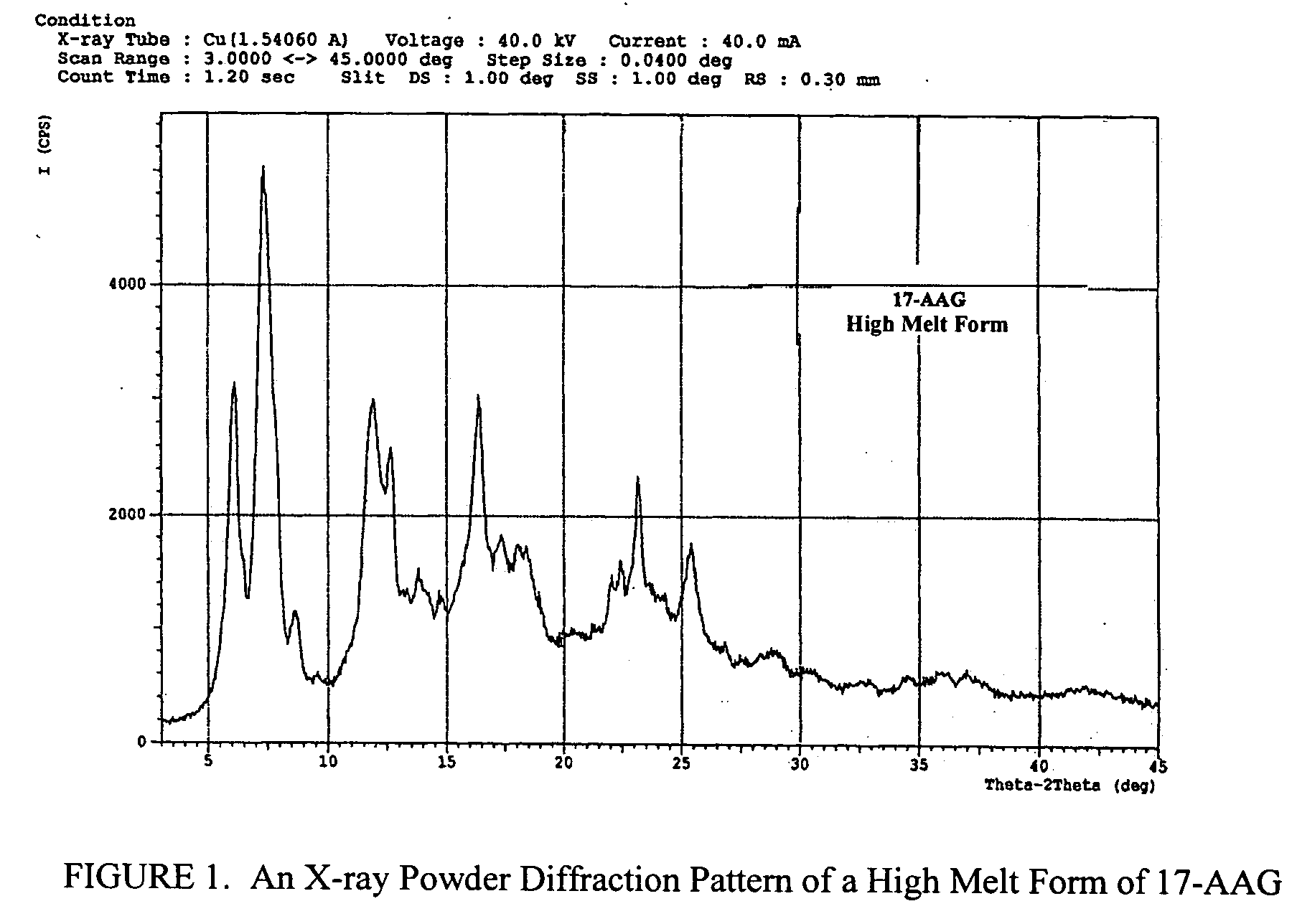
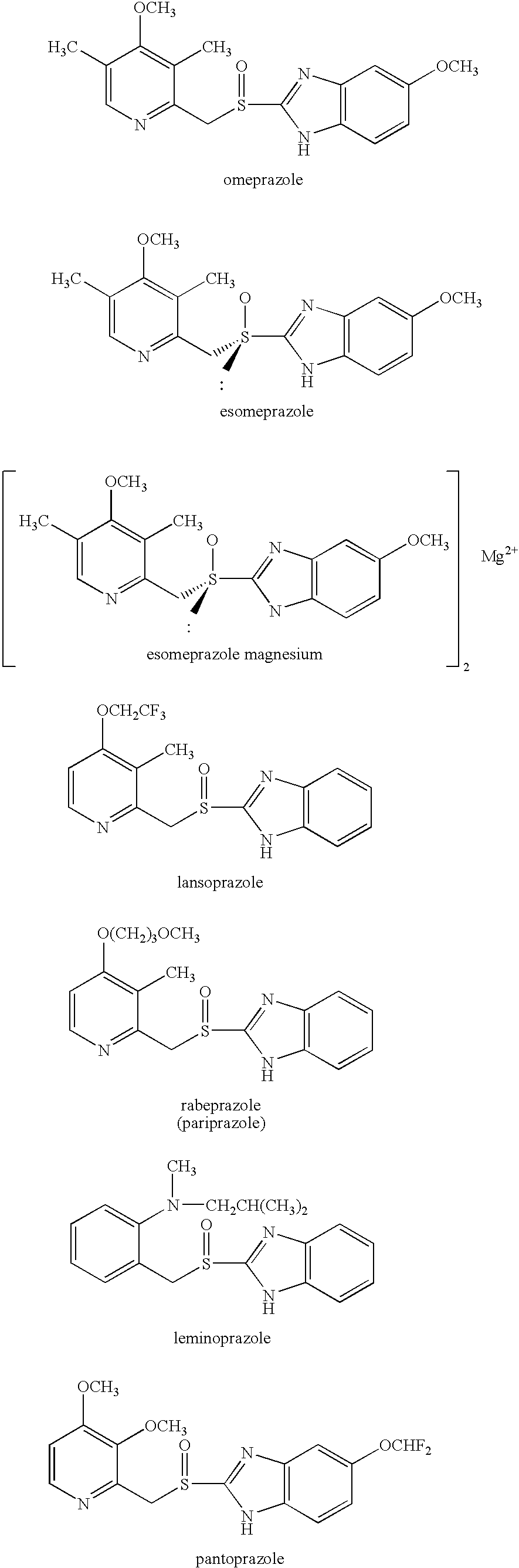
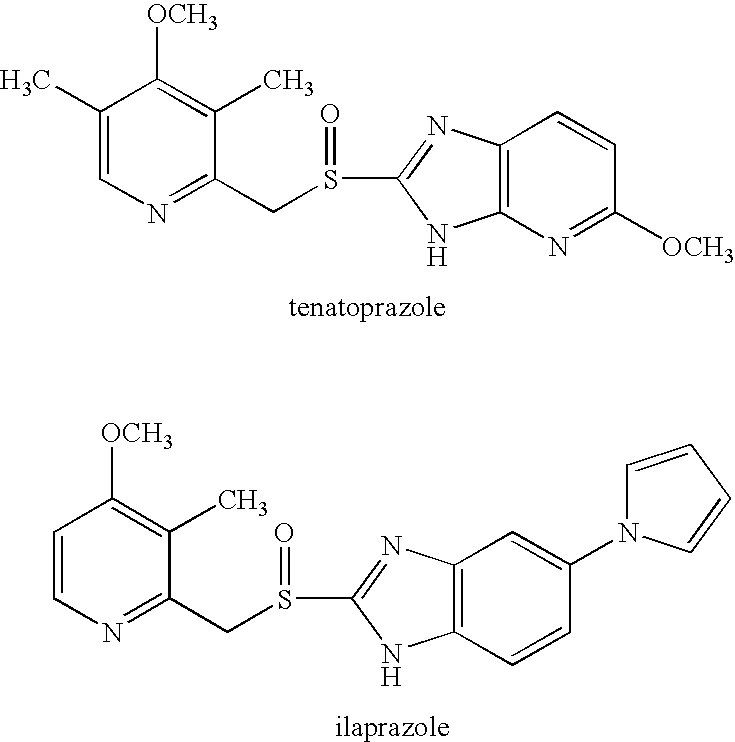
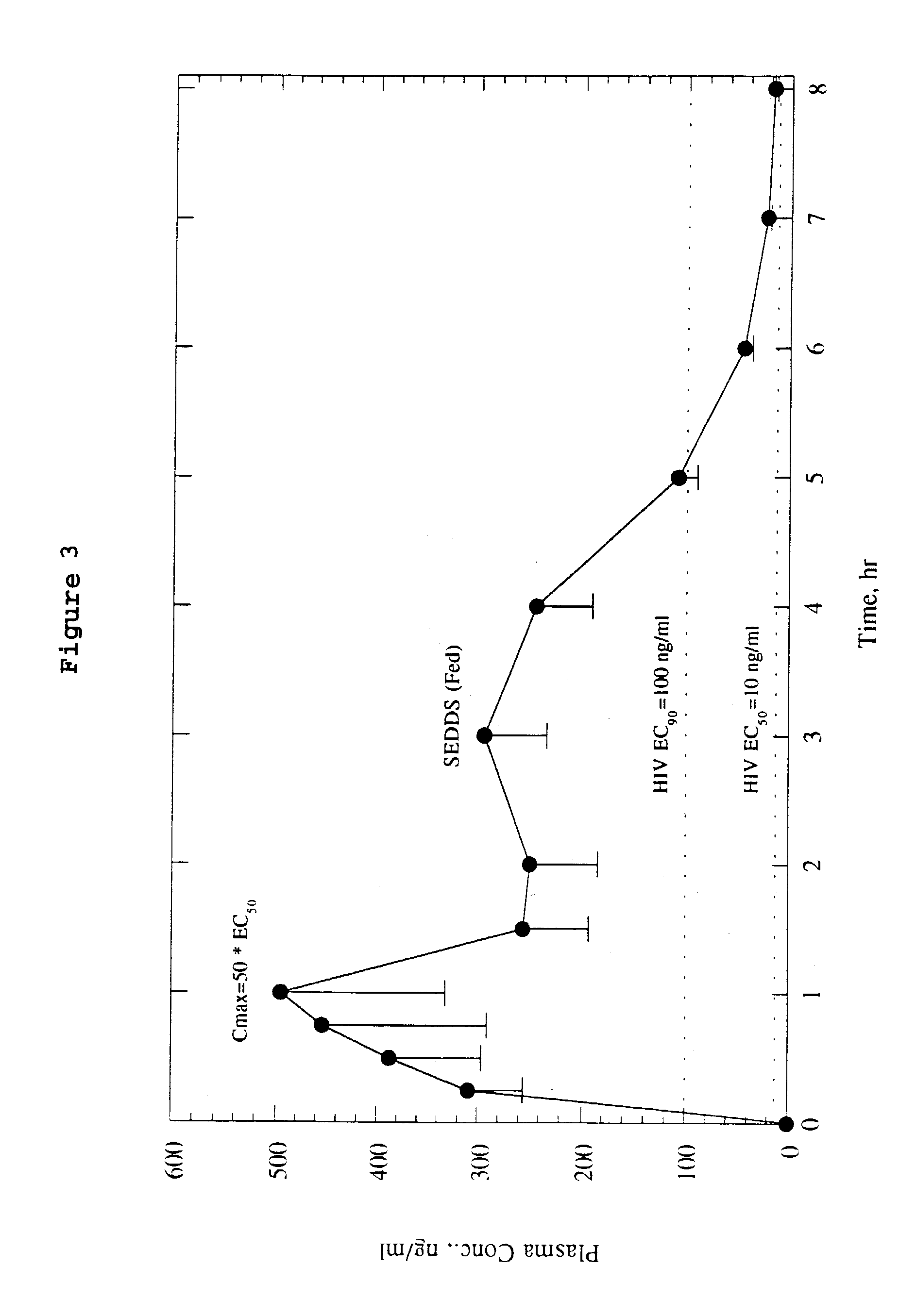
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
537 results about "Oral drug preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Oral pharmaceuticals formulation comprising paclitaxel, derivatives and methods of administration thereof
InactiveUS20040092428A1Improve bioavailabilityImprove oral bioavailabilityOrganic active ingredientsCyclic peptide ingredientsOral medicationBioavailability
The invention concerns excipients or combinations thereof suitable for preparing an oral formulation containing a pharmaceutical agent. More particularly, the invention is directed to stable, efficacious and bioavailable oral pharmaceutical formulations comprising paclitaxel, derivatives of paclitaxel and pharmaceutically acceptable salts thereof. The formulations of the invention increase bioavailability of paclitaxel when dissolved in the gastrointestinal system. