye use, which is characterized in that the components and the proportion of the weight of contents are: cyclosporine A: 0.05 to 0.5%; cellulose thickening agent: 0.3 to 2%; chrysanthemum or the extract with honeysuckle: 0.3 to 6%; oil phase: 0.25 to 7.0%; solubilizing agent emulsifier: 0.1 to 5.0%; glycerol: 1 to 4%; pH adjuster: proper amount; the allowance is purified water. The preparation method comprises the following steps: the cellulose thickening agent is added into water of proper amount, heated to 80 to 90 DEG C and stirred, dispersed and cooled to ambient temperature; the chrysanthemum or the extraction with honeysuckle is added to the cellulose solution, mixed well for later use; the cyclosporine A is dissolved in the oil phase; the mixed liquid is added to the obtained cyclosporine mixed liquid; then the ultrasonic, shearing and emulsification is made for 1 to 5 minutes; the pH value is adjusted to 7.2 to 7.8 by the pH adjuster; after the prescription amount of purified water is added to the full dose, finished product is obtained.
Owner:南京医科大学附属南京第一医院
Maintenance-and-amplification method and differentiation induction method for primordial germ cells/primordial germ cell-like cells
The present invention provides a method for expanding PGC / PGCLC, including culturing PGC / PGCLC in the presence of a phosphodiesterase 4 (PDE4) inhibitor and / or cyclosporine A, further in the presence of forskolin, and a method for inducing oocytes from PGC / PGCLC, including culturing PGC / PGCLC in the presence of bone forming protein (BMP) and retinoic acid (RA).
Owner:KYOTO UNIV
Cyclosporin A dispersion solid and its preparation method
InactiveCN1245212CIncrease dissolution ratePowder deliverySuppositories deliverySolubilityCaplet Dosage Form
A dispersing solid (capsule, tablet, particle, suppository and dripping pill) of cyclosporin A is prepared from cyclosporin A and carrier by solvent method, solvent fusion method, etc. It has high solubility of easy absorption.
Owner:FUDAN UNIV +1
In-situ gel containing cyclosporin micelle as sustained-release ophthalmic drug delivery system
PendingCN112516084AImproved membrane transportImprove stabilitySenses disorderAntipyreticOphthalmic drugOphthalmology
The invention provides an in-situ gel containing cyclosporin micelles as a sustained release ophthalmic drug delivery system. The in-situ gel contains 0.01 wt% to 5 wt% of an aqueous ophthalmic formulation of cyclosporin present in the form of a micelle with the particle size of no greater than 20 nm.
Owner:IVEW THERAPEUTICS (ZHUHAI) CO LTD
Cyclosporin ophthalmic emulsion and preparation method thereof
ActiveCN112569188AExtended release timePromote absorptionSenses disorderCyclic peptide ingredientsGlycerolPharmaceutical Substances
The cyclosporin eye emulsion is prepared from the following components in percentage by mass: 0.01 to 0.05 percent of cyclosporin, 1.5 to 3 percent of collagen, 1.2 to 1.5 percent of castor oil, 1.8 to 2.5 percent of glycerol, 0.04 to 0.08 percent of anthocyanin, 0.8 to 1.2 percent of polysorbate 80 and the balance of double distilled water. The cyclosporin ophthalmic emulsion is prepared throughhigh-speed dispersion and shear emulsification, and collagen and anthocyanin in the emulsion are combined for use, so that the slow-release effect of the medicine can be effectively enhanced, and theresidual quantity of the medicine in corneas is increased.
