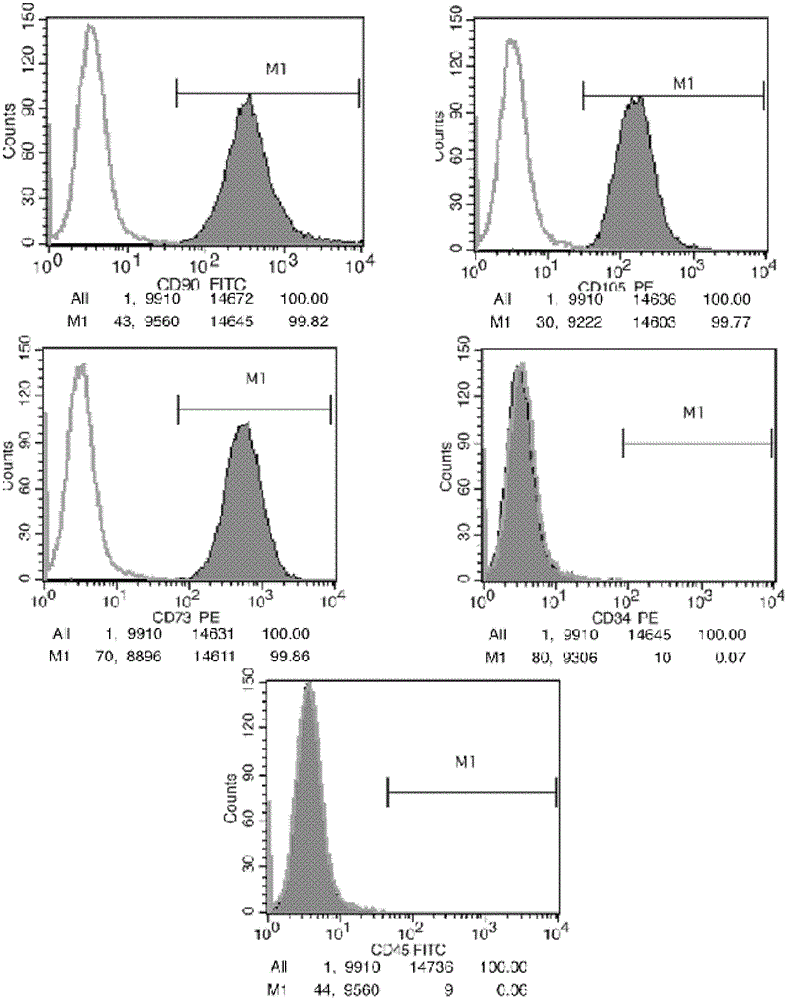
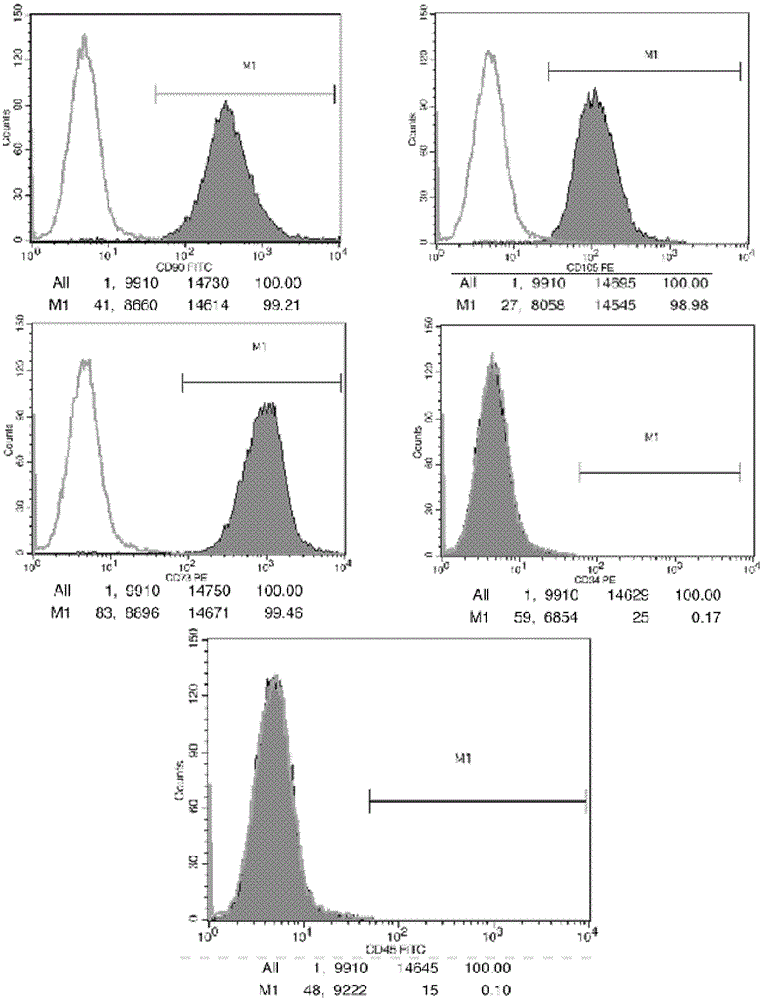
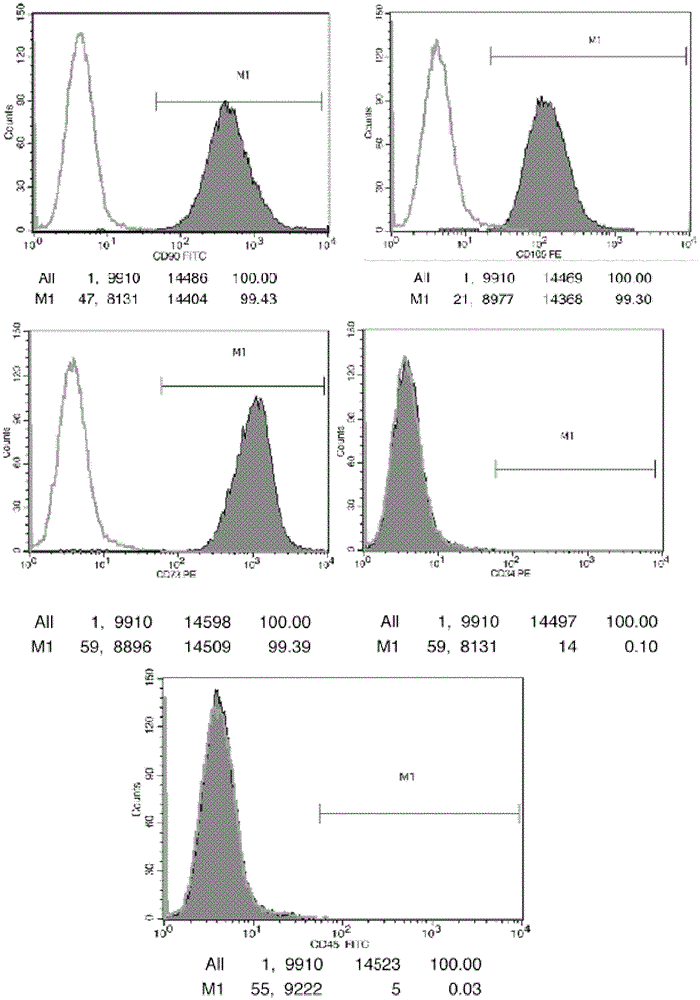
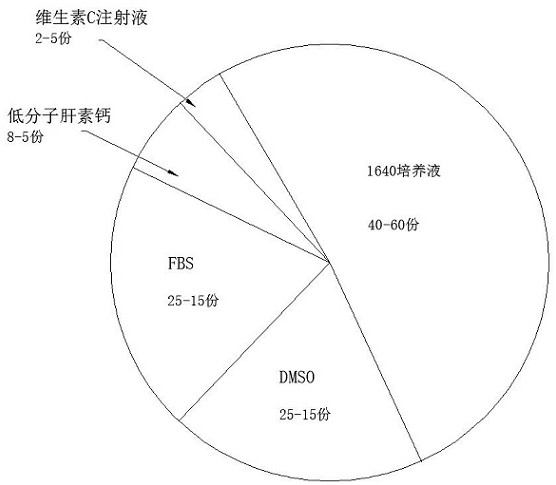
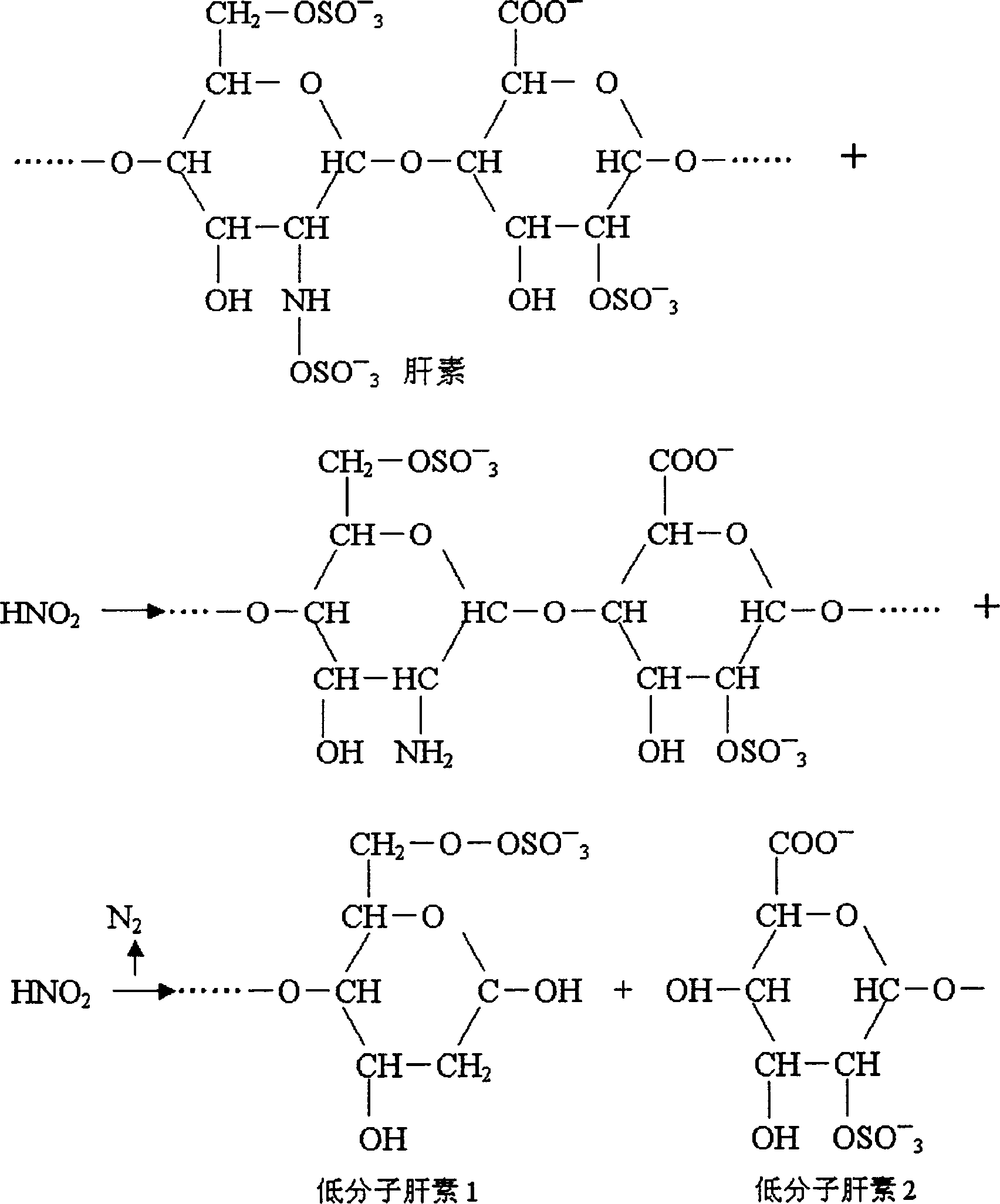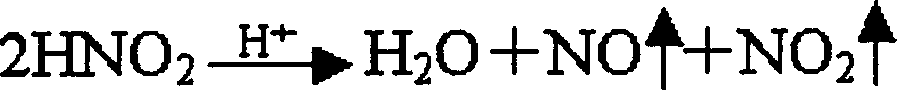Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
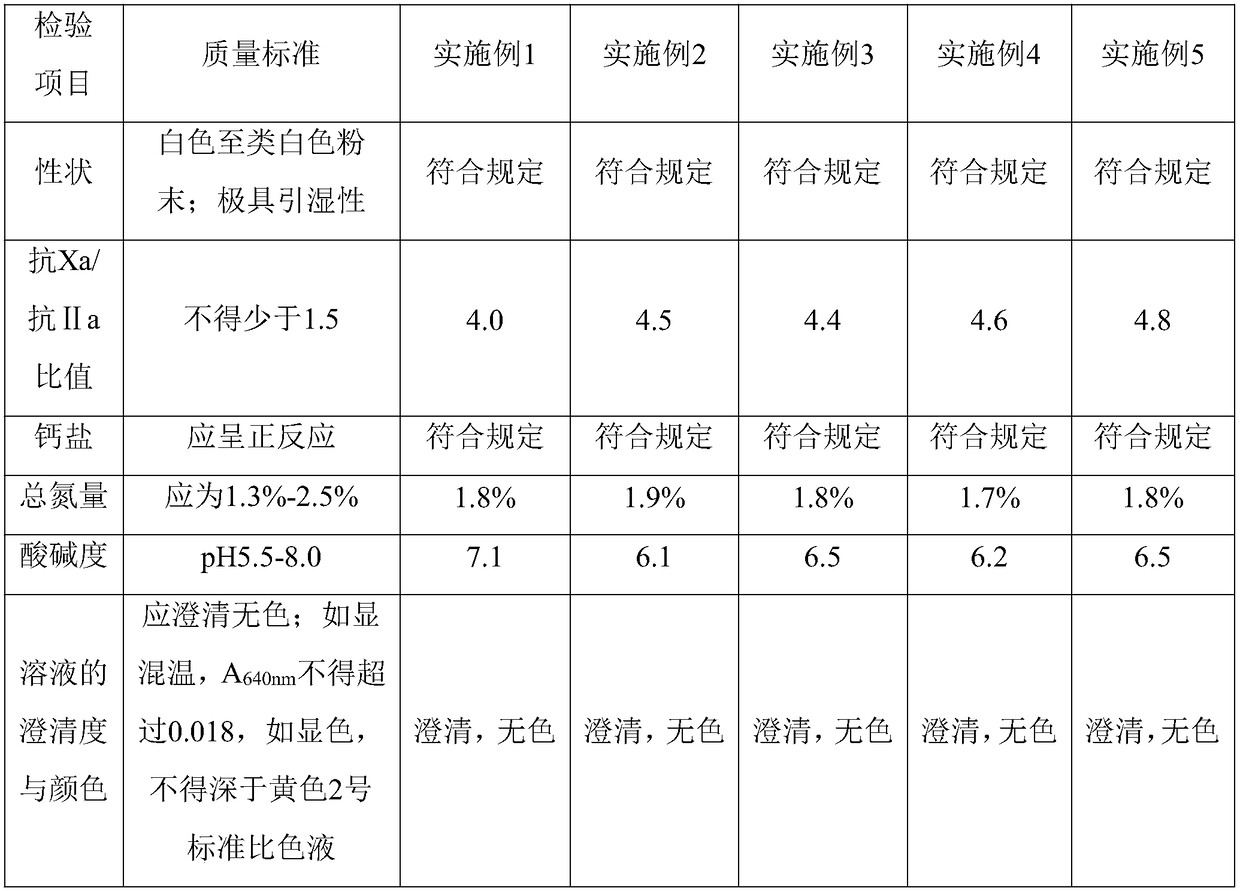
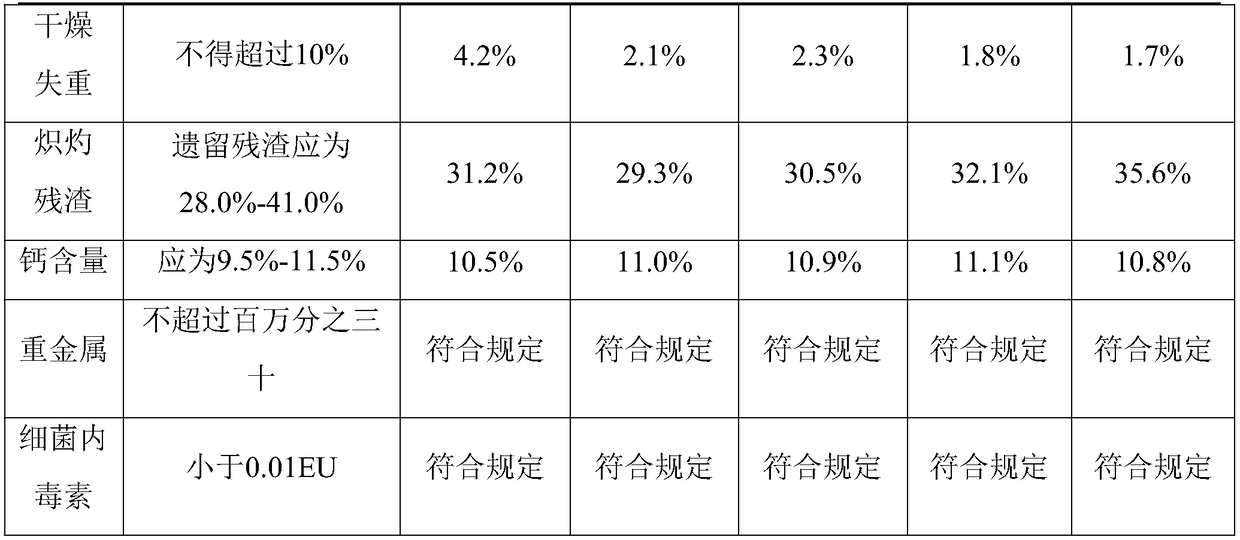
32 results about "Heparin Calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The calcium salt form of heparin. As a glycosaminoglycan anticoagulant, heparin calcium binds to antithrombin III to form a heparin-antithrombin III complex. The complex binds to and irreversibly inactivates thrombin and other activated clotting factors IX, X, XI, and XII and prevents the transformation of fibrinogen to fibrin. (NCI)
Technique for producing ultra-low molecular heparin sodium (calcium)
InactiveCN101519459AImprove securityGood and long-lasting antithrombotic effectPulmonary artery embolismDisease
Aiming at the conditions that heparin has severe bleeding side effects in clinical practice and clinical application thereof is restricted, the invention discloses a technique for producing ultra-low molecular heparin sodium (calcium). The technique comprises the following steps of: taking heparin sodium solution, adding sodium nitrite solution for cracking; adjusting the lysis buffer by using alkaline; absorbing impurities by using an anion-exchange column; washing for obtaining ultra-low molecular heparin calcium; carrying out filtration by using an ultrafiltration membrane and obtaining a precipitate by using alcohol; and after desalting, dehydration, re-precipitation, cooling and drying, obtaining a finished product of ultra-low molecular heparin calcium. The product has better and safer antithrombotic effect under low level of anticoagulation, and can be widely used for preventing and treating diseases such as deep vein thrombosis, pulmonary embolism, disseminated intravascular coagulation, and the like.
