Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
171 results about "Pioglitazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pioglitazone is a diabetes drug (thiazolidinedione-type, also called "glitazones") used along with a proper diet and exercise program to control high blood sugar in patients with type 2 diabetes.
Solid preparation comprising alogliptin and pioglitazone
ActiveUS20100092551A1High dissolution rateReduce adverse effectsBiocideOrganic active ingredientsDiabetes mellitusAlogliptin
A solid preparation containing compound (I), wherein the definition of compound (I) is as defined in the description, and pioglitazone, which is useful as a therapeutic drug for diabetes and the like and superior in the dissolution property, chemical stability and dissolution stability, is provided. A solid preparation containing the following first and second parts:(1) the first part containing compound (I) or a salt thereof and, as the first excipient, sugar or sugar alcohol; and(2) the second part containing pioglitazone or a salt thereof and, as the second excipient, sugar or sugar alcohol.
Owner:TAKEDA PHARMA CO LTD
Respiratory disease treatment
InactiveUS8236786B2Anti-inflammatory effect of the compound to be achieved more efficientlyNo additional benefitBiocidePowder deliveryEnantiomerInhalation
There is provided a pharmaceutical composition that is adapted for pulmonary administration by inhalation, which composition comprises a glitazone, such as pioglitazone or rosiglitazone, and one or more pharmaceutically acceptable carriers and / or excipients, and wherein the glitazone content of the composition consists of at least 95% by weight of the 5R enantiomer and less than 5% by weight of the 5S enantiomer. There is also provided a use and kit.
Owner:PULMAGEN THERAPEUTICS INFLAMMATION LTD
Methods and drug products for treating alzheimer's disease
Provided herein are drug products with low dose pioglitazone for use in the treatment (e.g., delay of onset) of cognitive impairment of the Alzheimer's type. Methods of manufacture thereof are also provided. Further provided are methods of treatment for Alzheimer's disease including administering a drug product with low dose pioglitazone. The methods may include determining whether the subject is at risk of developing Alzheimer's disease based upon the subject's age and TOMM40 523 genotype.
Owner:ZINFANDEL PHARMA
Slow release capsule of compound metformin pyrrolidone and preparation method
A slow-releasing capsule of compound fluamine pyrrolinone for treating diabetes is composed of the central slow-releasing fluamine microball or small tablet and the coated fast-releasing pyrrolinone layer. Its advantages are high curative effect and no toxic by-effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Coated preparation
ActiveUS20100166853A1Increase maximum blood concentration and AUCReduce deviationBiocideMetabolism disorderSolubilityBULK ACTIVE INGREDIENT
The present invention provides a preparation containing pioglitazone or a salt thereof as an active ingredient, which shows high bioavailability of pioglitazone and less interindividual variation in blood drug concentration, as well as a preparation with suppressed color change during preservation. The preparation contains a core containing a pharmaceutically acceptable organic acid with water solubility at 20° C. of not less than 10 mg / mL and pKa1 (a negative common logarithm of the first acid dissociation constant Ka1) at 25° C. of not more than 5, and a coating layer containing pioglitazone or a salt thereof. The coating layer may further contain mannitol or trehalose.
Owner:TAKEDA PHARMA CO LTD
Application of ferroptosis inhibitor
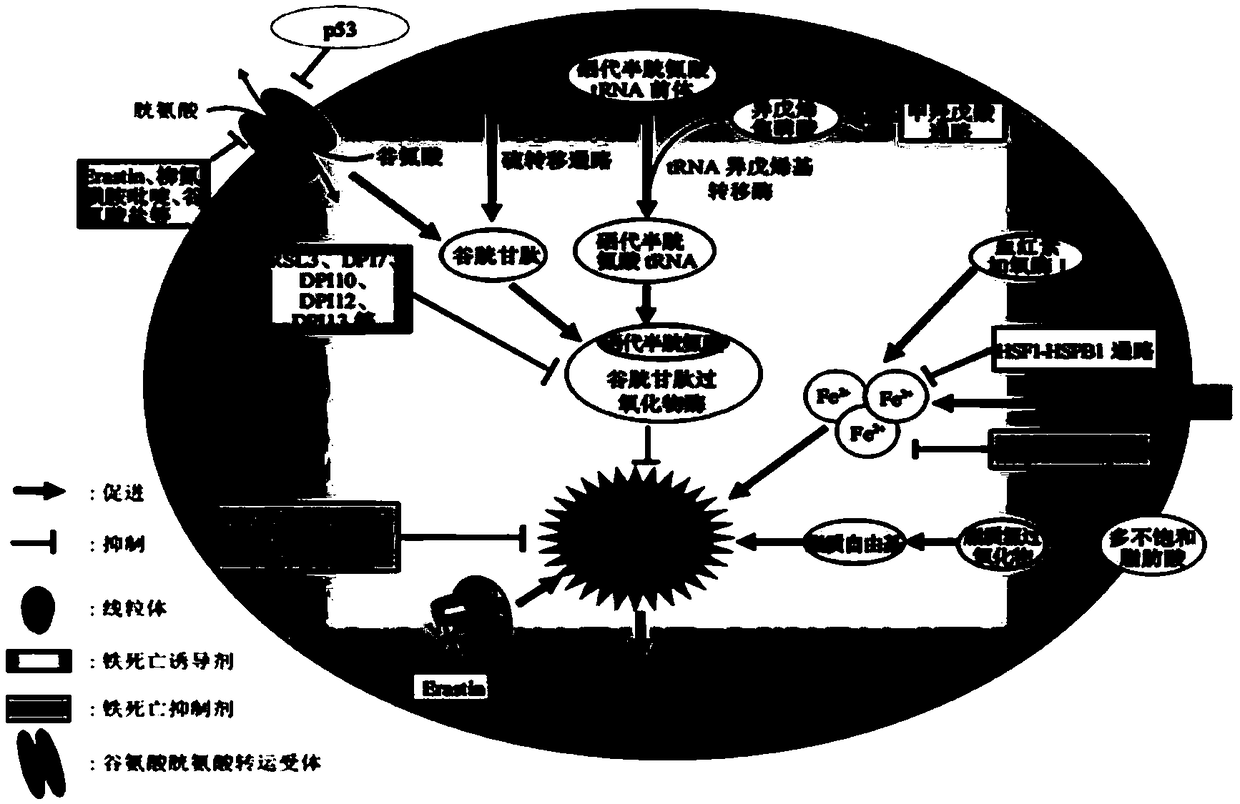
The invention relates to the technical field of medical science, in particular to new application of a ferroptosis inhibitor. The ferroptosis inhibitor is prepared from Liproxstatin-1, Ferrostatin-1,rosiglitazone, pioglitazone, troglitazone and Zileuton. The new application refers to that the inhibitor is applied to treating radiation-induced pulmonary injury diseases. The application has the advantages that (1) radioactive rays trigger radiation-induced pulmonary injury by mediating pulmonary alveoli epithelial cells to have ferroptosis, the ferroptosis specificity inhibitor can be used to prevent and treat radiation-induced pulmonary injury, and has the important meaning on development of new drug for preventing and treating radiation-induced pulmonary injury; and (2) it is found that radioactive rays can cause cell death through the mode of ferroptosis, and application of the ferroptosis inhibitor also has the significant theoretical meaning on guiding research for guiding radioactive rays to treat cancer mechanism.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Biomarkers For Assessing Altherosclerotic Potential
The invention also provides methods, apparatuses and reagents useful for predicting future atherosclerosis based on expression levels of genes selected from the set of 68 genes with differential expression in response to pioglitazone and rosiglitazone. The invention also discloses reagent sets and biomarkers for predicting progression of atherosclerosis induced by anti-diabetic therapy in a subject. In one particular embodiment the invention provides a method for predict whether a compound will induce atherosclerosis using gene expression data from sub-acute treatments.