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts of such derivatives to patients in need thereof. The formulations of the invention are particularly suitable for oral administration to mammals including humans.
Owner:TRANSFORM PHARMACEUTICALS INC
Oral pharmaceutical formulations containing non-steroidal anti-inflammatory drugs and acid inhibitors
InactiveUS20070154542A1AntipyreticDigestive systemSide effectNonsteroidal Antiinflammatory Drugs/NSAIDs
The present disclosure provides enteric coated capsules and orally dissolving films comprising non-steroidal anti-inflammatory drugs and acid inhibitors, as well as methods of treating treatment humans for pain and / or inflammation while reducing gastrointestinal side effects.
Owner:HORIZON PHARMA USA
Oral medicine for treating cardio-cerebral vascular disease and preparation process thereof
InactiveCN1502337AReduce the number of daily dosesMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismOral medicineDisease
The present invention discloses a slowly-released oral medicine preparation which is made up by using breviscapine as main raw material and has the functions of promoting blood circulation and removing blood stasis, removing obstruction in the channels to relieve pain for curing angiocardiopathy and cerebrovascular disease with obvious therapeutic effect and its preparation method. Said slowly-released tablet (by one tablet) contains 60-120 mg of breviscapine, 20-75 mg of diluent, 50-150 mg of filling agent and 10-50 mg of slow release material. Said invention also provides its preparation method and concrete steps.
Owner:CHENGDU LIST PHARMA
Oral pharmaceutical formulations for antidiabetic compounds
InactiveUS20100087481A1Improve solubilitySurprising chemical stabilityBiocideAntipyreticDiseaseEnergy homeostasis
Oral pharmaceutical preparations of salts and polymorphs of a compound useful in the treatment of inflammatory and metabolic conditions and diseases are provided herein. The oral pharmaceutical preparation is useful for the treatment or prevention of conditions and disorders associated with energy homeostasis such as type II diabetes, lipid metabolism, adipocyte differentiation and inflammation.