Owner:SUZHOU FAST BIOLOGICAL PHARMACY TECH
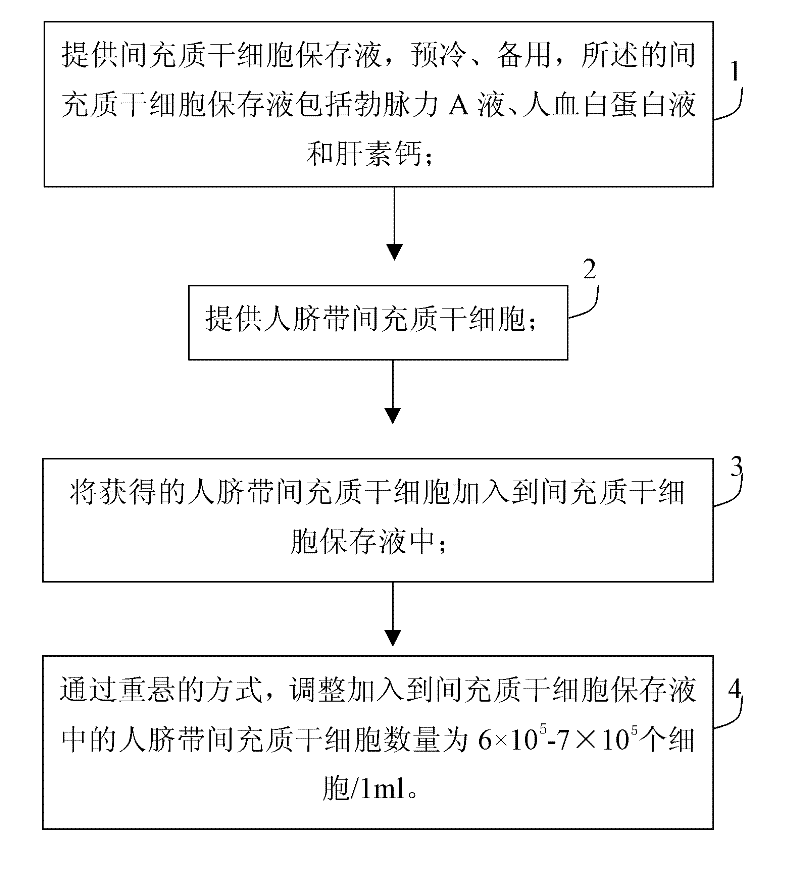
Human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and preparation method thereof
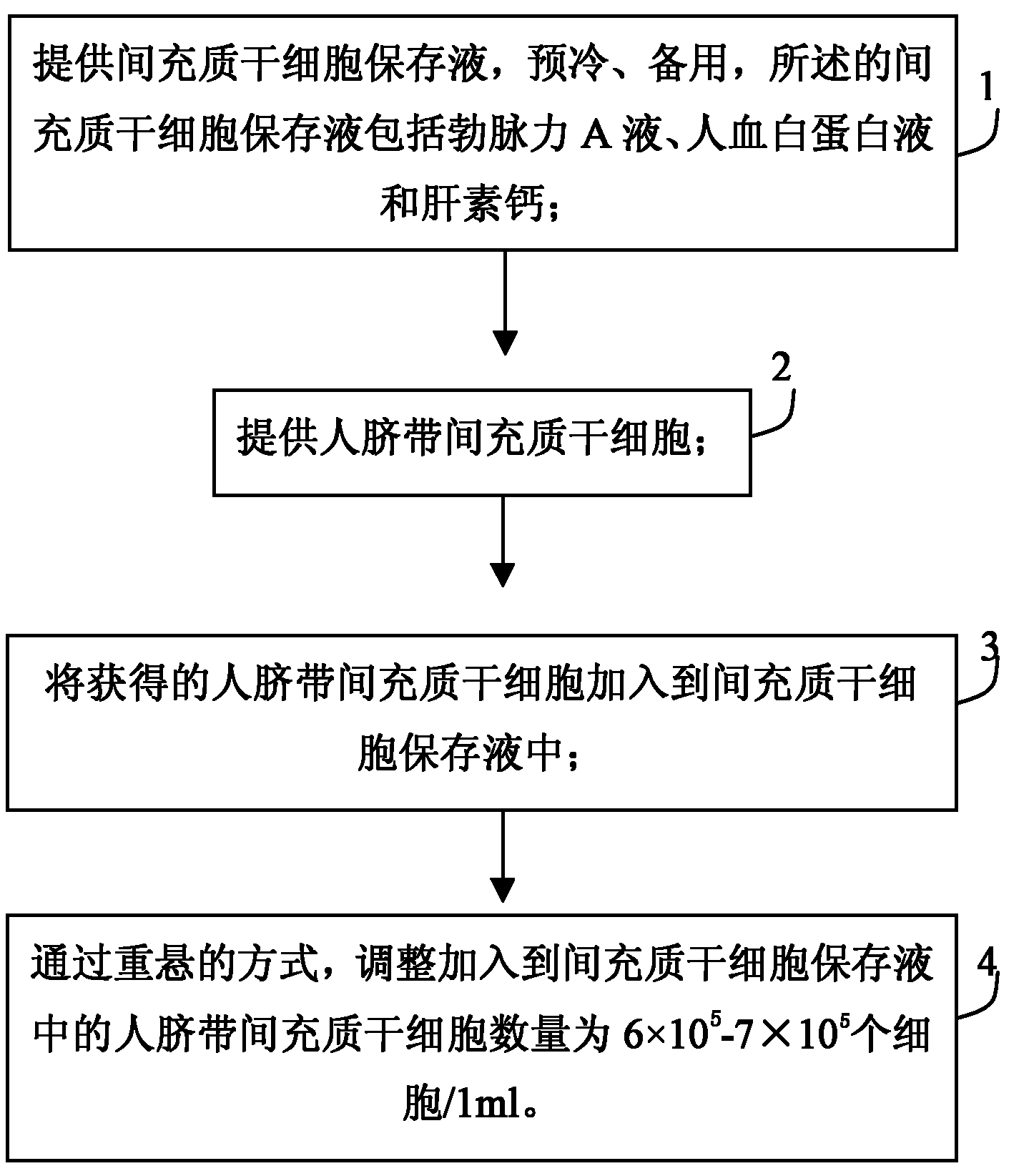
ActiveCN102008507ALong shelf lifeRepair damageDigestive systemUnknown materialsRingers solutionMedicine
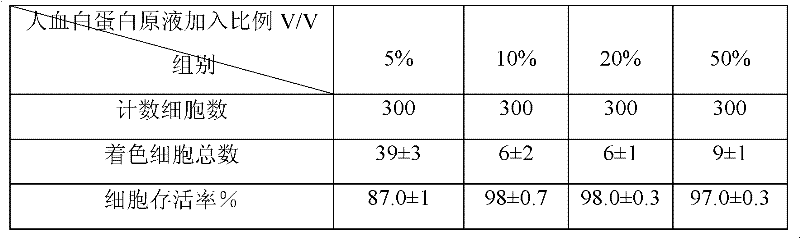
The invention discloses a human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and a preparation method thereof. The injection is composed of 10%-50% of human albumin stock solution, 49.5%-89.5% of acetated Ringer's solution and 0.5% of heparin calcium, wherein, the concentration of the human albumin stock solution is 10%, and 1ml of the injection contains 6*10<5>-7*10<5> HUMSCs. The preparation method comprises the following steps: providing an MSC preserving fluid and pre-cooling the MSC preserving fluid for later use, wherein, the MSC preserving fluid comprises the human albumin stock solution, the Acetated Ringer's solution and the heparin calcium; providing the HUMSCs and adding the HUMSCs to the MSC preserving fluid; and adjusting the number of the HUMSCs added to the MSC preserving fluid to be 6*10<5>-7*10<5> HUMSCs / ml by means of re-suspending. The injection provided by the invention has the advantages of obvious curative effect and high stability, and is convenient in storage and transport and safe in use, thus being capable of bringing gospel to patients with hepatic fibrosis.
