Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
203 results about "Sodium heparin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
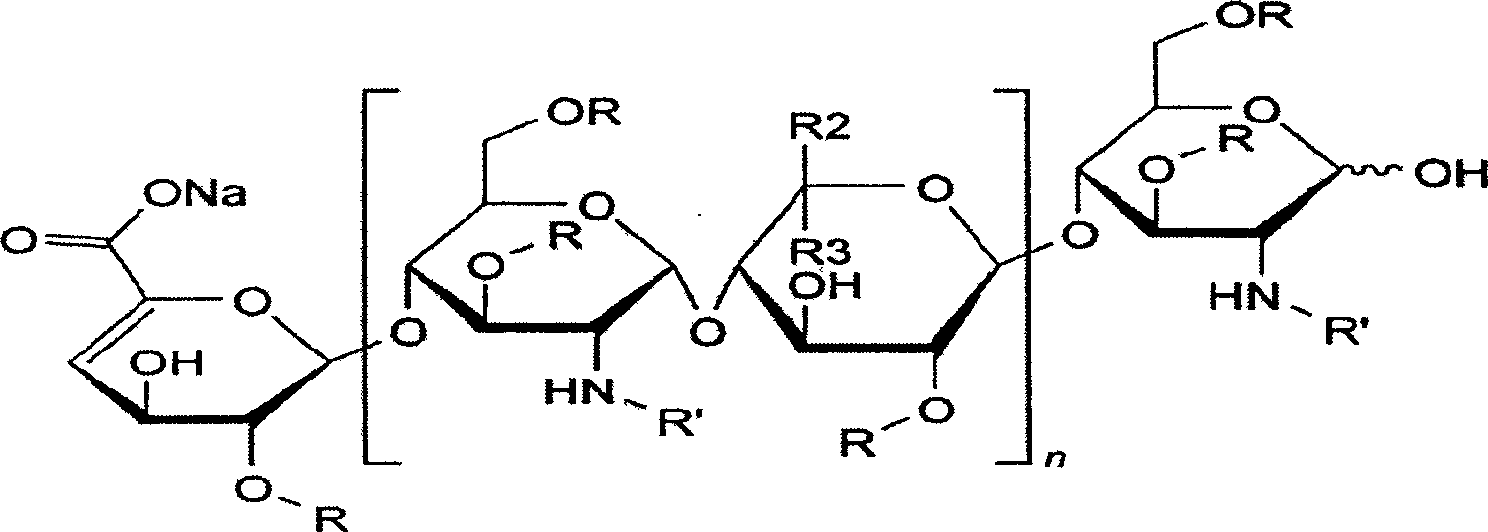
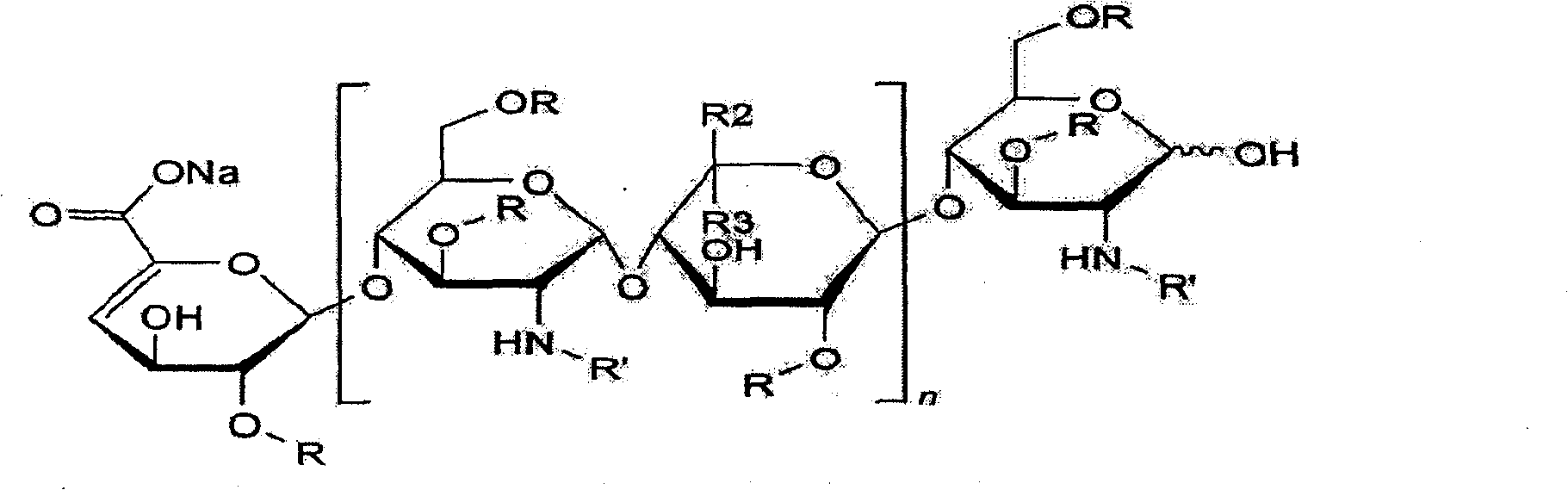
Structure of heparin sodium (representative subunits): HEPARIN SODIUM INJECTION is a sterile preparation of heparin sodium derived from porcine intestinal tissue, standardized for anticoagulant activity, in water for injection.
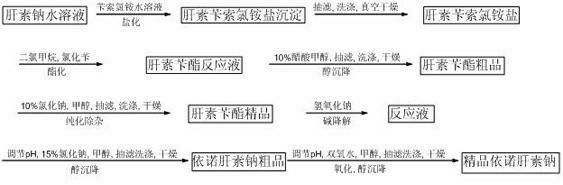
Heparin sodium and its preparing process
The invention discloses a heparin sodium and its preparing process for extracting sodium heparin from pig small intestine mucous membrane through the steps of, enzymolyzing 2709 proteinases to obtain the liquemine solution, charging sodium chloride, elevating temperature to condensate the animal protein in the pig small intestine mucous membrane, thus resulting ionic exchange for the obtained heparin sodium, performing elution with the strong alkalinity ion exchange resin after washing diluted sodium chloride solution and high concentration sodium chloride solution, subjecting the eluted heparin sodium in alcohol solution for settlement and drying to obtain the liquaemin sodium crude product, acidifying the soluble heparin crude product to remove acidic proteins, then performing purification operation of double oxidization.
Owner:张国良
Preparation method for producing heparin sodium by using porcine small intestines
The invention discloses a preparation method for separating biochemical medicaments from mucous membranes of porcine small intestines, in particular a preparation method for producing heparin sodium by using porcine small intestines. The preparation method for producing the heparin sodium by using the porcine small intestines is characterized by comprising the following steps of: preparing intestinal mucosa, and performing enzymolysis, adsorption, elution, precipitation, desalination, dehydration and drying to obtain the heparin sodium. The method has the advantages that: the heparin sodium has high yield, low activity loss and stable quality; the waste liquor is pollution-free in discharge and can be recycled; the problem of stench in a heparin sodium workshop is solved by adopting a workshop waste gas purification treatment device; and 2,000 to 2,400 pieces of porcine small intestines are needed for producing 0.1 billion units of heparin sodium by using the conventional process, only 1,400 to 1,600 pieces of porcine small intestines are needed for producing 0.1 billion units of heparin sodium by using the preparation method, and the effect and the activity of the heparin sodium are greatly improved, so that the production cost is greatly reduced, and the profit can be improved by over 40 percent.