Owner:LEE KATHLEEN M
Pharmaceutical formulation of omeprazole
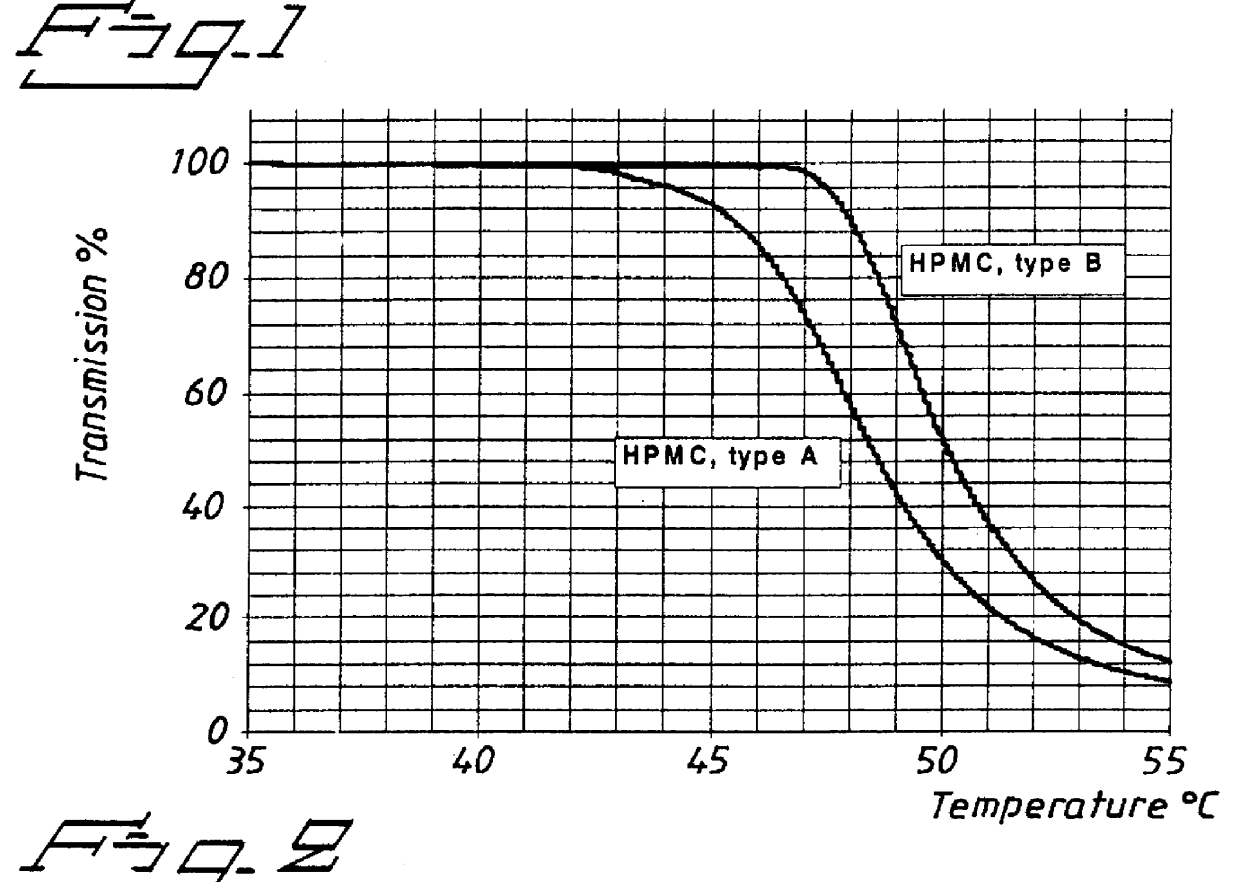
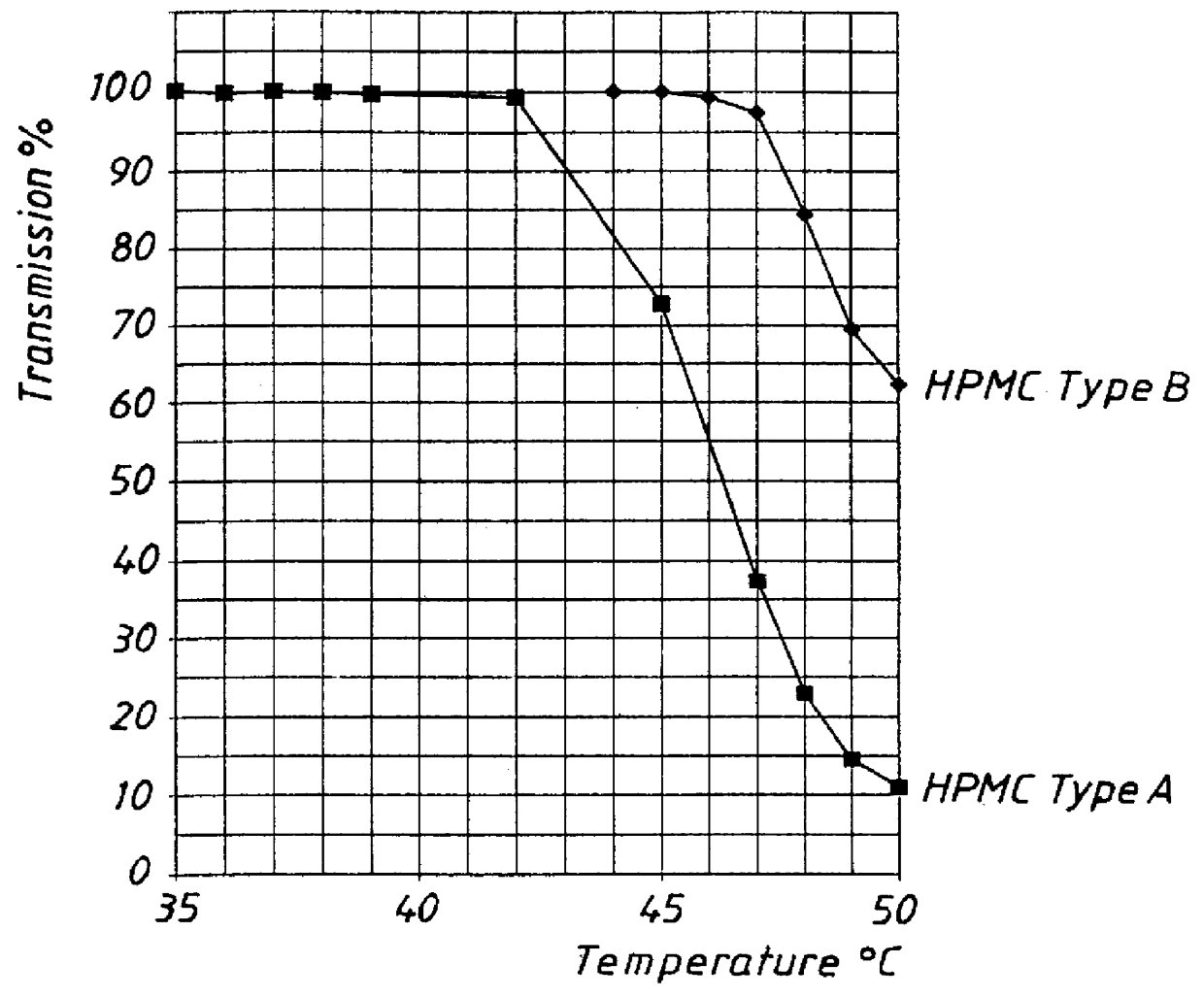
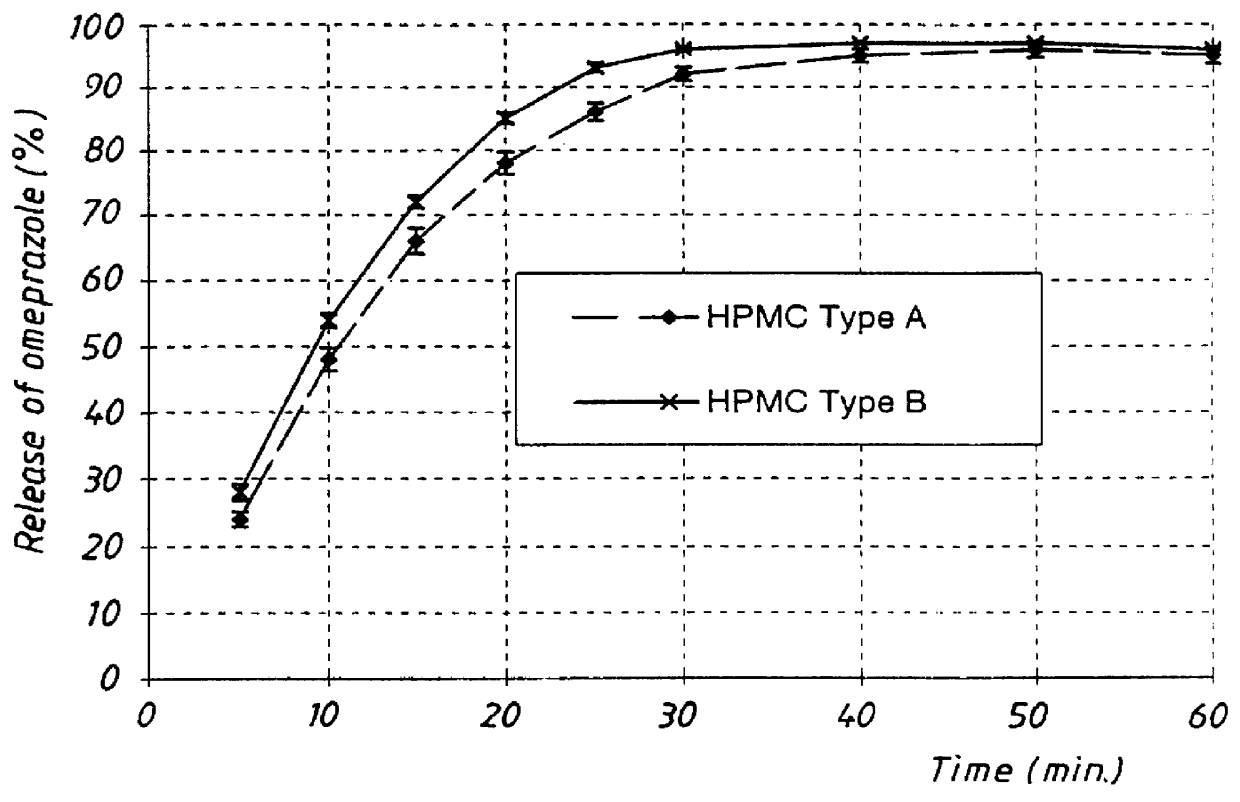
PCT No. PCT / SE98 / 00922 Sec. 371 Date Jun. 8, 1998 Sec. 102(e) Date Jun. 8, 1998 PCT Filed May 18, 1998 PCT Pub. No. WO98 / 53803 PCT Pub. Date Dec. 3, 1998An enteric coated oral pharmaceutical formulation comprising as active ingredient a compound selected from the group of omeprazole, an alkaline salt of omeprazole, the (-)-enantiomer of omeprazole and an alkaline salt of the (-)-enantiomer of omeprazole, wherein the formulation comprises a core material of the active ingredient and optionally an alkaline reacting compound, the active ingredient is in admixture with a pharmaceutically acceptable excipient, such as for instance a binding agent, and on said core material a separating layer and an enteric coating layer. A hydroxypropyl methylcellulose (HPMC) of low viscosity with a specific cloud point is used in the manufacture of pharmaceutical formulations. Furthermore, the application describes the processes for their preparation and the use of the claimed formualtions in medicine.
Owner:ASTRAZENECA AB
New Combination Dosage Form
InactiveUS20070122470A1Salicyclic acid active ingredientsBiocideGastrointestinal complicationsSalicylic acid
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Oral pharmaceutical formulations and methods for producing and using same
InactiveUS20060067953A1Reduce the environmentLow toxicityBiocideSolution deliveryCompound (substance)Phospholipid
Owner:CONFORMAL THERAPEUTICS CORP (US)
Oral pharmaceutical preparation for colon-specific delivery
InactiveUS20100209520A1Sufficient therapeutic effectPrecision releaseBiocideNervous disorderInter layerAnionic polymers
The present invention relates to an oral pharmaceutical preparation having an excellent capability of delivering a drug to colon, more specifically an oral pharmaceutical preparation for delivering a drug to colon and comprising a core comprising at least a pharmaceutically acceptable vehicle, an inner layer covering said core and comprising said drug, an intermediate layer covering said inner layer and comprising a cationic polymer soluble or swellable at a pH of not more than 6.6, and an outer layer covering said intermediate layer and comprising an anionic polymer soluble at a pH of not less than 7.0.