Owner:中源协和生物细胞存储服务(天津)有限公司
Mesenchymal stem cell injection, preparation method and application thereof in preparing medicine for treating diabetes
InactiveCN102920735AIncrease productionEasy quality controlCell dissociation methodsPeptide/protein ingredientsVitamin CSide effect
The invention discloses a mesenchymal stem cell injection, a preparation method and application of the mesenchymal stem cell injection in preparing a medicine for treating diabetes; the mesenchymal stem cells are derived from human umbilical cord and placenta; the mesenchymal stem cell injection consists of components: human mesenchymal stem cells, human albumin, low molecular heparin calcium, compound amino acid, vitamin C of 0.5% and dissolving medium; and the dissolving medium can be a compound electrolyte solution or glucose or normal saline. According to the mesenchymal stem cell injection, the preparation method and the application of the mesenchymal stem cell injection in preparing the medicine for treating the diabetes, the mesenchymal stem cell injection is used for repairing injured islet beta cells, and the blood sugar is reduced by secretion of the endogenous insulin, therefore, a purpose of foundational treating the diabetes is achieved. A disease course of the diabetes can be reversed, patients are helped in escaping out of inconvenience and toxic and side effects of taking the endogenous drug and injecting the insulin as well as serious complications caused by poor control of the blood sugar; and the 1-type and 2-type diabetes are treated thoroughly.
Owner:青岛奥克生物开发有限公司
Hepatic stem cell preserving solution and applications of hepatic stem cell preserving solution
ActiveCN102948413AImprove stabilityGood curative effectDead animal preservationSodium acetateSide effect
The invention discloses hepatic stem cell preserving solution and applications of the hepatic stem cell preserving solution. The hepatic stem cell preserving solution provided by the invention is prepared by fixing the volume of injection solution including 0.1 to 1g of human albumin, 2.60 to 4.97g of sodium chloride, 2.48 to 4.74g of sodium gluconate, 1.82 to 3.40g of sodium acetate, 0.18 to 0.35g of potassium chloride, 0.15 to 0.28g of magnesium chloride, and 0.4 to 0.7mL of heparin calcium based on effective dose by water to be reach to 100mL. The hepatic stem cell preserving solution provided by the invention is used for storing hepatic stem cells, and the survival rate of the hepatic stem cell is more than 85% and even more than 95% within 12 hours. The cell suspension which is obtained by floating the hepatic stem cells on the hepatic stem cell preserving solution can be used as the medicine for treating diabetes mellitus, and has the characteristics of being high in stability, good in curative effect, high in safety, being without toxic or side effect, convenient to store and transport, applicable to massive clinical use, and broad in application prospect. The hepatic stem cell preserving solution brings good news for diabetic patients, and provides a new way for clinical stem cell use.
Owner:北京清美联创干细胞科技有限公司
Room-temperature transport liquid for MSC (mesenchymal stem cells)
InactiveCN108432742AOvercome the disadvantages of transportationExcellent biological properties preservation effectDead animal preservationHuman albuminSimple component
The invention discloses a room-temperature transport liquid for MSC (mesenchymal stem cells). The room-temperature transport liquid comprises raw materials in percentage by mass as follows: 25%-40% ofa trehalose liquor, 20%-40% of a red blood cell stock liquor, 1%-10% of heparin calcium and 20%-45% of a human albumin injection. Various defects of traditional MSC transport are overcome, room-temperature mass transport is realized, huge waste of culture media and liquid nitrogen in the traditional transport mode is avoided, and cost is reduced effectively. Meanwhile, the room-temperature transport liquid contains simple components, is convenient to prepare and is pollution-free, and has excellent biological characteristic preservation effect on the MSC.
Owner:FOSHAN UNIVERSITY
Umbilical cord mesenchymal stem cell injection liquid, preparation method and application thereof
InactiveCN106237313AGreat differentiation potentialImprove proliferative abilityOrganic active ingredientsPeptide/protein ingredientsVitamin CHuman albumin
The invention relates to the technical field of stem cells and especially relates to an umbilical cord mesenchymal stem cell injection liquid, a preparation method and an application thereof. The injection liquid comprises umbilical cord mesenchymal stem cells, human albumin, low molecular heparin calcium, vitamin C, panax japonicus majoris saponin and a solvent. The injection liquid is prepared from the umbilical cord mesenchymal stem cells, so that the materials are convenient to obtain and the method does not violate ethical issue. The injection liquid can effectively prolong preservative time of the cells and the preserved stem cells have high activity, so that the injection liquid is convenient to use. After refusion, the injection can promote differentiation and maturation of the mesenchymal stem cell to liver cells, thus reducing incidence rate of GVHD.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Hepatic stem cell preserving solution and applications of hepatic stem cell preserving solution
ActiveCN102948413BImprove stabilityGood curative effectDead animal preservationSodium acetateSide effect
The invention discloses hepatic stem cell preserving solution and applications of the hepatic stem cell preserving solution. The hepatic stem cell preserving solution provided by the invention is prepared by fixing the volume of injection solution including 0.1 to 1g of human albumin, 2.60 to 4.97g of sodium chloride, 2.48 to 4.74g of sodium gluconate, 1.82 to 3.40g of sodium acetate, 0.18 to 0.35g of potassium chloride, 0.15 to 0.28g of magnesium chloride, and 0.4 to 0.7mL of heparin calcium based on effective dose by water to be reach to 100mL. The hepatic stem cell preserving solution provided by the invention is used for storing hepatic stem cells, and the survival rate of the hepatic stem cell is more than 85% and even more than 95% within 12 hours. The cell suspension which is obtained by floating the hepatic stem cells on the hepatic stem cell preserving solution can be used as the medicine for treating diabetes mellitus, and has the characteristics of being high in stability, good in curative effect, high in safety, being without toxic or side effect, convenient to store and transport, applicable to massive clinical use, and broad in application prospect. The hepatic stem cell preserving solution brings good news for diabetic patients, and provides a new way for clinical stem cell use.
Owner:北京清美联创干细胞科技有限公司
Culture medium for adipose derived stem cells
InactiveCN106222136AKeep aliveReduce apoptosis rateCulture processSkeletal/connective tissue cellsCholesterolVascular endothelium
The invention discloses a culture medium for adipose derived stem cells. The culture medium for the adipose derived stem cells comprises RPMI-1640 medium, and further comprises 2 to 2.5g / L of D-glucose, 30 to 45mg / L of cholesterol, 0.5 to 0.8g / L of potassium chloride, 0.1 to 0.3 mu g / L of sodium selenite, 8 to 10 mu g / L of taurine, 10 to 20 mu g / L of heparin calcium, 2.2 to 2.5g / L of human serum albumin, 20 to 50mg / L of vascular endothelial growth factors, 3 to 6mg / L of fibroblast growth factors, 40 to 80mg / L of platelet-derived growth factors, 20 to 40mg / L of glutathione, 50 to 100mg / L of vitamins, and 100 to 200mg / L of plant extracts. The culture medium for the adipose derived stem cells disclosed by the invention is free of components of animal origin, has no residues or pollution, has good cell anchorage dependence and high proliferation rate, improves the pathogenic microorganism resistance of the culture medium, and further lowers the cost of the culture medium.