Owner:ENTELOS HLDG
Method for identifying whether blood-glucose-lowering traditional Chinese medicine contains pioglitazone or rosiglitazone or not
ActiveCN103954608ARapid identificationQuick judgmentRaman scatteringSurface-enhanced Raman spectroscopyGlucose lowering
The invention provides a method for identifying whether a blood-glucose-lowering traditional Chinese medicine contains pioglitazone or rosiglitazone or not. The method comprises the following steps: carrying out thin layer chromatography on a blood-glucose-lowering traditional Chinese medicine to be detected; finding a suspected spot at a corresponding Rf value part of the pioglitazone and the rosiglitazone. The method is characterized by further comprising the following three steps: 1, collecting a series of one-dimensional surface enhanced Raman spectrums of the suspected spot to obtain a dynamic spectrum pattern of the blood-glucose-lowering traditional Chinese medicine to be detected; 2, pre-treating the dynamic spectrum pattern and drawing to obtain a two-dimensional correlation surface enhanced Raman spectrum of the blood-glucose-lowering traditional Chinese medicine to be detected; 3, judging whether the blood-glucose-lowering traditional Chinese medicine contains the pioglitazone or the rosiglitazone or not according to the two-dimensional correlation surface enhanced Raman spectrum of the blood-glucose-lowering traditional Chinese medicine to be detected. According to the technical scheme provided by the invention, whether the blood-glucose-lowering traditional Chinese medicine contains the pioglitazone or the rosiglitazone or not can be identified rapidly; the detection result is accurate and reliable, the cost is low and the operation is convenient.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