Owner:山东绅联药业股份有限公司
Recombined human alkaline fibroblast growth factor gelling agent and process for preparing the same
InactiveCN1733294AReduce formationEffective function recoveryPeptide/protein ingredientsPharmaceutical delivery mechanismMANNITOL/SORBITOLEffective treatment
The invention relates to a recombinant human basic fibroblast growth factor gelling agent and the process for preparation, which comprises recombinant human basic fibroblast growth factor (rh-bFGF) stock solution 0.1-100% (W / W), medicinal auxiliary material 0-99.9% (W / W), the stock solution comprises effective treatment dose of recombinant human basic fibroblast growth factor (rh-bFGF) 0.01-1% (ug / g), NaCl 0.01-20% (W / W), phosphates cushioning liquid 2m Mol - 200m Mol, and right amount of water for injection, the medicinal auxiliary materials can be selected from: carbomer 940NF, sodium hyaluronate, trehalose, mannitol, dextran 40 and sodium heparin.
Owner:朗肽生物制药股份有限公司
Biomaterial for preventing accretion after surgery and preparation method
InactiveCN1539515AStrong adhesionHigh mechanical strengthSurgical drugsCoatingsTissue repairFreeze-drying
A biologic material for preventing adhesion after operation is prepared from chitosan and sodium hyaluronate through preparing their solutions, proportionally mixing, defoaming, loading in mould, freeze drying to become film or tube, immersing the mixed liquid, flushing by distilled water drying and sterilizing. It is possible to attach the antiadhering medicine to it. Its advantages are high attachment, mechanical strength and antibacterial power, and better biocompatibility and bioactivity.
Owner:刘永庆
Method for extracting sodium heparin
The invention relates to a method for extracting sodium heparin, which comprises the following steps: (1) enzymic hydrolyzing intestinal mucosa in a conventional method, and adopting enzyme preparation for the hydrolysis: mixture which is formed by mixing prolease, papain and lipase at a mass ratio of 1 to 4: 1 to 3: 0.1 to 0.3, and selecting the prolease from one of 2709 enzyme, AS1.398 enzyme and pancreatin; (2) resin adsorbing in a conventional method; (3) resin eluenting in a conventional method; (4) settling out the sodium heparin in a conventional method; (5) purifying the sodium heparin in a conventional method; (6) drying the sodium heparin in a conventional method to obtain the pure sodium heparin. An improved solution is characterized in that precipitator is added before filtering and hydrolyzing the mixture in the step 1. The method has simple process, short production period, simple and convenient operation and less investment, and is applicable to the industrialized mass production. The purity of the heparin can reach 100 to 120IU / mg, the extraction efficiency can reach 100 million IU / 1700 to 1800 chatterlings, the extraction efficiency can be improved by 20 to 30 percent, consumed salt can be reduced by 30 to 50 percent, water can be reduced by 50 to 70 percent, energy can be reduced by 30 to 40 percent, and the recycling rate of the crude protein can reach 60 percent.