Owner:ANHUI HUIEN BIOTECH
Stem cell preparation for treating diabetes mellitus and preparation method of stem cell preparation
InactiveCN103845362AImprove stabilityGood curative effectMetabolism disorderArtificial cell constructsInsulin Secreting CellBlood sugar
The invention provides a stem cell preparation for treating diabetes mellitus. The stem cell preparation comprises: stem cells separated and extracted from human blood or tissues and organs and / or islet-like cells induced and differentiated from the stem cells and a cell preservation solution containing 0.5% of heparin calcium, wherein the cell content is 1*10<5> to 5*10<7> / ml. The invention also discloses a preparation method of the stem cell preparation. The method comprises the following steps: (1) separation and purification of the stem cell; (2) in vitro culture of the stem cells or in vitro induction and differentiation of the stem cells to form islet-like cells; (3) diluting the cell obtained in the step (2) by using the cell preservation solution to prepare a stem cell preparation; (4) quality detection of the stem cell preparation. The stem preparation is transplanted into the body and can be differentiated into insulin-secreting cells to secret insulin, and the blood sugar concentration of a person with diabetes is effectively reduced. Thus, a new way is provided for overcoming treatment of the diabetes dependent on the insulin and curing the diabetes once and for all.
Owner:上海坤爱生物科技股份有限公司
Eye drop
InactiveCN106581300AGood treatment effectEasy to useSenses disorderHydroxy compound active ingredientsHuman bodyEye/ear drops
The invention relates to the technical field of medicines, and concretely relates to an eye drop. The eye drop comprises, by weight, 15-20 parts of a pearl liquid, 5-8 parts of taurine, 8-10 parts of vitamins, 5-10 parts of aspartic acid, 10-15 parts of Semen Cassiae, 5-8 parts of orange peel, 5-10 parts of heparin calcium, 3-5 parts of a mulberry liquid, 3-5 parts of Polygonum multiflorum, 5-8 parts of a green tea extract liquid, 3-5 parts of an animal internal organ extract liquid, 5-8 parts of a ginseng extract liquid, 10-15 parts of borneol and 3-5 parts of antibiotics. The raw materials of the eye drop are most from natural plants and Chinese herbal medicines, and no antiseptics, hormones or other substances harmful to human bodies are added, so the eye drop has the advantages of no dependence of people, substantial treatment effect, and convenience and comfortableness in use.
Owner:青岛益邦瑞达生物科技有限公司
Preserving fluid for mesenchymal stem cells, preparation method and application thereof
The embodiment of the invention provides a preserving fluid for mesenchymal stem cells, a preparation method and an application thereof. The preserving fluid for mesenchymal stem cells includes multiple electrolytes injection, insulin, estrogen, progestational hormone, sodium selenite, heparin calcium and human serum albumin. The preserving fluid composed of the components is capable of supportinglife state of stem cells, especially umbilical cord mesenchymal stem cells, is capable of preventing cell viability from quickly decreasing and even preventing cells from death, and has the advantageof capability of long-term preserving stem cells.
Owner:药鼎(北京)国际细胞医学技术有限公司
Human fat mesenchymal stem cell transportation fluid and preparation method thereof
The invention discloses a human fat mesenchymal stem cell transportation fluid and a preparation method thereof. The transportation fluid is prepared from the following components in percentage by mass: 30-50 percent of fetal calf serum, 10-20 percent of DMSO, 5-10 percent of 0.2% heparin calcium solution and 20-40 percent of 0.2% trehalose solution. The human fat mesenchymal stem cells are storedby adopting the transportation fluid, the cell death speed can be slowed, and the proliferation and differentiation activity of the fat mesenchymal stem cells can be maintained.
Owner:JIANGSU RE STEM BIOTECH
Herapin calcium eye drops
The heparin calcium eye drops are mainly used for curing the disease of allergenic epipephysitis, and can be used for quickly removing the symptom of eye-itching, congestion and lachrymation, and its therapeutic effect is reliable, and it is convenient for use and has no adverse reaction. Said invention is a stable solution made up by using heparin calcium as main effective component, and adding chloromycetin eye drops of distilled water as diluent through processes of co-mixing and mutual solution according to a certain proportion.
Owner:黄建林
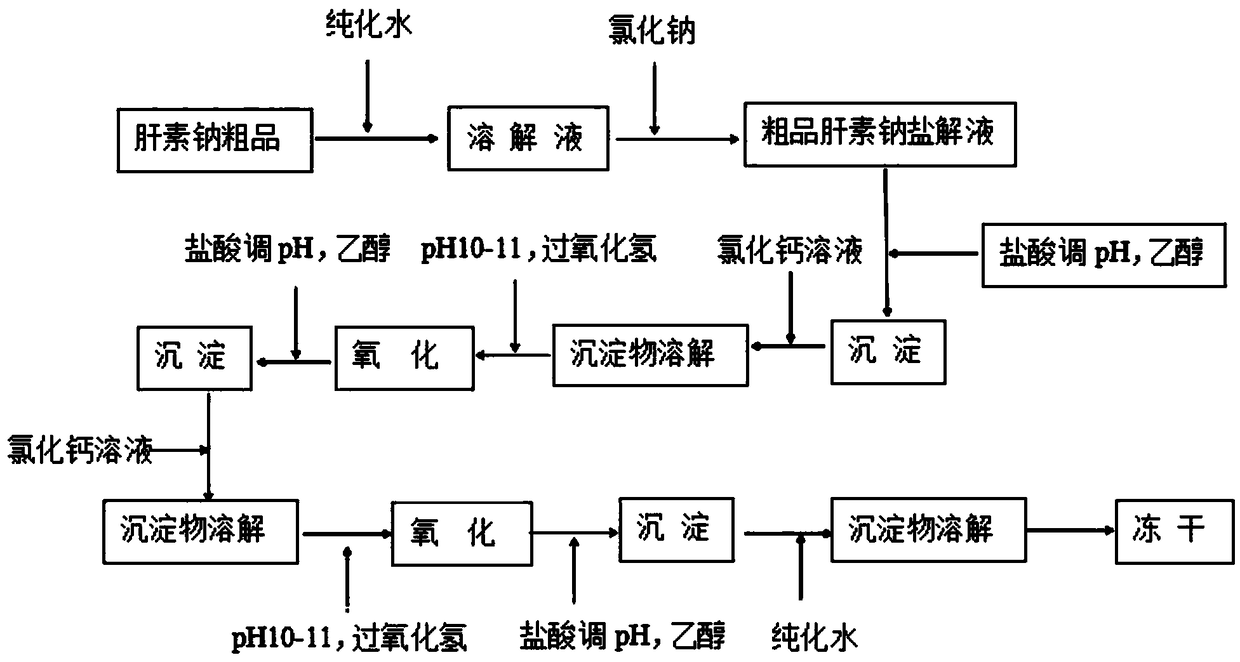
Method of producing heparin calcium from crude heparin sodium
The invention discloses a method of producing heparin calcium from crude heparin sodium. The method comprises the steps, such as, with crude heparin sodium as a raw material, salt decomposition, precipitating, dissolving, oxidizing, precipitating, dissolving, oxidizing, precipitating, dissolving, and lyophilizing, and the finished heparin calcium having stable and controllable quality is obtained.The method of producing heparin sodium uses crude heparin sodium as a raw material; calcium conversion is achieved during oxidative cleaning and decoloring by hydrogen peroxide addition; the problemof the prior art is solved that calcium-sodium exchange of cation exchange resin has high resin activation requirement, has the need for massive water and operational complexity and high production cost. The method has the advantages that the production steps are simplified, less impurity is introduced, finish heparin calcium of high purity, high yield, high activity and controllable quality stability can be acquired, the product quality is higher than that provided in existing standards of EP (European pharmacopeia); the method is simple to perform, low in production cost and easy to implement for industrial large-scale production, and has important application prospect.
Owner:HUBEI YINUORUI BIOLOGICAL PHARMA
Heparin calcium compound and preparation method thereof
InactiveCN102603923AChange the status quo of low purityGuarantee product qualityBlood disorderExtracellular fluid disorderSide effectUltrafiltration
The invention discloses a heparin calcium compound and a preparation method thereof. The preparation method comprises the following steps of: dissolving a low molecular heparin calcium rough product in water; dropwise adding non-redox acid with stirring; adding active carbon to adsorb; then filtering; ultrafiltering filtrate with an ultrafiltration membrane and concentrating; adding calcium oxide or calcium hydroxide into concentrated solution and violently stirring; adding neutral aluminum oxide into the solution and uniformly stirring; adding to the upper end of a neutral aluminum oxide preparative chromatographic column to separate and purify; collecting eluent; adding ethanol into the eluent; performing gradient cooling to separate precipitate; and drying the precipitate to obtain refined low molecular heparin calcium. The method has the advantages of simple process, convenience for operation, low cost, suitability for mass production, improved purity of the low molecular heparin calcium compound and capabilities of guaranteeing the safety of the heparin calcium compound in an anti-coagulation medicament, improving the quality of a preparation product and reducing the toxic or side effect.