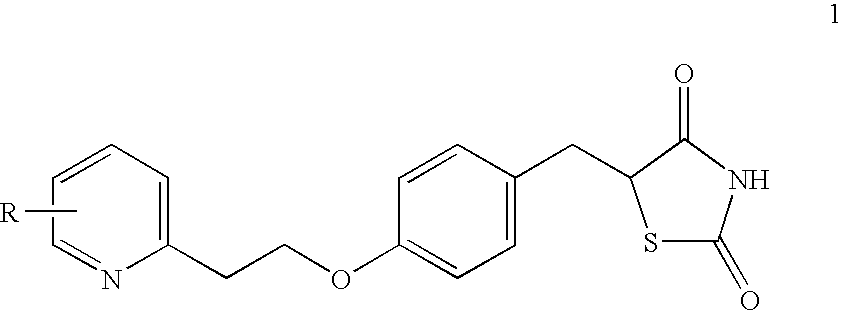
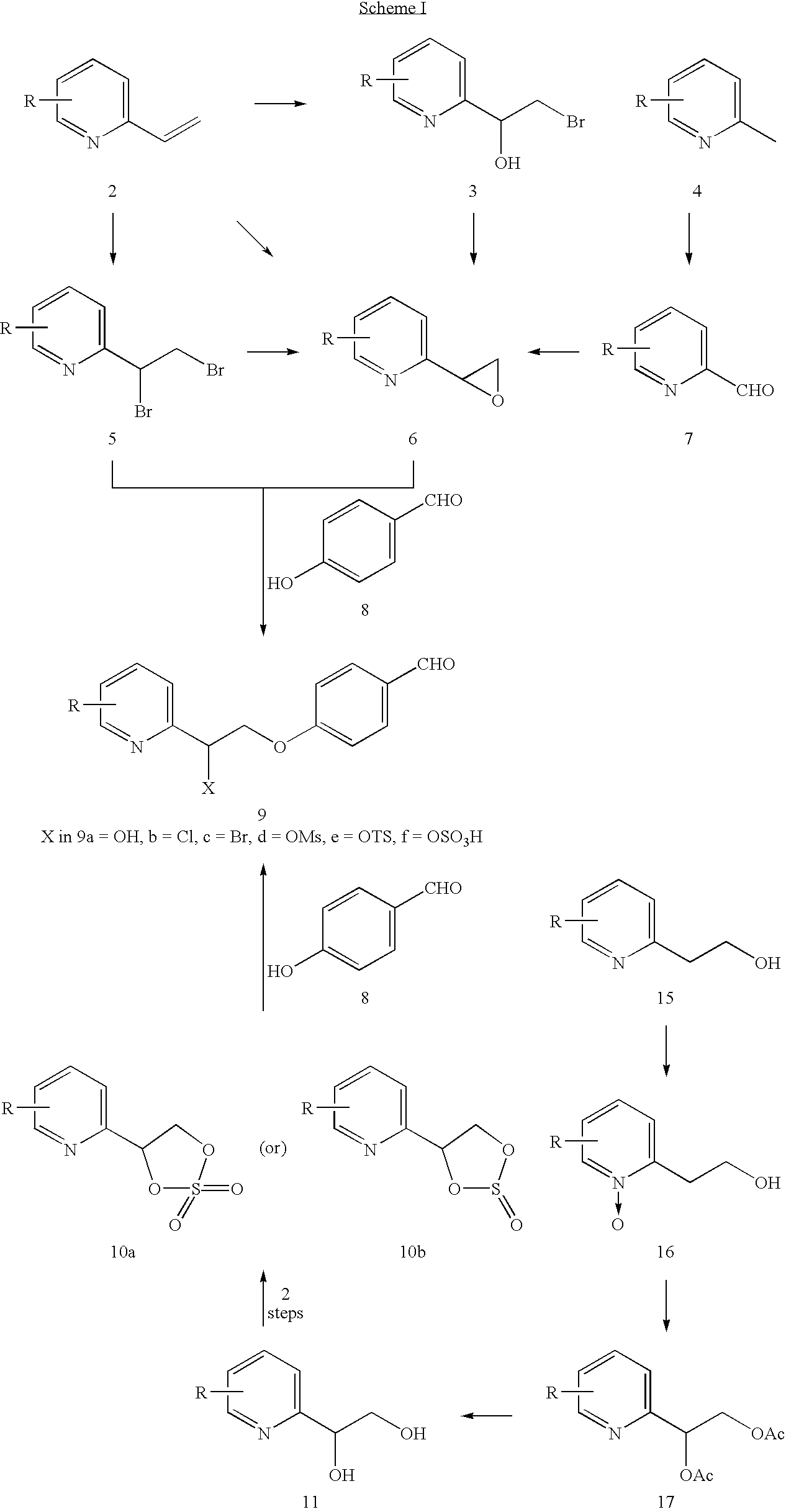
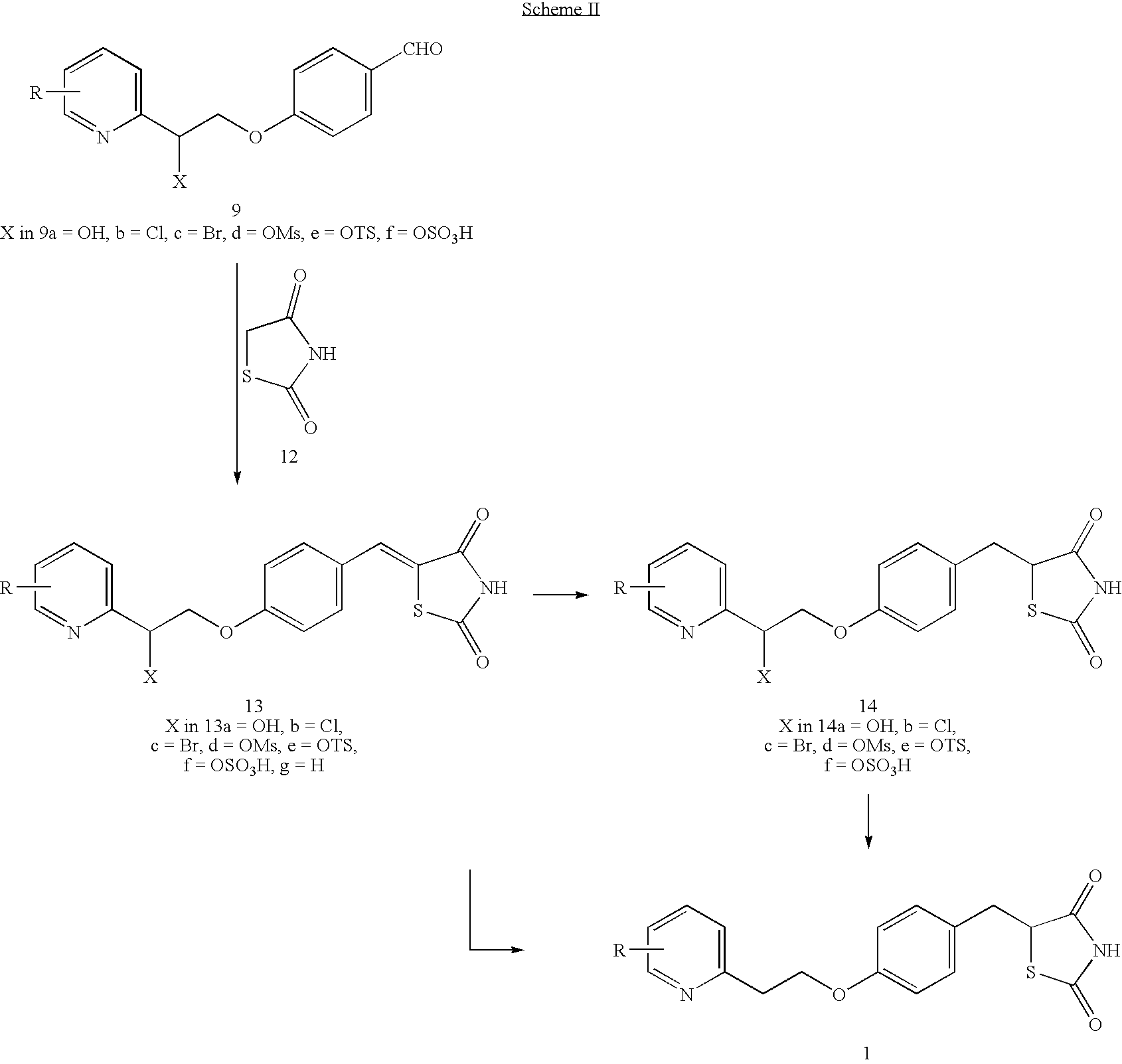
Novel process to prepare pioglitazone via several novel intermediates
InactiveUS20080004446A1High yieldEasy to operateOrganic chemistryMetabolism disorderThiazolidinedioneMedicinal chemistry
A process for preparing thiazolidinediones, preferably pioglitazone, is described. Also described are intermediates involved in synthesizing thiazolidinediones, and processes for preparation and use in medicine.
Owner:CADILA HEALTHCARE LTD
Novel process to prepare pioglitazone via several novel intermediates
InactiveUS20060167061A1High yieldBetter commercial viabilityBiocideOrganic chemistryThiazolidinedioneMedicinal chemistry
A novel process for preparing thiazolidinediones, preferably Pioglitazone, as described. Also described are novel intermediates involved in its synthesis and process for their preparation and use in medicine.
Owner:CADILA HEALTHCARE LTD
Respiratory disease treatment
InactiveUS20110053986A1Anti-inflammatory effect of the compound to be achieved more efficientlyNo additional benefitBiocidePowder deliveryEnantiomerInhalation
There is provided a pharmaceutical composition that is adapted for pulmonary administration by inhalation, which composition comprises a glitazone, such as pioglitazone or rosiglitazone, and one or more pharmaceutically acceptable carriers and / or excipients, and wherein the glitazone content of the composition consists of at least 95% by weight of the 5R enantiomer and less than 5% by weight of the 5S enantiomer. There is also provided a use and kit.