Owner:AMATERASPHARMA INC
Medicinal composition for preventing or treating metabolic syndrome
ActiveCN1891229AImprove resistance statusReduce microalbuminuriaOrganic active ingredientsMetabolism disorderPharmaceutical formulationExcipient
The present invention relates to a medicine, preparation for preventing and curing metabolic syndrome. Said oral preparation includes biguanide sugar-reducing medicine or its medicinal salt, vitamins B or its related compound with similar bio-activity and medicinal carrier or excipient. Besides, said invention also provides its preparation method and concrete steps.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Sustained-release, oral pharmaceutical formulations and methods of making and using same
A sustained-release, oral pharmaceutical formulation of tramadol comprising a compound formed in situ of tramadol or a tramadol salt and a pharmaceutically acceptable acidic substance. The compound formed in situ has a desired water solubility. Also provided are methods of treatment using the pharmaceutical formulations. Method for preparing such formulations are also provided. The preparation method comprises repeatedly mixing tramadol or its salt with the acidic substance, and moistening the mixture and formulating the mixture under an energy input, such as heat or pressure. Optionally, drying, repeated granulating, extrudation and pelleting may also be included.
Owner:GRUNENTHAL GMBH
Oral Pharmaceutical Dosage Form Comprising as Active Ingredients a Proton Pump Inhibitor together with Acetyl Salicyclic Acid
InactiveUS20100178334A1Salicyclic acid active ingredientsBiocideSalicylic acidGastrointestinal complications
The present invention relates to an oral pharmaceutical preparation for use in the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid. The present preparation comprises a fixed oral dosage form comprising a proton pump inhibitor in combination with acetyl salicylic acid. Furthermore, the present invention refers to a method for the manufacture thereof and the use thereof in medicine. The present invention also relates to a specific combination comprising esomeprazole, or an alkaline salt thereof or a hydrated form of any one of them, and acetyl salicylic acid for use as a medicament for the prevention of thromboembolic vascular events, such as myocardial infarction or stroke, and for the prevention and / or reduction of gastrointestinal complications associated with the use of acetyl salicylic acid.
Owner:ASTRAZENECA AB
Self-emulsifying drug delivery system
Oral pharmaceutical formulation which improves the bioavailability of pharmaceuticals which are substantially water and oil insoluble is disclosed. In addition to the pharmaceutical, the formulation includes an emulsifier, an oil and an solubilizer. Alternatively, the formulation includes an aqueous solution of solubilizer.
Owner:GD SEARLE & CO
Traditional Chinese medicinal composition for treating alcoholic fatty liver and preparation method thereof
The invention discloses a traditional Chinese medicinal composition for treating alcoholic fatty liver and a preparation method thereof. The traditional medicinal composition contains the following raw materials of: by weight, 15-35 parts of Artemisia capillaries, 15-30 parts of Radix Puerariae, 15-35 parts of Poria cocos, 10-25 parts of couchgrass root, 10-25 parts of Herba Eupatorii, 10-25 parts of cassia seed, 4-10 parts of green tea, 5-12 parts of haw, 5-12 parts of red sage root, 5-12 parts of sweet wormwood, 5-12 parts of cortex lycii, 8-15 parts of burdock, 4-10 parts of Caulis Bambusae In Taeniam, 4-10 parts of polygonatum rhizome, 4-10 parts of ligustrum lucidum, 4-10 parts of Ligusticum wallichii and 2-8 parts of licorice. The traditional Chinese medicinal composition can be made into any clinically acceptable oral medicinal preparation including pill, particulate agent, capsules or tablet and the like. It shows through clinical observation result that the traditional Chinese medicinal composition has substantial curative effects for alcoholic fatty liver. Safety observation indicates that the traditional Chinese medicinal composition is safe in clinic usage and has no toxic and side effect.
Owner:黄文珍
Trimetazidine formulation with different release profiles
InactiveUS20110274751A1Reduce treatment onset timeAvoid stickingOrganic active ingredientsPill deliveryControl releaseImmediate release
A multilayered solid oral pharmaceutical formulation of trimetazidine or a pharmaceutically acceptable salt or polymorph of trimetazidine wherein one layer of said formulation provides controlled release, while the other layer provides immediate release.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Oral nicotine formulation buffered with amino acid
InactiveCN101795710ASolve the problem of bad tasteOrganic active ingredientsNervous disorderNicotine dependenceParkinson's disease
A pharmaceutical oral formulation for delivering nicotine in any form to a subject by transmucousal uptake in the oral cavity comprising nicotine in any form, wherein said oral formulation is buffered with at least one amino acid, preferably at least one endogenous amino acid. Also contemplated is a method for the oral delivery of nicotine in any form, a method for the reduction of the urge to smoke or use tobacco as well as methods for manufacturing the oral formulation, the use of said oral formulation for obtaining transmucousal uptake of nicotine in the oral cavity of a subject, and use of nicotine for the production of an oral formulation as per above for the treatment of a disease selected from the group consisting of tobacco or nicotine dependence, Alzheimer's disease, Crohn's disease, Parkinson's disease, Tourette's syndrome, ulcerous colitis and post-smoking-cessation weight control.