Owner:灵康药业集团股份有限公司
Process for preparing low moledule heparin calcium of low nitrite content
InactiveCN1268650CLow costSimple processOrganic active ingredientsBlood disorderSodium nitriteHeparin sodium
Owner:ZHAOKE PHARMA HEFEI
Endometrium stem cell protecting liquid and preparation method thereof
InactiveCN109566597AImprove recovery rateLower metabolic rateDead animal preservationReduction rateMedicine
The invention provides endometrium stem cell protecting liquid and a preparation method thereof. The protecting liquid comprises 1-5 parts of heparin calcium, 20-40 parts of low-molecular dextran, 1-2parts of papaverine, 5-10 parts of mycose, 800-1000 parts of water and 4-6 parts of a traditional Chinese medicine extract. The endometrium stem cell protecting liquid does not contain serum, is lowin price, after stored endometrium stem cells are cryopreserved and recovered, the recovery rate is high, and along with passing of the time, the reduction rate of the recovery rate of the endometriumstem cells is reduced.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Transporting fluid for human umbilical cord mesenchymal stem cells
ActiveCN109006803AImprove survival rateDoes not affect multilineage differentiation abilityDead animal preservationMedicineHuman albumin
The invention relates to a transporting fluid for human umbilical cord mesenchymal stem cells. According to the invention, heterophyllin B with a concentration of 5 [mu]M is added on the basis of theprior art, so the shortcoming that conventional transporting fluids is obviously reduced in cell viability after suspension for 72 h can be effectively overcome. The transporting fluid of the invention is prepared by uniformly mixing, by mass, 30% of a trehalose solution (a PBS solution of trehalose) with a concentration of 0.2%, 25% of a red blood cell storage solution, 5% of a heparin calcium solution (a PBS solution of heparin calcium) with a concentration of 0.2% and 40% of human albumin injection at room temperature, and further comprises heterophyllin B with a concentration of 5 [mu]M. The transporting fluid of the invention can improve the survival rate of human umbilical cord mesenchymal stem cells having been transported for 72 hours, and does not affect the multi-directional differentiation ability of the human umbilical cord mesenchymal stem cells.
Owner:江苏赛亿细胞技术研究院有限公司
A kind of mesenchymal stem cell preservation solution and its preparation method and application
ActiveCN102365933BProlong the duration of activityEasy to prepareDead animal preservationSkeletal/connective tissue cellsBlood plasmaUmbilical cord
The invention belongs to the field of biomedicine and discloses a mesenchymal stem cell preservation solution and its preparation method and application. The mesenchymal stem cell preservation solution contains human AB type umbilical cord blood plasma and 70- 80AXaIU / ml calcium low molecular weight heparin; the human AB type umbilical cord blood plasma and mesenchymal stem cells come from the same provider. The mesenchymal stem cell preservation solution of the present invention can prolong the activity maintenance time of mesenchymal stem cells (maintain more than 90% of the activity for 72 hours at 4-15°C), and the mesenchymal stem cells are stored in the preservation solution of the present invention for 72 hours. , the cell morphology is normal, does not affect its proliferation and expansion ability, and does not affect the phenotypic characteristics of mesenchymal stem cells. The mesenchymal stem cell preservation solution of the present invention is safe and reliable in clinical application, and the AB-type umbilical cord blood plasma and umbilical cord mesenchymal stem cells are derived from the same placenta and belong to autologous plasma, which reduces the possibility of exogenous virus contamination.
Owner:广州市天河诺亚生物工程有限公司
Cryopreservation solution and cryopreservation method for immune cells
InactiveCN113615681AImprove cryopreservation effectSimple recipeDead animal preservationAntioxidantCryopreservation
The invention relates to a cryopreservation solution and a cryopreservation method for immune cells. The cryopreservation solution comprises FBS, DMSO, a 1640 culture solution, low-molecular heparin calcium and an antioxidant. The cryopreservation method of the cryopreservation solution for immune cells comprises the following steps: 1, storing: putting the immune cells into a freezing tube, then adding a freezing medium, slowly blowing and beating, uniformly mixing, and putting the freezing tube into a cooling chamber; 2, cooling for the first time: cooling to 3 DEG C and keeping the temperature for 4 minutes; then cooling to -3 DEG C at a speed of -2 DEG C / min; and finally, reducing the temperature to -40 DEG C at the speed of -11 DEG C / min; 3, raising the temperature to -30 DEG C at the speed of -16 DEG C / min for the first time; 4, cooling for the second time: keeping the temperature at -30 DEG C for 4 minutes; then cooling to -50 DEG C at the speed of -2 DEG C / min; finally, reducing the temperature to -80 DEG C at the speed of -10 DEG C / min; and keeping at -80 DEG C for 5 days; and 5, storing: finally storing in a liquid nitrogen tank for cryopreservation.
Owner:郑州源创基因科技有限公司
Dog mesenchymal stem cell injection and preparation method and application thereof
InactiveCN106540244AImprove stabilityGood curative effectPeptide/protein ingredientsUnknown materialsSide effectCurative effect
The invention provides dog mesenchymal stem cell injection and a preparation method and application thereof. The dog mesenchymal stem cell injection comprises dog mesenchymal stem cells, heparin calcium, dog blood albumin and an electrolyte solution. The mesenchymal stem cells from dog bone marrow are utilized ingeniously, and combined with other compositions to be prepared into an injection form, namely the dog mesenchymal stem cell injection. The traditional therapeutic method for a dog skin tissue injury is broken. A stem cell therapy is brought in the field of pet medical treatment, the dog mesenchymal stem cell injection has the advantages that stability is high, the curative effect is good, usage is safe, long-term preservation and transportation are achieved, no toxic and side effect exists, and the foundation is laid for large-scale clinic use.
Owner:FOSHAN UNIVERSITY
Human placenta mesenchymal stem cell transporting liquid
ActiveCN109122662AImprove survival rateDoes not affect multilineage differentiation abilityDead animal preservationMesenchymal stem cellHuman albumin
The invention relates to human placenta mesenchymal stem cell transporting liquid. Adding 5micron heterophyllin C on the basis of the prior art effective overcomes the defect of evident cell viabilitylowering after suspension of the transporting liquid for 72h in the prior art. The transporting liquid is prepared from, by mass, 30% of 0.2% trehalose solution (PBS solution of trehalose), 25% of red blood cell storage liquid, 5% or 0.2% heparin calcium solution (PBS solution of heparin calcium) and 40% of human albumin injection by well mixing at the normal temperature and contains 5micron heterophyllin C. The transporting liquid has the advantage that viability of human placenta mesenchymal stem cells in transporting for 72h is improved without affecting the multi-directional differentiation performance.
Owner:深圳华大基因细胞科技有限责任公司
Human amniotic fluid mesenchymal stem cell transport fluid
ActiveCN109042627AImprove survival rateDoes not affect multilineage differentiation abilityDead animal preservationCyclic peptideMotility
The invention relates to human amniotic fluid mesenchymal stem cell transport fluid. On the basis of the prior art, 5 microM radix pseudostellariae cyclic peptide A is added, so that the weakness of the prior art that the cell motility rate after the transportation fluid is suspended for 72 hours is apparently reduced can be effectively overcome. The formula of the transport fluid is as follows: the human amniotic fluid mesenchymal stem cell transport fluid is prepared by uniformly mixing 30 percent of 0.2 percent trehalose solution (PBS solution of trehalose), 25 percent of red blood cell storage fluid, 5 percent of 0.2 percent heparin calcium solution (PBS solution of heparin calcium) and 40 percent of human blood albumin injection at normal temperature, and the human amniotic fluid mesenchymal stem cell transport fluid comprises 5 microM radix pseudostellariae cyclic peptide A. The transport fluid not only can improve the motility rate of the human amniotic fluid mesencymal stem cells after being transported for 72 hours, but also does not influence the multi-directional differentiation capability.
Owner:辽宁美全生物科技有限公司
Method for preparing nadroparin calcium and dalteparin sodium
ActiveCN112830980AChemical industryChemical/physical/physico-chemical microreactorsUltrafiltrationPhysical chemistry
The invention belongs to the field of preparation of low-molecular-weight heparin, and relates to a method for preparing nadroparin calcium and dalteparin sodium. Specifically, the invention relates to a method for preparing low-molecular-weight heparin, which comprises the steps of heparin sodium cracking, reduction, neutralization and ultrafiltration in a microchannel reactor, and the low-molecular-weight heparin is selected from nadroparin calcium and dalteparin sodium. According to the method, the selectivity of heparin sodium cracking reaction is improved, and the probability of repeated cracking is reduced, so that the increase of micromolecular heparin is avoided, the load of later ultrafiltration molecular weight screening is reduced, and the yield of the product is improved.
