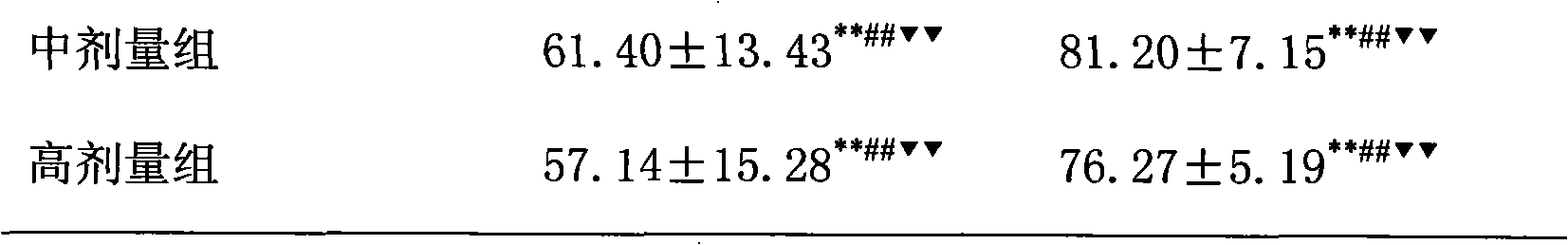New purpose of pharmaceutical composition containing pioglitazone and heparin or low molecular heparin
A technology of low molecular weight heparin and pioglitazone hydrochloride, applied in the field of medicine, to achieve the effect of reducing fat deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
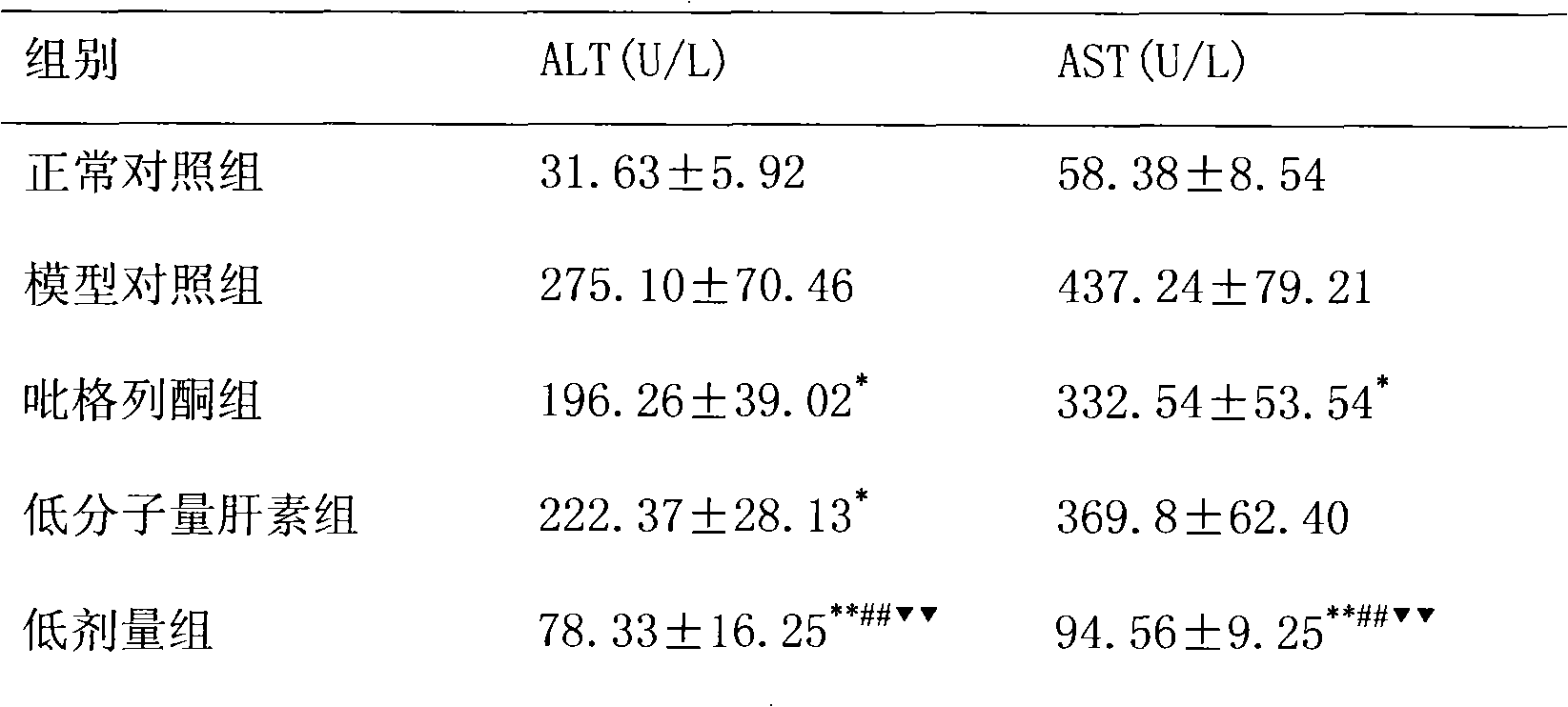
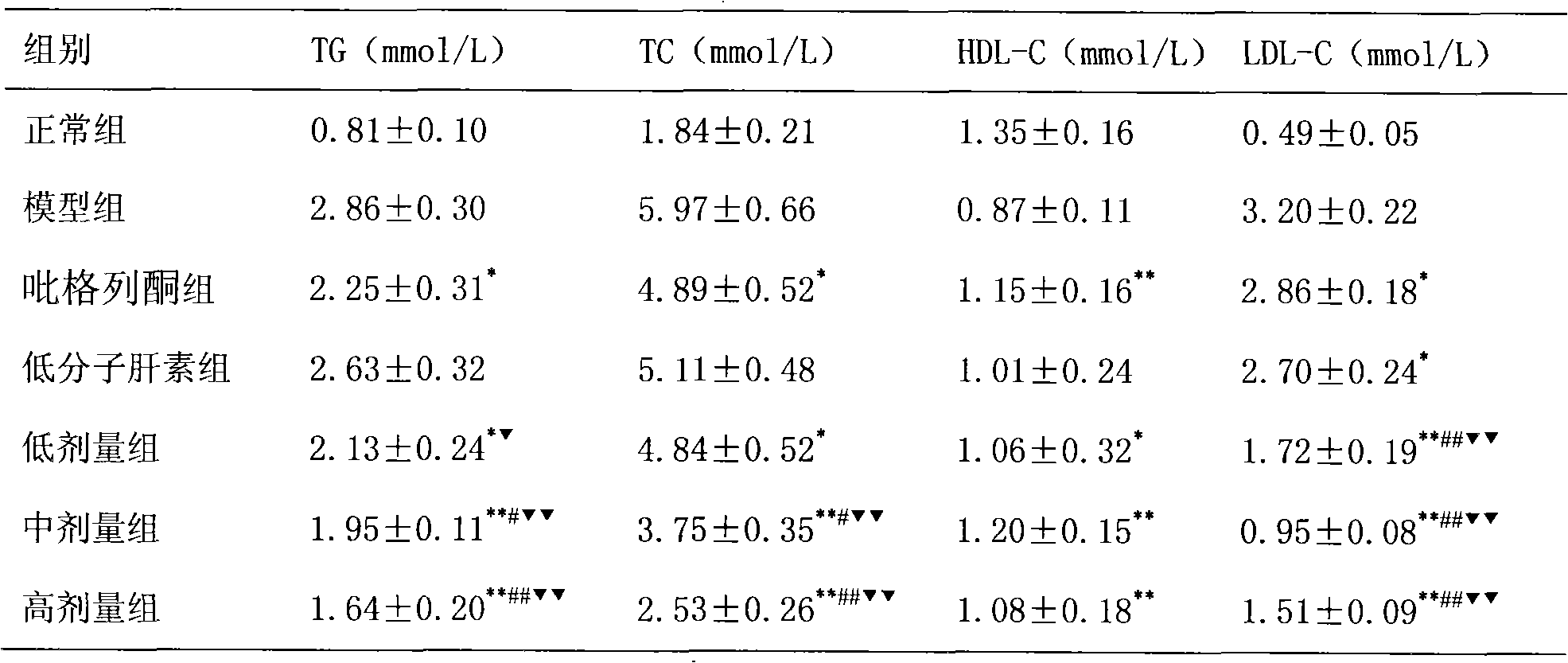
[0018] The treatment of embodiment 1 pioglitazone and low molecular weight heparin to non-alcoholic fatty liver
[0019] 1 Preparation of non-alcoholic fatty liver model
[0020] SD rats, male, weighing 220±20g, were modeled by intragastric administration of high-fat emulsion combined with intraperitoneal injection of tetracycline. Except for the normal control group, the other groups received intraperitoneal injection of tetracycline 150 mg / kg on the first day of modeling, and then intraperitoneal injection of tetracycline 110 mg / kg once every 6 days, a total of 6 times. Except for the normal group, each group was fed with high-fat emulsion at 9:30 am every day, with a dose of 10ml·kg -1 d -1 . High-fat emulsion formula: 20g lard, 10g cholesterol, 5g fructose, 5g sucrose, 1g bile salt, 1g sodium glutamate, 15ml propylene glycol, a little soil temperature 80, distilled water to 100ml. Gastrointestinal administration of high-fat milk continued for 8W.
[0021] 2 Grouping a...
Embodiment 2
[0055] The treatment of embodiment 2 pioglitazone and heparin to non-alcoholic fatty liver
[0056] 1 Preparation of non-alcoholic fatty liver model
[0057] With embodiment 1.