Owner:PULMAGEN THERAPEUTICS INFLAMMATION LTD
Culture medium for inducing adipogenic differentiation of muscle derived stem cells of skeletal muscles, and application and adipogenic differentiation method thereof
ActiveCN106754664AIncreased rate of adipogenic differentiationCulture processSkeletal/connective tissue cellsDexamethasoneMethyl xanthine
The invention relates to the field of medicine, and discloses a culture medium for inducing adipogenic differentiation of muscle derived stem cells of skeletal muscles, and an application and an adipogenic differentiation method thereof. The culture medium disclosed by the invention comprises 3-isobutyl-1-methylxanthine, insulin, indomethacin, dexamethasone, pioglitazone and FBS in a basic culture medium. The components of the culture medium are optimized and are induced to differentiate, and the conventional component in the existing mesenchymal stem cells is replaced by pioglitazone, so that an effect of significantly improving the adipogenic differentiation rate of the muscle derived stem cells of skeletal muscles is realized.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Fine particle size pioglitazone
The present invention provides pioglitazone of defined particle size distribution. The Pioglitazone of defined particle size can be formulated into a wide variety of dosage forms.
Owner:TEVA PHARM USA INC
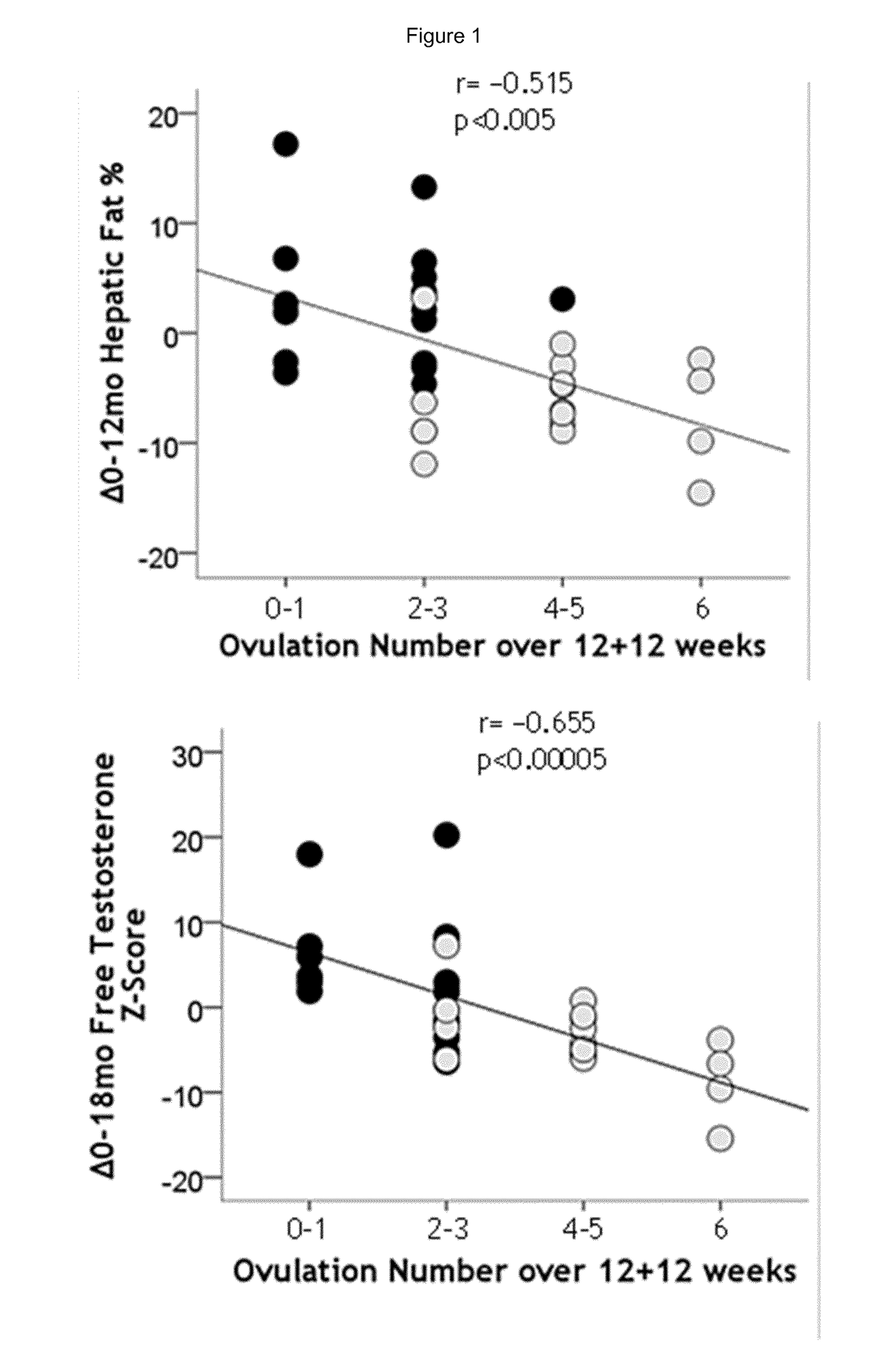
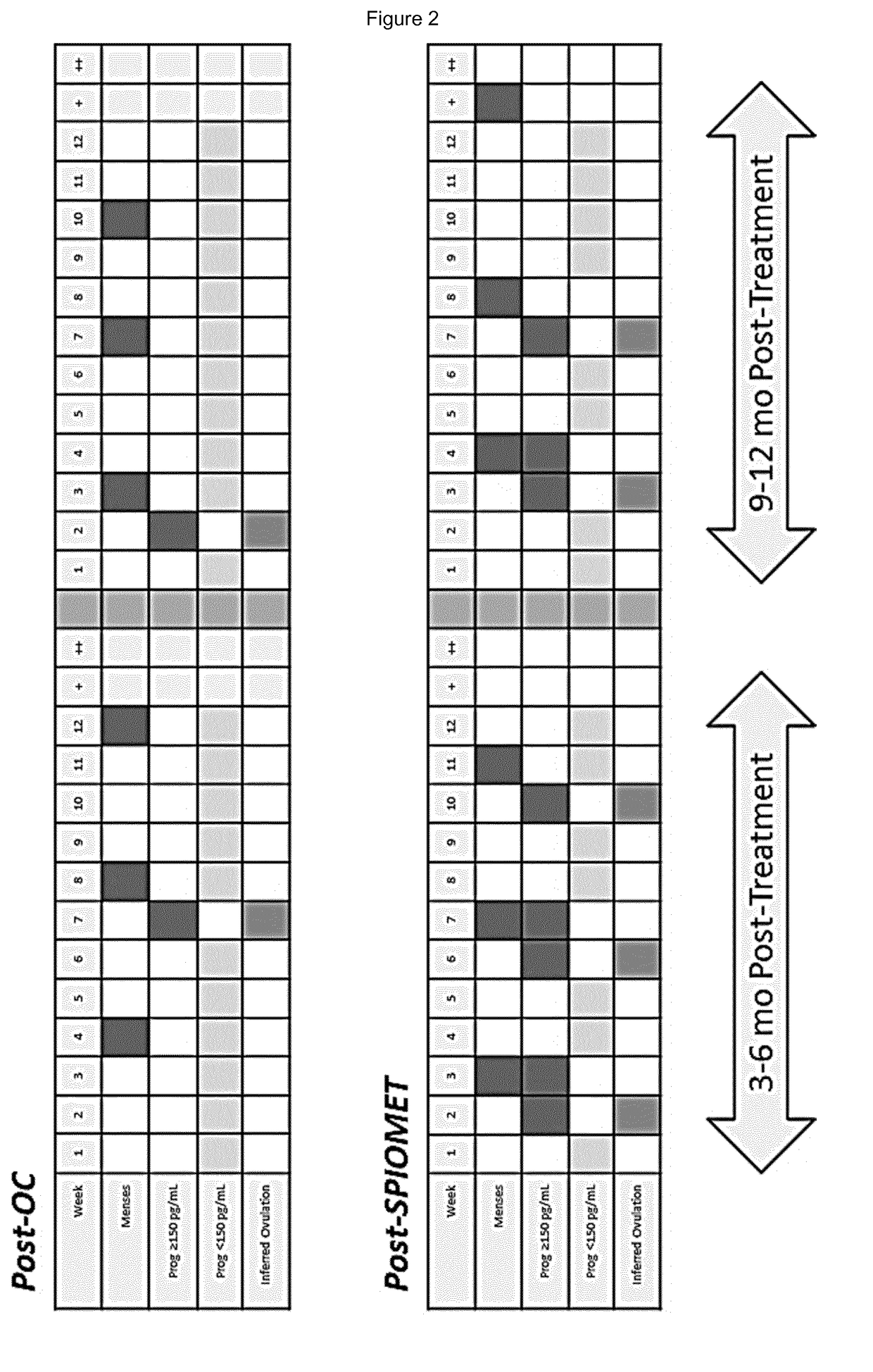
Treatment of hepatic steatosis related oligo-ovulation
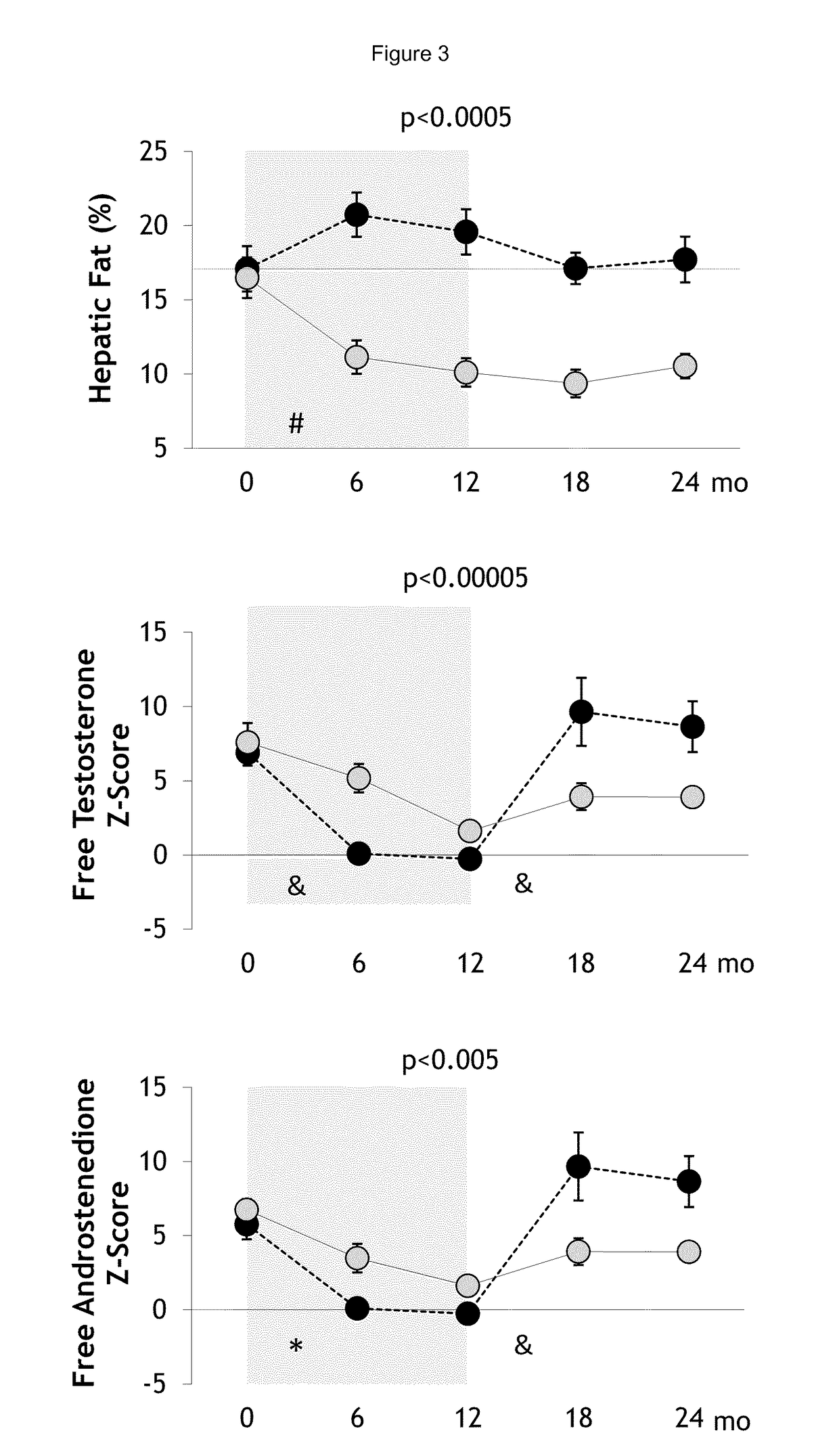
PendingUS20190060328A1Decreased visceralReduce liver fat contentOrganic active ingredientsDigestive systemOvulation timesSteatosis
The present invention relates to a method and composition for use in treating a condition that benefits from the reduction of hepatic and / or visceral fat, such as polycystic ovary syndrome in adolescent girls or women of childbearing age, involving the use of spironolactone, pioglitazone and metformin.