Owner:SHENZHEN SCIPROGEN BIO PHARMA
Human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and preparation method thereof
InactiveCN102008507BLong shelf lifeRepair damageDigestive systemSkeletal/connective tissue cellsRingers solutionMedicine
The invention discloses a human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and a preparation method thereof. The injection is composed of 10%-50% of human albumin stock solution, 49.5%-89.5% of acetated Ringer's solution and 0.5% of heparin calcium, wherein, the concentration of the human albumin stock solution is 10%, and 1ml of the injection contains 6*10<5>-7*10<5> HUMSCs. The preparation method comprises the following steps: providing an MSC preserving fluid and pre-cooling the MSC preserving fluid for later use, wherein, the MSC preserving fluid comprises the human albumin stock solution, the Acetated Ringer's solution and the heparin calcium; providing the HUMSCs and adding the HUMSCs to the MSC preserving fluid; and adjusting the number of the HUMSCs added to the MSC preserving fluid to be 6*10<5>-7*10<5> HUMSCs / ml by means of re-suspending. The injection provided by the invention has the advantages of obvious curative effect and high stability, and is convenient in storage and transport and safe in use, thus being capable of bringing gospel to patients with hepatic fibrosis.
Owner:中源协和生物细胞存储服务(天津)有限公司
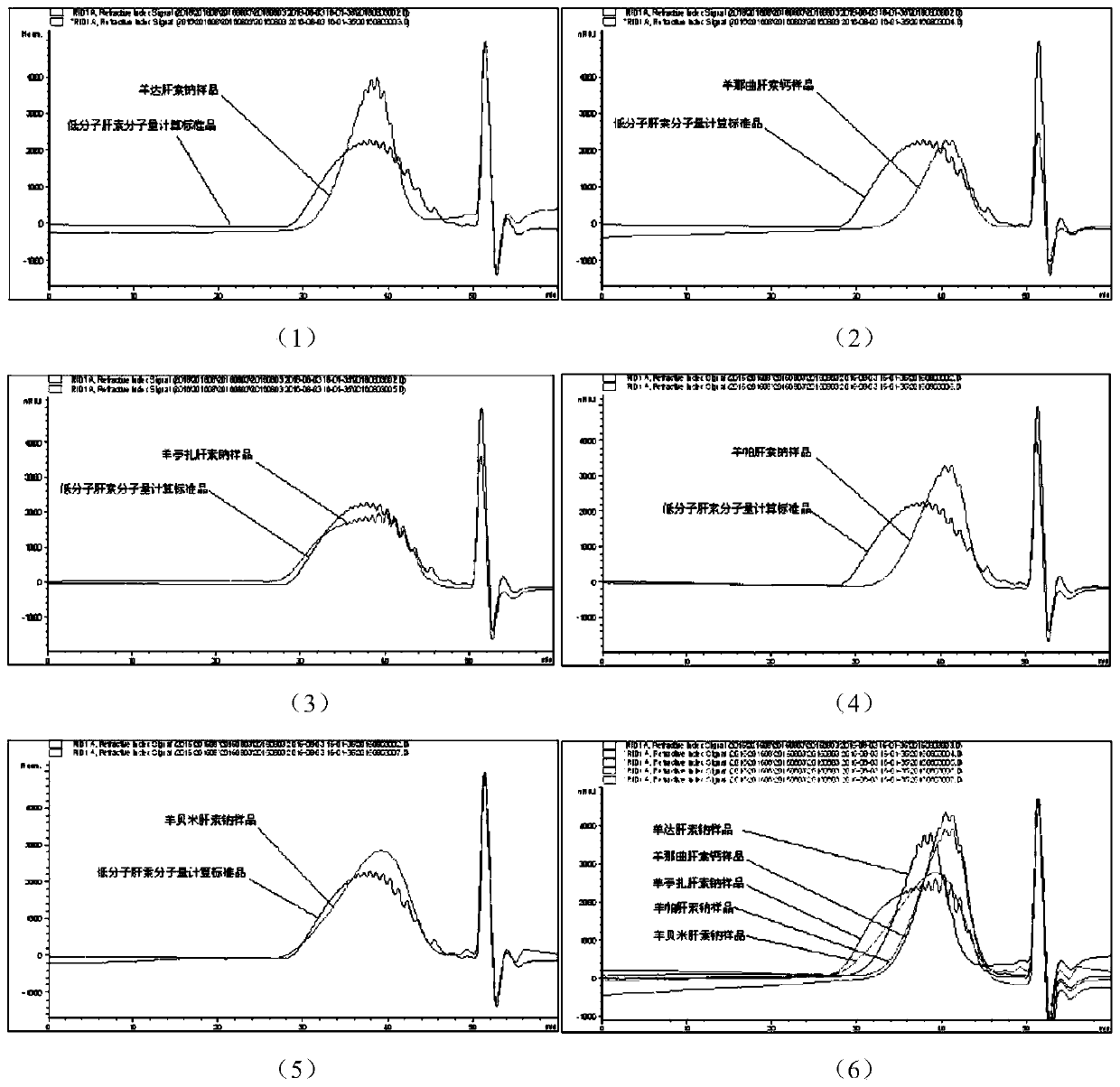
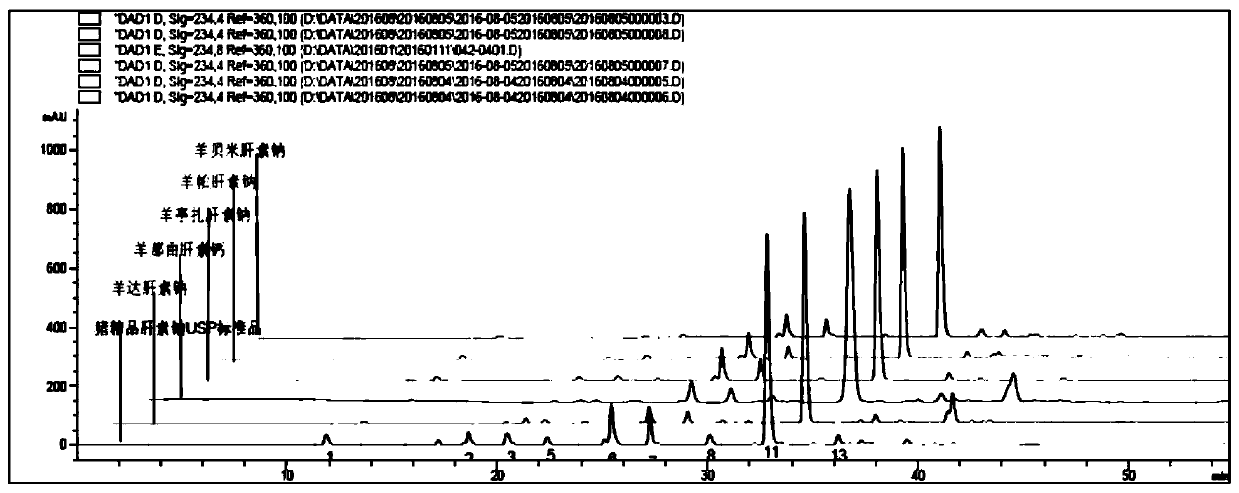
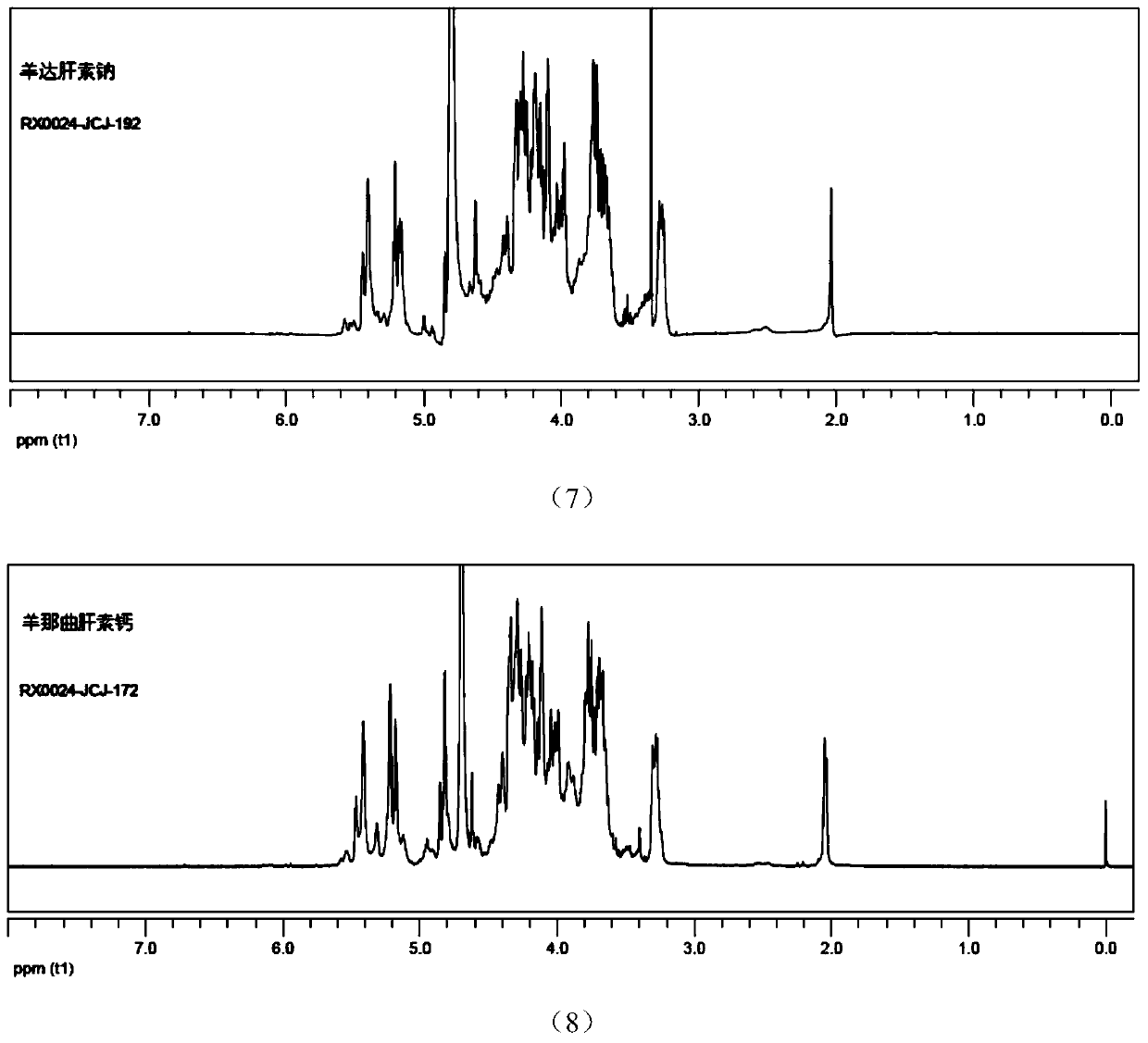
Sheep-derived low-molecular-weight heparin and its preparation method and application
ActiveCN107759712BRich sourcesAbundant productionOrganic active ingredientsBlood disorderDrug biological activitySodium heparin
The invention discloses low-molecular-weight heparin derived from sheep, including sheep dateparin sodium, sheep nadroparin calcium, sheep tinzaparin sodium, sheep betaparin sodium and sheep bemiparin sodium. The low molecular weight heparins of sheep origin above all have common provenance characteristics. In terms of chemical structure, the content of the main disaccharide ΔUA2S‑GlcNS6S (ΔIS) is between 66%‑74%. Sheep low-molecular-weight heparin and pig-derived low-molecular-weight heparin have similar physical and chemical properties and biological activities, which expands the source of low-molecular-weight heparin drugs. The preparation method of sheep low-molecular-weight heparin introduced by the invention is simple and easy, and the process is stable. The obtained product is refined and can fully meet the requirements of various pharmacopoeias for the current low-molecular-weight heparin. Sheep low-molecular-weight heparin also has halal properties that porcine-derived low-molecular-weight heparin does not have. It has a huge market in the majority of Muslim populations, countries and regions, and can be used in anticoagulant, antithrombotic, anti-inflammatory, anticancer and halal drugs.
Owner:SUZHOU RONGXI BIOTECH CO LTD +1
Heparin calcium compound and preparation method thereof
InactiveCN102603923BIncrease dissolution rateHigh purityBlood disorderExtracellular fluid disorderSide effectUltrafiltration
The invention discloses a heparin calcium compound and a preparation method thereof. The preparation method comprises the following steps of: dissolving a low molecular heparin calcium rough product in water; dropwise adding non-redox acid with stirring; adding active carbon to adsorb; then filtering; ultrafiltering filtrate with an ultrafiltration membrane and concentrating; adding calcium oxide or calcium hydroxide into concentrated solution and violently stirring; adding neutral aluminum oxide into the solution and uniformly stirring; adding to the upper end of a neutral aluminum oxide preparative chromatographic column to separate and purify; collecting eluent; adding ethanol into the eluent; performing gradient cooling to separate precipitate; and drying the precipitate to obtain refined low molecular heparin calcium. The method has the advantages of simple process, convenience for operation, low cost, suitability for mass production, improved purity of the low molecular heparin calcium compound and capabilities of guaranteeing the safety of the heparin calcium compound in an anti-coagulation medicament, improving the quality of a preparation product and reducing the toxic or side effect.