Owner:MCNEIL AB
Stable pharmaceutical formulations
A stable oral pharmaceutical formulation comprising ramipril or its pharmaceutically acceptable salt and a stabilizing amount of an ammoniomethacrylate copolymer in a pharmaceutically acceptable carrier medium is described.
Owner:SUN PHARMA INDS
Oral pharmaceutical formulation comprising cannabinoids and poloxamer
PendingUS20210059976A1Improve bioavailabilityTotalPowder deliveryNervous disorderPharmaceutical formulationCannabidiol
The present invention relates to a novel cannabinoid oral pharmaceutical dosage form, based on a Type IV or Type IV-like formulation, as classified using the Lipid Formulation Classification System. The formulation comprises a combination of at least two cannabinoids. The first cannabinoid is selected from the group consisting of tetrahydrocannabinol (THC) and analogues thereof; and the second cannabinoid is selected from the group consisting of cannabidiol (CBD) and analogues thereof.
Owner:GW RES LTD
Oral pharmaceutical preparations decreased in bitterness by masking
InactiveUS7727552B1Suppress bitternessBitter tastePowder deliveryNervous disorderOral medicineCarrageenan
A composition of an oral medicine or an oral medicine which can prevent an unpleasant taste of the medicine is herein disclosed. It is granules, powders, syrups and the like which is prevented from an unpleasant taste, comprising a basic medicine having an unpleasant taste and an anionic polymer such as carrageenan.
Owner:EISIA R&D MANAGEMENT CO LTD
Oral medicinal preparation of tadalafil
ActiveCN103191075AImprove complianceImprove bioavailabilityOrganic active ingredientsPill deliveryTadalafilOrally disintegrating tablet
The invention relates to an oral medicinal preparation of tadalafil and application of the preparation in treatment of male erectile dysfunction. The preparation is characterized in that the main medicne is tadalafil or salt thereof, and the particular formulation can be orally disintegrating tablets, chewable tablets, oral membranes and other preparations capable of being quickly released in the oral cavity; tadalafil is an insoluble medicament, and is low in bioavailability, so that the administrated infective dose is large, multiple adverse responses can be generated, and visual impairment or loss can be caused due to unreasonable administration. Regarding the researches of tadalafil medicinal preparation for improving medicament bioavailability and reducing occurrence of adverse responses, the wide clinical application of tadalafil in treatment of male erectile dysfunction is particularly important. Aiming at the fact that the tadalafil medicinal preparation brings great favor to broad ED patients in improvement of the compliance when the tadalafil medicinal preparation is taken by a patient, particularly in administration in an environment without water and in a non-swallow mode. The oral medicinal preparation is prepared through massive studies based on patients.
Owner:NANJING CHIA TAI TIANQING PHARMA
Oral pharmaceutical formulations of acid-labile active ingredients and process for making same
InactiveUS20050031696A1Inhibit gastric acid secretionBiocidePowder deliveryBULK ACTIVE INGREDIENTActive ingredient
An oral pharmaceutical formulation in dosage form of acid-labile compounds, methods of treating using the formulation and a process for its production are described.
Owner:DR REDDYS LAB LTD
Pulsatile release compositions and methods for enhanced intestinal oligonucleotide drug absorption
Delayed release oral pharmaceutical formulations and methods for enhanced intestinal drug absorption. The formulation comprises a first population of carrier particles comprising a drug and a penetration enhancer which are released at a first location in the intestine, and a second population of carrier particles comprising a penetration enhancer and a delayed release coating or matrix. This penetration enhancer is released at a second location in the intestine downstream from the first location and enhances absorption of the drug when it reaches the second location.
Owner:IONIS PHARMA INC
Fasudic hydrochloride oral formulation
ActiveCN1813762AEasy to useImprove bioavailabilityOrganic active ingredientsPill deliveryDiseaseOral drug preparation
The present invention relates to a fashudil hydrochloride oral preparation for curing ischemic cerebrovascular disease due to cerebrovascular spasm after subarachnoid hemorrhage. It can be made into tablet, capsule and granule preparation.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Moxifloxacin oral preparation and preparation method thereof
ActiveCN101890169ALow priceEasy to buyAntibacterial agentsOrganic active ingredientsMedicineMoxifloxacin
The invention relates to an oral pharmaceutical preparation containing moxifloxacin, salts and / or hydrates of the moxifloxacin, soluble starch and pregelatinized starch. The preparation is characterized by containing 2.9%-14.5% of soluble starch and 1.4%-6.5% of pregelatinized starch, wherein all percents are calculated on the basis of the weight of the pharmaceutical preparation. The invention also relates to a preparation method of the preparation and an application of the preparation for treating or preventing bacterial infection of people or animals.