Owner:HOSPITAL SANT JOAN DE DEU +1
Detection method of rosiglitazone or pioglitazone in Chinese patent medicine and health food
The invention relates to a detection method for qualitatively detecting whether thiazolidinedione medicines are doped in a Chinese patent medicine and a health food which have a hypoglycemic effect. The method comprises the following steps: 1, taking a sample to be measured, adding dichloromethane according to a case that 5-10mL of dichloromethane corresponds with 1g of the sample, strongly shaking for at least 40s, and filtering; 2, adding a small amount of a hydrochloric acid solution with the concentration of 0.08-0.12mol / L to a filtrate obtained in step 1, up-down overturning at least eight times, and standing for two-phase layering; and 3, adding a proper amount of a phosphomolybdic acid solution with the concentration of 0.04-0.1g / mL to an upper layer obtained in step 2 drop by drop. If deposition or turbidity appears, it shows that rosiglitazone or pioglitazone is doped in the sample. A corresponding kit comprises a reagent 1: dichloromethane; a reagent 2: the hydrochloric acid solution with the concentration of 0.08-0.12mol / L; and a reagent 3: the phosphomolybdic acid solution with the concentration of 0.04-0.1g / mL. The detection method of the invention has the advantages of accuracy and reliability, and the kit is convenient for carrying and carrying out onsite detection.
Owner:广东省药品检验所
New purpose of pharmaceutical composition containing pioglitazone and heparin or low molecular heparin
ActiveCN101884643AHighlight substantiveSignificant progressOrganic active ingredientsDigestive systemFatty liverPyrrole
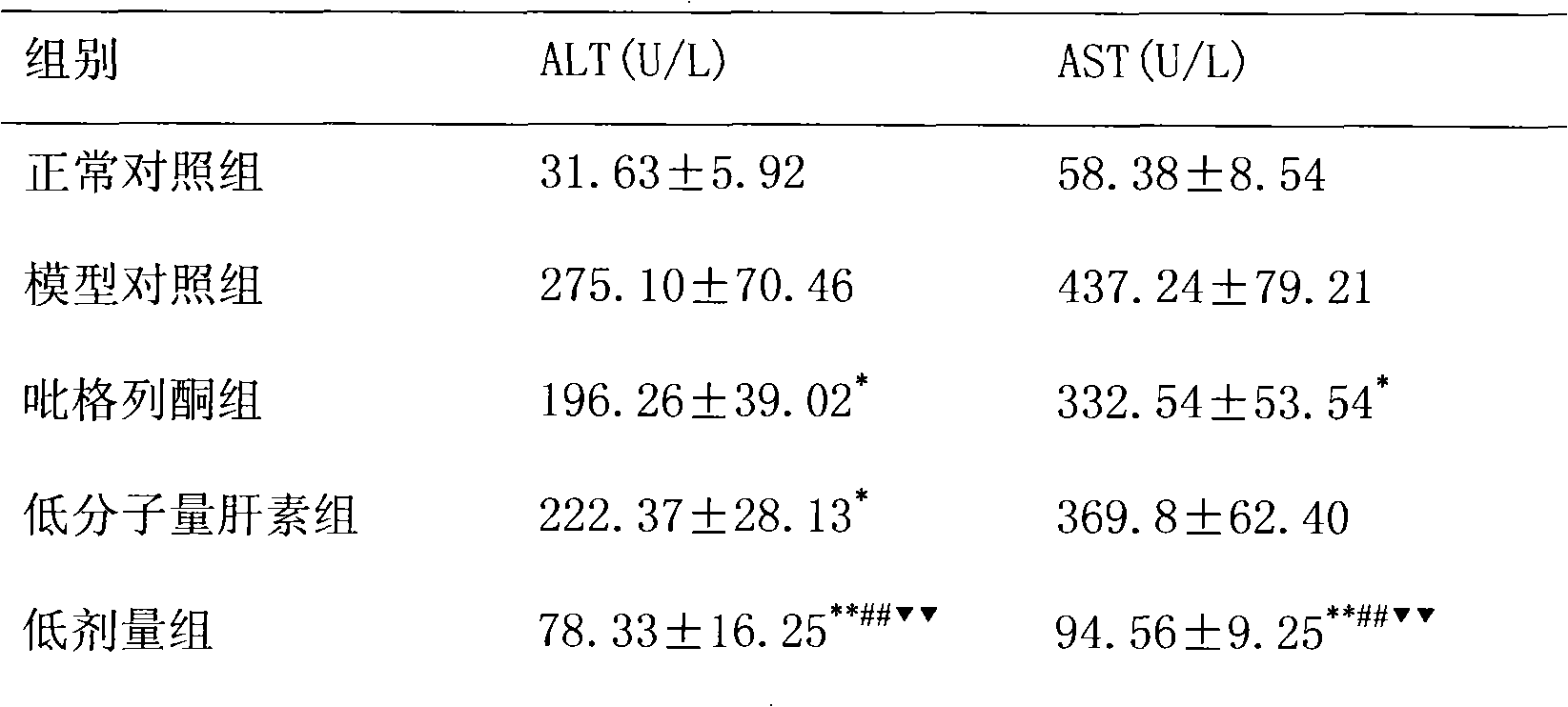
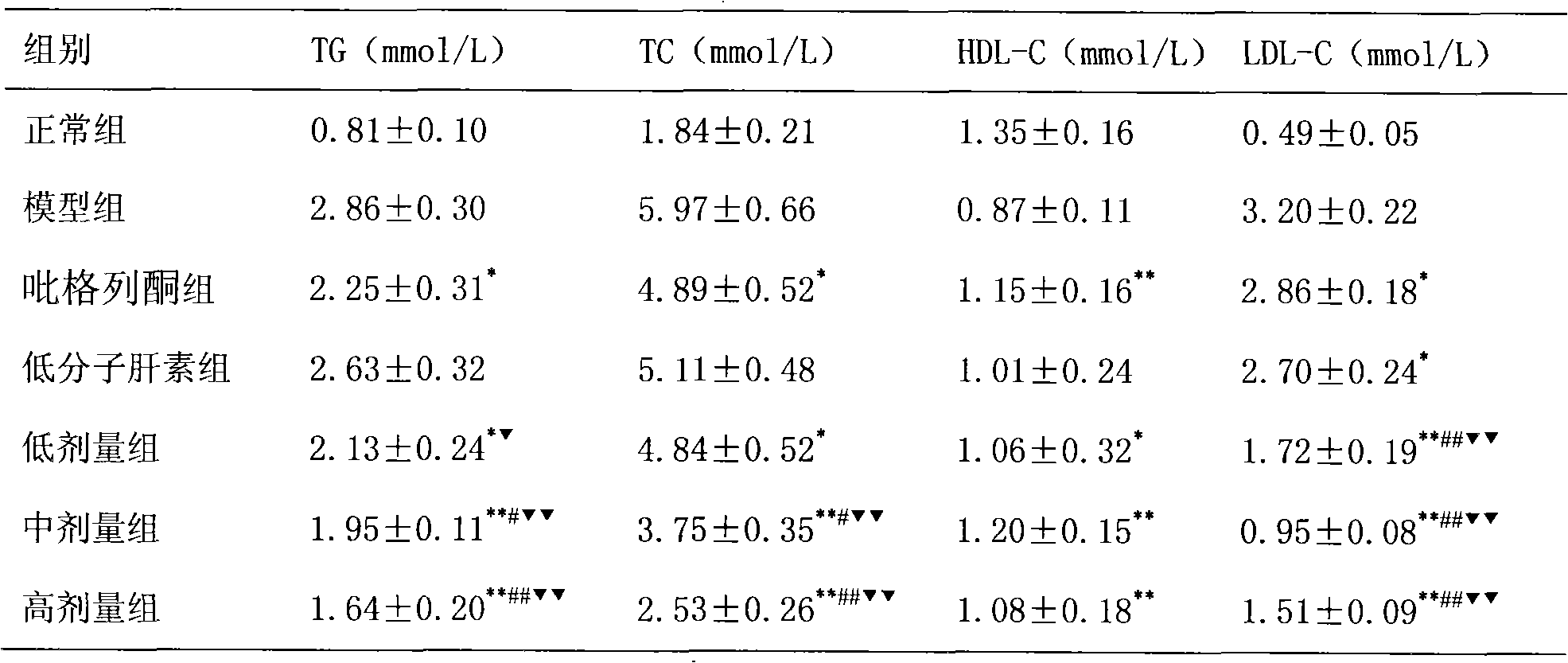
The invention relates to the application of pioglitazone and heparin or low molecular heparin or pharmaceutically acceptable salts thereof or derivatives thereof to preparing a pharmaceutical composition, in particular to the application to preparing a medicament for preventing or treating fatty liver. the purpose of the pioglitazone and the heparin or the low molecular heparin or the pharmaceutically acceptable salts thereof in lessening fat deposition in rat hepatocytes and the synergy of a pioglitazone and heparin or low molecular heparin compound in preventing or treating the rat fatty liver are proved by animal tests. The invention provides a new candidate medicament for searching a medicament for lessening fat deposition in hepatocytes and treating diseases caused by clinic lipid metabolism, such as fatty liver, and the like and therefore enriches the prior art.
Owner:LUNAN PHARMA GROUP CORPORATION
Methods and drug products for treating Alzheimer's disease
Provided herein are drug products with low dose pioglitazone for use in the treatment (e.g., delay of onset) of cognitive impairment of the Alzheimer's type. Methods of manufacture thereof are also provided. Further provided are methods of treatment for Alzheimer's disease including administering a drug product with low dose pioglitazone. The methods may include determining whether the subject is at risk of developing Alzheimer's disease based upon the subject's age and TOMM40 523 genotype.