Owner:灵康药业集团股份有限公司
Nonirritating eye drops for myopia
InactiveCN106620052AAvoid magnificationNo side effectsOrganic active ingredientsSenses disorderEye/ear dropsSide effect
The invention relates to the technical field of medicines, particularly nonirritating eye drops for myopia. The eye drops include, by weight, 20-25 parts of mulberry fruit, 15-20 parts of barbary wolfberry fruit, 15-20 parts of bitter apricot seeds, 20-25 parts of abalone shell, 15-20 parts of ethylparaben, 5-10 parts of glossy privet fruit, 10-15 parts of heparin calcium, 15-20 parts of ethylparaben, 3-8 parts of Chinese magnolia vine fruit, 20-25 parts of a plant extract liquid, 5-10 parts of isoproterenol, 2-4 parts of an osmotic pressure conditioning agent and 45-55 parts of distilled water. A plurality of the traditional Chinese medicines are adopted as raw materials of the eye drops, and the eye drops are safe, nonirritating and free of side effects, and have a nourishing effect and an effect of preventing the myopic degree from being deepened for patients with myopia. The adopted isoproterenol can rapidly relax ciliary muscle, and can effectively prevent mydriasis of eyes. The eye drops are convenient to use and are particularly suitable for relieving eye fatigue and preventing the myopic degree from being deepened for patients with myopia.
Owner:青岛鹏通瑞德电气科技有限公司
Eye drops
InactiveCN108283676ANo dependencyGood treatment effectSenses disorderHydroxy compound active ingredientsEye/ear dropsTreatment effect
The invention discloses eye drops. The eye drops comprise the following raw materials in parts by weight: 15-20 parts of liquid pearl, 5-8 parts of taurine, 2-5 parts of wolfberry, 1-5 parts of plantain seed, 1-5 parts of Chinese magnoliavine, 8-10 parts of vitamin, 5-10 parts of aspartic acid, 10-15 parts of cassia seed, 5-8 parts of orange peel, 1-3 parts of Chinese angelica, 1-3 parts of radixbupleuri, 5-10 parts of heparin calcium, 3-5 parts of mulberry liquid, 3-5 parts of fleece-flower root, 1-2 parts of poria cocos, 5-8 parts of a green tea extract, 5-8 parts of a ginseng extract, 10-15 parts of borneol, and 3-5 parts of antibiotic. The eye drops have the advantages that the raw materials select natural plants and Chinese herbal medicines, harmful substances such as an antiseptic and hormone on human body are not added, dependence cannot be generated for human, treatment effect is obvious, and usage is convenient and comfortable.
Owner:HUZHOU ZHIWEI TECHNICAL SERVICE CO LTD
Method and system for determining anti-Xa factor titer of low-molecular-weight heparin calcium injection
PendingCN114279989AEasy to operateImprove work efficiencyPreparing sample for investigationColor/spectral properties measurementsMethyl palmoxirateInjection solution
The invention relates to the field of chemical measurement, in particular to a method and system for measuring the anti-Xa factor titer of a low-molecular-weight heparin calcium injection.The method comprises the steps that 100 microliters of a low-molecular-weight heparin calcium solution and 100 microliters of an antithrombin solution are taken and evenly mixed, balancing is conducted for 1 minute at the temperature of 37 DEG C, and a first mixed solution is obtained; adding 200 [mu] l of an FXa solution into the first mixed solution, uniformly mixing, and balancing at 37 DEG C for 1 minute to obtain a second mixed solution; adding 500 [mu] l of a chromophoric substrate solution into the second mixed solution, mixing, and balancing at 37 DEG C for 4 min to obtain a test solution; continuously measuring the absorbance of the test solution at the wavelength of 405nm by using an ultraviolet spectrophotometer and taking water as a blank, recording the current absorbance every one minute, totally 5 minutes, and calculating the absorbance change rate; 100 [mu] l of a trihydroxymethyl aminomethane-sodium chloride buffer solution and 100 [mu] l of a test solution are taken to replace a low-molecular-weight heparin calcium solution to be operated according to the steps, the absorbance change rates are calculated respectively, operation is more convenient, and the working efficiency is improved.
Owner:江苏大同盟制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com