[0058] 2 Grouping and administration
[0059] After one week of adaptive feeding, the model rats were randomly divided into normal group, model group and drug treatment group. The specific grouping information is shown in the table below, with 10 rats in each group. Every day at 14:30 in the afternoon, the medicine was administered into the stomach, and the dosage was as follows:
[0060] Normal control group: the same volume of sodium carboxymethylcellulose;
[0061] Model control group: the same volume of sodium carboxymethyl cellulose;
[0062] Pioglitazone group: 1.5mg / (kg.d) pioglitazone hydrochloride;
[0063] Heparin group: 6mg / (kg.d) heparin sodium;
[0064] Compound group: 1.5mg / (kg.d) pioglitazone+6mg / (kg.d) heparin sodium;
[0065] 3 detection indicators
[0066] After the last admi...
Embodiment 3
[0089] The preparation of embodiment 3 compound capsules
[0091] Pioglitazone Hydrochloride 20g
[0092] 180g pregelatinized starch
[0093] Mannitol 160g
[0094] Lactose 80g
[0095] 6% PVP in 95% ethanol solution
[0096] Micronized silica gel 4.5g
[0097] Preparation process: pass the heparin calcium, pioglitazone hydrochloride, pregelatinized starch, mannitol, lactose and micropowder silica gel in the prescription through a 100-mesh sieve, mix well, add 6% PVP in 95% ethanol solution to granulate, and bake at 60°C Dry, sieve through a 18-mesh sieve, and fill the capsules.
[0098] The heparin calcium in this embodiment can also be replaced by low molecular weight heparin calcium or low molecular weight heparin sodium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com