Owner:ZINFANDEL PHARMA
A process for the preparation of substituted phenyl ether compounds and rosiglitazone
A novel process for the preparation of a compound of the formula (II), which is useful as intermediate compound for the preparation of thiazolidinedione derivatives, such as rosiglitazone, pioglitazone, troglitazone and ciglitazone, is disclosed. The novel process comprising reacting a compound of the formula (III) with a compound of the formula (IV) in a mixture of a non-polar water immiscible organic solvent and water (two phase system) with an alkali metal hydroxide or an alkali metal carbonate as a base in the presence of a phase transfer catalyst. In the first aspect of the present invention comprising reacting 2-(N-methyl-N-(2- pyridyl) ethanol with 4-fluorobenzaldehyde in the mixture of a non-polar water immiscible organic solvent, preferably toluene, and water with an alkali metal hydroxide or an alkali metal carbonate as a base, preferably potassium hydroxide, in the presence of a phase transfer catalyst, e.g. tetra n-butylammonium hydrogensulphate or benzyltriethylammonium chloride, to obtain 4-[2-(N-methyl-N-(2- pyridyl)amino)ethoxy]benzaldehyde, which is the key intermediate for preparing rosiglitazone and its salts, e.g. maleate salt or phosphate salt, useful in the treatment of Type II diabetes.
Owner:SANDOZ AG
Method for preparing metformin hydrochloride controlled-release pellet preparation
InactiveCN103417496AUniform absorption rateSmall body bioavailabilityOrganic active ingredientsMetabolism disorderSide effectClinical efficacy
The invention relates to a method for preparing a metformin hydrochloride controlled-release pellet preparation. A metformin hydrochloride controlled-release pellet is composed of a drug-contained pellet core and a controlled-release layer. The drug-contained pellet is composed of metformin hydrochloride, a filling agent and an adhesive. The controlled-release layer is composed of a controlled-release material and a pore-foaming agent. The metformin hydrochloride controlled-release pellet is mixed with pioglitazone hydrochloride controlled-release pellets to obtain the compounded metformin hydrochloride / pioglitazone hydrochloride controlled-release pellet preparation which achieves the ideal effect of regulating a blood sugar level, can reduce toxic and side effects of drugs, can achieve the effect of continuously and stably reducing the blood sugar level in the body, and facilitate improvement of the compliance of a patient. The compounded metformin hydrochloride controlled-release pellet preparation has high bioavailability and stable blood concentration. Clinical effects are effectively improved and a better dosage regimen and a better treatment effect are provided for domestic and overseas diabetics.