Owner:WUHAN CONFORM PHARMA CO LTD
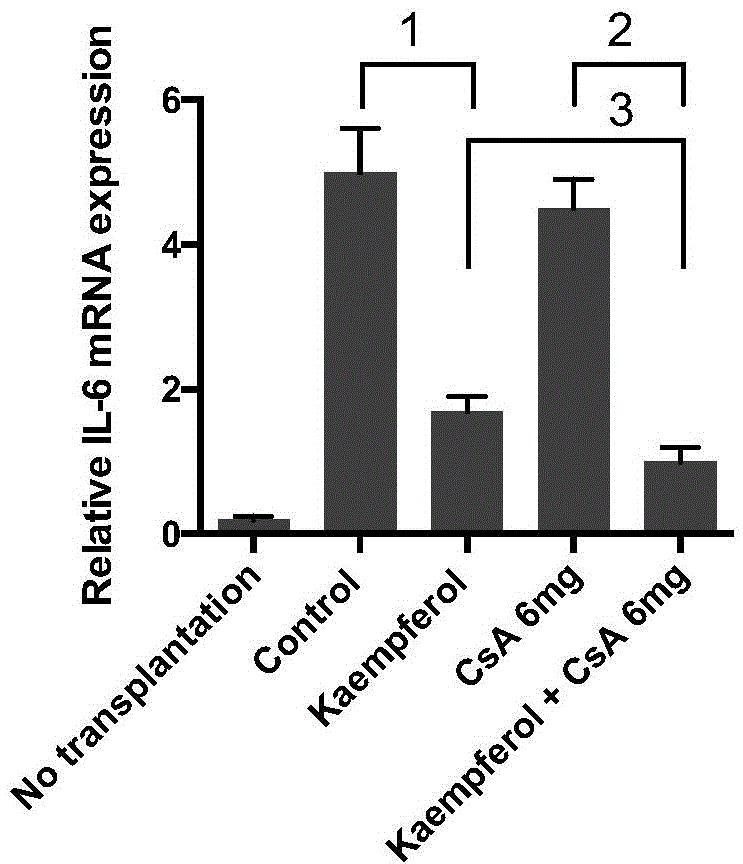
Application of kaempferol in medicines for inhibiting rejection reaction of receptor on organ transplantation
InactiveCN104434908AProlong survival timeLong-term activityOrganic active ingredientsCyclic peptide ingredientsSide effectPharmacologic action
The invention discloses an application of kaempferol in medicines for inhibiting rejection reaction of a receptor on organ transplantation. The invention discloses pharmacological effects of kaempferol in inhibiting rejection reaction of organ transplantation in the body of a receptor for the first time; and moreover, optimal effects can be achieved when kaempferol is combined with low-dose cyclosporin for a short time, and long-term survival of an islet graft in the receptor can be induced, so that various side effects caused by using full-dose cyclosporin for a long time can be avoided, the economic burden of patients brought by long-term anti-rejection treatment can also be lightened, and the long-term high-quality life of organ transplantation patients can be further facilitated. The invention also discovers an immune adjustment action mechanism of kaempferol, namely that kaempferol can be used for significantly increasing the number of regulatory T lymphocytes in the body of the receptor and reducing the gene expression of an islet graft IL-6 in the body of the receptor, so that the effects of resisting inflammation and resisting rejection reaction can be achieved.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Method for detecting cyclosporine pharmaceutical preparation and auxiliary materials thereof
PendingCN113804802AEfficient separationImprove stabilityComponent separationPharmaceutical formulationChromatography column
The invention provides a method for detecting a cyclosporine pharmaceutical preparation, which comprises the following steps of: 1) treating the cyclosporine pharmaceutical preparation with a diluent to obtain a cyclosporine pharmaceutical preparation solution to be detected; and (2) detecting the cyclosporine pharmaceutical preparation solution to be detected obtained in the step (1) through using a gel chromatography exclusion method, wherein a chromatographic column adopted in the gel chromatography exclusion method detection is formed by connecting at least two water-soluble GPC chromatographic columns in series. The method can effectively separate povidone, OP-40 and polyoxyethylene hydrogenated castor oil, has the outstanding advantages of being good in stability, easy and convenient to operate, rapid, high in efficiency, good in sensitivity and the like, can achieve effective separation when the concentration of povidone, OP-40 and polyoxyethylene hydrogenated castor oil is low, and can provide a basis for further research on specific properties of any two or three of povidone, OP-40 and polyoxyethylene hydrogenated castor oil, such as cyclosporin eye drops.
Owner:WUHAN WUYAO SCI & TECH
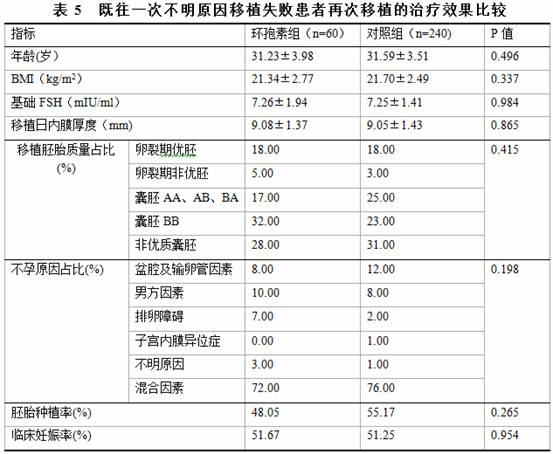
Application of cyclosporine A in preparation of medicine for improving embryo implantation rate of patient suffering from repeated embryo implantation failure
ActiveCN113769065AImprove biological behaviorImprove planting rateCyclic peptide ingredientsSexual disorderBiotechnologyEmbryo transplantation
The invention discloses application of cyclosporine A in preparation of a medicine for improving the embryo implantation rate of a patient suffering from repeated embryo implantation failure. In the prior art, the cyclosporine A is used as an immunosuppressor or a medicine for preventing miscarriage and preventing abortion, the application proposes that the cyclosporine A can improve the embryo planting rate for the first time, and particularly discloses improvement of the embryo planting rate of secondary embryo transplantation of the patient suffering from the repeated embryo implantation failure, and the research result is different from that in the prior art. Specifically, according to the technical scheme, clinical application of the cyclosporine A can be effectively guided, and side effects caused by improper medication are avoided.