Owner:JIANGSU YABANG QIANGSHENG PHARMA
Experimental apparatus for simulating in-vivo dissolution and absorption process of oral drug preparation
InactiveCN108088971AEnhanced flushing actionIncrease medium flow rateTesting medicinal preparationsMedicineDissolution
The invention discloses an experimental apparatus for simulating an in-vivo dissolution and absorption process of an oral drug preparation. The experimental apparatus comprises a dissolution pool, wherein a drug basket frame is arranged inside the dissolution pool in a suspending manner, a liquid inlet on the bottom of the dissolution pool is communicated with an outlet of a confluence valve by virtue of a liquid inlet main path, a liquid outlet connector inserted in the dissolution pool transports a dissolution medium in the dissolution pool to a distribution valve in real time by virtue of aliquid outlet main path, the distribution valve is divided into two liquid outlet branches, one liquid outlet branch is separately communicated with a sample collector (for implementing the online automatic sample collection) and a waste liquid collection bottle by virtue of a sample collection three-way valve, the other liquid outlet branch is communicated with one inlet of the confluence valveto form a circulating loop, another inlet of the confluence valve is communicated with a dissolution medium source by virtue of a medium input pipeline, the liquid inlet main path and the liquid outlet main path are respectively provided with a liquid inlet pump and a liquid outlet pump, and the flow rate of the liquid inlet is the same with an output amount of the liquid outlet connector. By adopting the experimental apparatus, the dissolution and absorption process of a drug in a living body can be simulated, and the synchronous evaluation of the dissolution and absorption of the drug can berealized.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
Sustained-release, oral pharmaceutical formulations and methods of making and using same
A sustained-release, oral pharmaceutical formulation of tramadol comprising a compound formed in situ of tramadol or a tramadol salt and a pharmaceutically acceptable acidic substance. The compound formed in situ has a desired water solubility. Also provided are methods of treatment using the pharmaceutical formulations. Method for preparing such formulations are also provided. The preparation method comprises repeatedly mixing tramadol or its salt with the acidic substance, and moistening the mixture and formulating the mixture under an energy input, such as heat or pressure. Optionally, drying, repeated granulating, extrudation and pelleting may also be included.
Owner:GRUNENTHAL GMBH
Chinese medicinal composition for treating toothache and preparation method thereof
InactiveCN101940658ANo adverse reactionReliable clinical useAntipyreticAnalgesicsSide effectGLYCYRRHIZA EXTRACT
The invention discloses a Chinese medicinal composition for treating toothache and a preparation method thereof. The Chinese medicinal composition comprises the following components in part by weight: 10 to 25 parts of Chinese angelica, 8 to 15 parts of rehmanniae vaporata, 4 to 12 parts of asarum, 3 to 10 parts of red paeony root, 2 to 8 parts of dahurian angelica root, 2 to 8 parts of incised notopterygium rhizome, 5 to 15 parts of Szechuan lovage rhizome, 2 to 8 parts of kudzuvine root, 2 to 9 parts of motherwort fruit and 1 to 6 parts of liquorice. The Chinese medicinal composition can be prepared into any clinically acceptable orally-taken medicinal preparation by common methods for preparing Chinese medicaments; and the Chinese medicinal composition has the effects of resisting inflammation and easing pain and dispelling wind and clearing heat. Clinical observation results prove that the Chinese medicinal composition has obvious effect of treating toothache of various syndrome types and can quickly stop pain; and the Chinese medicinal composition also has the advantages of safe clinical application and no toxic or side effect, and can address both the symptoms and root causes.
Owner:黄文珍
Pioglitazone hydrochloride sustained-release dropping pill and preparation method thereof
InactiveCN101269040AIncrease surface areaHas a wetting effectOrganic active ingredientsMetabolism disorderPharmaceutical formulationBlood drug concentration
The invention discloses to a drug compound for treating diabetes and particularly relates to a drug compound oral pharmaceutical formulation adopting pioglitazone as the ingredient. The drug compound aims to supplement the deficiency of the prior oral pharmaceutical formulation used for treating Type-2 Diabetes and provide a drug compound oral pharmaceutical formulation, sustained-release pioglitazone dropping pill which has high bioavailability, controllable release time, long-acting effect, low frequency of drug taking, steady plasma concentration, low cost and absence of contamination during the production. The sustained-release pioglitazone dropping pill adopts pioglitazone as the chemical ingredient and is prepared jointly with the medicinal carriers of hydrophilic frame ingredients and hydrophobic frame ingredients used as the stroma.
Owner:北京博智绿洲医药科技有限公司
Traditional Chinese medicine composition for treating hemorrhoids and preparation method thereof
InactiveCN102000175ASafe for clinical useRapid hemostasisAnthropod material medical ingredientsCardiovascular disorderOral medicineSide effect
The invention discloses a traditional Chinese medicine composition for treating hemorrhoids and a preparation method thereof. The traditional Chinese medicine composition comprises the following ingredients in parts by weight: 10 to 25 parts of cimicifuga foetida, 8 to 16 parts of radix bupleuri, 10 to 20 parts of radix scutellariae, 4 to 10 parts of rhubarb, 4 to 10 parts of angelica, 2 to 8 parts of sanguisorba, 2 to 8 parts of glabrous sarcandra herb, 1 to 6 parts of yerbadetajo herba ecliptae and 1 to 6 parts of liquorice. The traditional Chinese medicine composition can be prepared into any one clinically acceptable oral medicine preparation according to the conventional preparation method of the traditional Chinese medicine preparation, has the effects of removing pathogenic heat from blood, stopping bleeding for convergence and activating blood circulation to dissipate blood stasis, can fast realize the hemostasis convergence on various haemorrhoids such as internal hemorrhoids, external hemorrhoids, mixed hemorrhoids and the like, has exact and reliable curative effect, treats both the secondary and primary symptoms, is safe in clinical application and has no any toxic or side effect.
Owner:黄文珍
Oral drop pill in use for clearing away heat and toxic material and preparation method
InactiveCN1660368AIncrease surface areaHas a wetting effectUnknown materialsAntiinfectivesOral DropsToxic material
A Chinese medicine in the form of dripping pill for treating cold, fever, sore throat, etc is prepared from scutellaria root, forsythia fruit, isatis leaf and liquorice root.
Owner:北京博智绿洲医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com