Owner:中国人民解放军第150中心医院
Processes for making thiazolidinedione derivatives and compounds thereof
A compound of the formula: wherein A represents a ring group connected to the oxygen atom by a C1 to C6 hydrocarbon chain, R is hydrogen or a C1-C4 alkyl, and Q is hydrogen, or an amine protecting group such as acetyl, trifluoroacetyl, benzoyl, benzyl, or trityl, is useful in making thiazolidinedione derivatives such as pioglitazone, rosiglitazone and troglitazone.
Owner:SYNTHON BV
Pioglitazone hydrochloride
Provided is a novel crystal form of pioglitazone hydrochloride and a method for making it. Also provided is a method for making a known crystal form of pioglitazone hydrochloride.
Owner:ETHICON ENDO SURGERY INC
Pioglitazone hydrochloride
Provided is a novel crystal form of pioglitazone hydrochloride and a method for making it. Also provided is a method for making a known crystal form of pioglitazone hydrochloride.
Owner:TEVA PHARM USA INC
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
Pharmaceutical composition for treating diabetes and complication thereof
ActiveCN102228457AImprove blood sugarLower blood fatOrganic active ingredientsNervous disorderDiseaseDiabetic complication
The invention discloses a pharmaceutical composition for treating diabetes and complication thereof, and particularly relates to a composition containing arctigenin and PioglitaZone or pharmaceutically acceptable salts thereof and to the purpose of the composition in preparing medicines for treating diabetes, diabetic complication and diseases related to diabetes.
Owner:江苏叠石桥家纺产业集团有限公司
Processes for preparing pioglitazone and its pharmaceutically acceptable salts
InactiveUS20090118514A1Simple and cost-effectiveEasy to operateOrganic chemistryNitrateCalcium Chloride Hexahydrate
Owner:DR REDDYS LAB LTD +1
Pioglitazone hydrochloride solid dispersoid and medicinal compound thereof as well as preparation methods and applications thereof
InactiveCN102389396AImprove solubilityImprove wettabilityOrganic active ingredientsPowder deliveryLiquid stateDissolution
The invention discloses a pioglitazone hydrochloride solid dispersoid and a medicinal compounds thereof as well as preparation methods and applications thereof. The pioglitazone hydrochloride solid dispersoid contains pioglitazone hydrochloride and PEG6000, with the weight ratio of 1:1. 5-9. The preparation method of the pioglitazone hydrochloride solid dispersoid comprises the following steps: A1. heating the PEG6000 to 60-80 DEG C, so as to enable the PEG6000 to be in a liquid state; A2. under the condition of constantly stirring the solution, adding the pioglitazone hydrochloride with proportional amount, stirring and dispersing for 20-40 minutes; and A3. quickly pouring a mixture on a metal panel with fast heat conduction to form a thin solid layer, immediately placing the thin solid layer in an environment with temperature below -20 DEG C, quenching for 50-70 minutes, taking out, smashing and screening with a 100-mesh sieve. The invention has the beneficial effects that the solid dispersoid can greatly improve the dissolution rate of the pioglitazone hydrochloride in a dissolution medium, so as to increase bioavailability. A solid dispersing agent can be further made into the medicinal compounds in forms of tablets and capsules.
Owner:CHENGDU HENGRUI PHARMA
Pioglitazone for use in the treatment of adrenoleukodystrophy
Owner:INSTITUCIO CATALANA DE RECERCA I ESTUDIS AVANCATS +2
Method for obtaining pioglitazone as an antidiabetic agent
A method for obtaining antidiabetic of formula (I), wherein the method comprises condensing of a 4-derivatized phenol or phenolate of general formula (II), wherein R is an amino group-containing organic residue, selected from the group comprising a residue of the following formula —NHR3, wherein R3 is hydrogen or a protecting group, which is removed before further treatment, and a residue of general formula (A), wherein Rb represents a carboxy group either in the free acid form or in the form of a salt or ester or another functional derivative or the nitrile group CN, and M represents a hydrogen or alkali metal atom, with a pyridine base of general formula (III, wherein Z is a leaving group other than a halogen, wherein, before or after carrying out the condensation, the following operations are carried out: (a) diazotizing the amino group present in organic residue R; (b) converting the diazotised residue R into a derivative of 2-halopropionate or 2-halopropionitrile of formula (B), wherein Rb is as defined above and X is a halogen; (c) cyclizing the derivative of 2-halopropionate or 2-halopropionitrile with thiourea; (d) hydrolysing the resulting imine thus giving pioglitazone of formula (I)
Owner:ZENTIVA AS
Processes for making thiazolidinedione derivatives and compounds thereof
The present invention provides a compound of the formula (I): wherein A represents a ring group connected to the oxygen atom by a C1 to C6 hydrocarbon chain, R is hydrogen or a C1-C4 alkyl, and Q is hydrogen, or an amine protecting group such as acetyl, trifluoroacetyl, benzoyl, benzyl, or trityl, is useful in making thiazolidinedione derivatives (formula (II)), such as pioglitazone, rosiglitazone and troglitazone.
Owner:SYNTHON BV
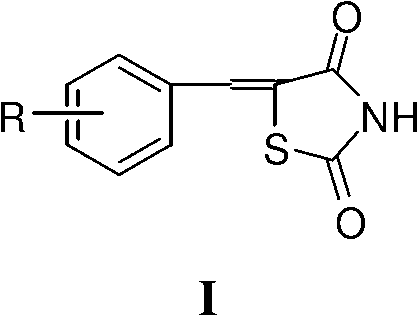
Application of 5- aryl (heterocycle) methylenethiazolidine-2,4-dione in preparation of PPAR (Peroxisome Proliferator Activated Receptor) agonist
InactiveCN102727489APossesses PPAR agonistic activityAgonistic activityOrganic active ingredientsAntimycoticsDiabetes mellitusAryl
The invention provides application of 5-arylmethylenethiazolidine-2,4-dione shown as the general formula I and 5-heterocyclemethylenethiazolidine-2,4-dione shown as the general formula II in preparation of a PPAR (Peroxisome Proliferator Activated Receptor) agonist, wherein 5-arylmethylenethiazolidine-2,4-dione and 5-heterocyclemethylene-thiazolidine-2,4-dione both has PPAR agonistic activity, the relative agonist ratio of a part of compounds to pioglitazone which is an existing PPARgamma agonist is higher than 100% and can be 239.77% at most, and therefore the part of compounds can be developed into high-efficiency low-toxicity antidiabetic drug possibly or can be used as antidiabetic leading molecules to be applied to the further structural optimization.
Owner:SOUTHWEST UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com