Owner:SUZHOU MUNICIPAL HOSPITAL
Method for constructing immune healthy synovial sarcoma xenograft mouse model
ActiveCN110637783AImproved issues with no immune systemCompounds screening/testingAnimal husbandrySynovial sarcomaTumour immunotherapy
The invention provides a method for constructing an immune healthy synovial sarcoma xenograft mouse model; the method comprises cultivating a generation P0, to be specific, resuscitating a tumor tissue block of a synovial sarcoma patient stored in a liquid nitrogen environment, then transplanting the tumor tissue block into an immunodeficient mouse under a sterile condition, and pretreating a micewith normal immunity by cyclosporine; culturing a generation Px, to be specific, inoculating the tumor tissue block, grown in a mouse from any of the generations P0 to Px, into the mouse pretreated with cyclosporine to form a generation Px mouse; and culturing a generation Py, to be specific, inoculating a synovial sarcoma tumor tissue block, grown in a mouse from any of the generations Px to Py-1, into the mouse with normal immunity until tumor tissues grow in the mouse, so that culturing of the generation Py is completed. The invention solves the problem that the traditional PDX (patient-derived xenograft) model has no immune system, provides an in-vivo model for the research on tumor immunotherapy, and widens the application range of the PDX model.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Chitin derivative and its preparation and its uses in preparation of medicines
InactiveCN1269844CLow toxicityNo hemolytic reactionOrganic active ingredientsChitosan derivativeToxin
A natural and biodegradable N-long chain alkyl-O-sulfochitosan, its preparing process, and its application as nano carrier which features low toxin, no hemolytic reaction and solubilization action to the medicine difficult to dissolve are disclosed. It can be used to prepare intravenous injection, such as the micellar medicine containing the component difficult to dissolve: taxol (or cyclosporin A) polymer.
Owner:南京亚东启天药业有限公司 +1
Preparation process for cyclosporine A-pH sensitive nanoparticle
InactiveCN104434804AThe preparation process is fastImprove conversion ratePowder deliveryCyclic peptide ingredientsPh sensitive nanoparticlesOrganosolv
Belonging to the field of chemical technologies, the invention relates to a preparation process for a cyclosporine A-pH sensitive nanoparticle, and more specifically relates to improvement of the preparation process of the cyclosporine A-pH sensitive nanoparticle. The preparation process for the cyclosporine A-pH sensitive nanoparticle is characterized by low cost and high synthesis rate, and the product has good intestinal absorption effect. The preparation process for the cyclosporine A-pH sensitive nanoparticle includes the steps of: weighing F68 and dissolving it in 125mL distilled water to serve as the water phase; dissolving 50mg of CyA and a proper amount of EudragitS100 in anhydrous ethanol to prepare an organic phase; injecting the organic phase into the water phase stirred at a speed of 400r.min with a No. 7 support marrow puncture needle quickly; and then conducting stirring continuously and transferring the mixture into 60DEG C water bath, and volatilizing the organic solvent completely, thus obtaining a CyA-NP colloidal solution.
Owner:李志刚
Cyclosporine formulations for use in the treatment of bronchiolitis obliterans syndrome (BOS)
ActiveUS20210077573A1Preventing and delaying progressionDispersion deliveryInorganic non-active ingredientsInhalationCiclosporin
The present invention relates to a composition comprising cyclosporine A (CsA) for use in the prevention of bronchiolitis obliterans syndrome (BOS) in a double lung transplanted patient, or for the treatment of BOS or for the prevention or delay of the progression of BOS in a double lung transplanted patient being diagnosed with BOS, wherein the composition is administered to said patient by inhalation of said composition in aerosolized form comprising a therapeutically effective dose of cyclosporine A.
Owner:BREATH THERAPEUTICS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com