Owner:GUANGYUAN HAITIAN IND
Production method of nadroparin calcium
ActiveCN103275246AEfficient degradationAvoid the tedious process of repeated impurity removalUltrafiltrationHalf-life
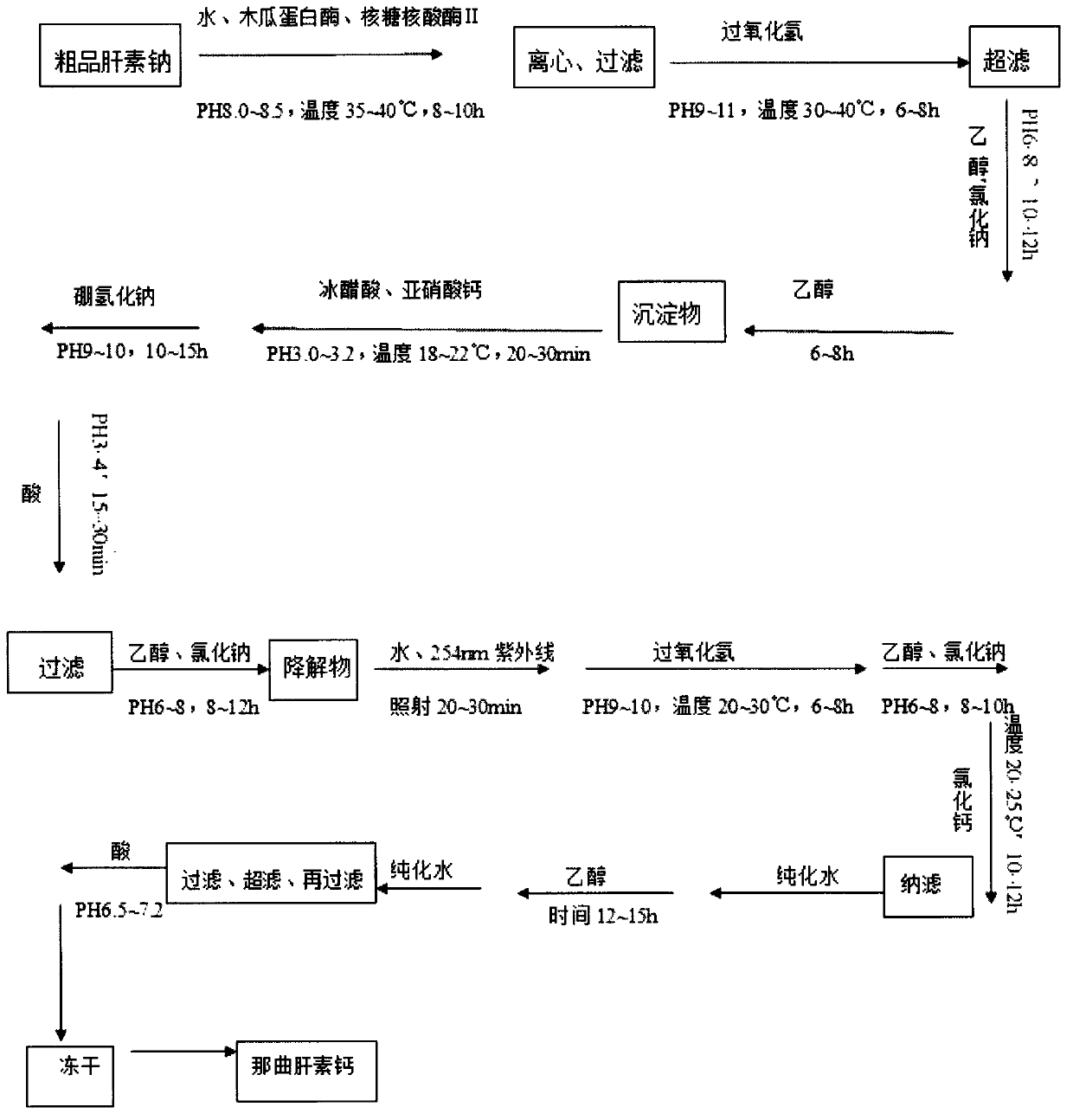
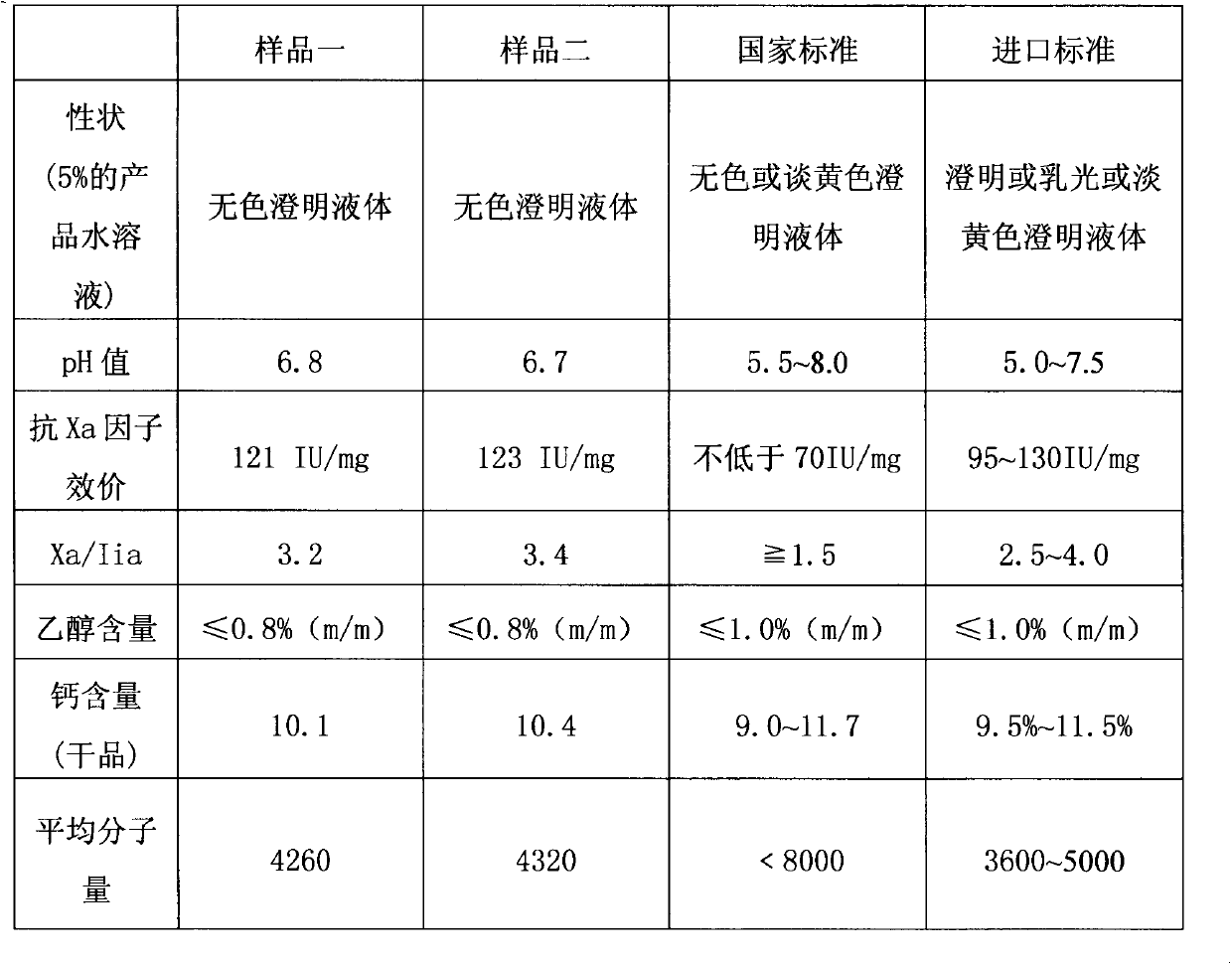
The invention discloses a production method of nadroparin calcium and belongs to the field of biomedicine. According to the method, crude heparin sodium is taken as raw material. Basing on enzymolysis and improved nitrite degradation method, nadroparin calcium high-quality product with particular average molecular weight (3800 to 5000Da) is prepared by enzymolysis, oxidative decolorization, ultrafiltration and impurity removal, ethanol precipitation and impurity removal, degradation, reduction, ultraviolet radiation, oxidation, Calcium replacement, nanofiltration, Ultrafiltration and refining, freeze-drying and other steps. The method provided by the invention has advantages of simple preparation technology, high bioavailability of the product, long half life in vivo, small hemorrhagic tendency and the like.
Owner:山东辰龙药业有限公司
Method for preparing enoxaparin sodium
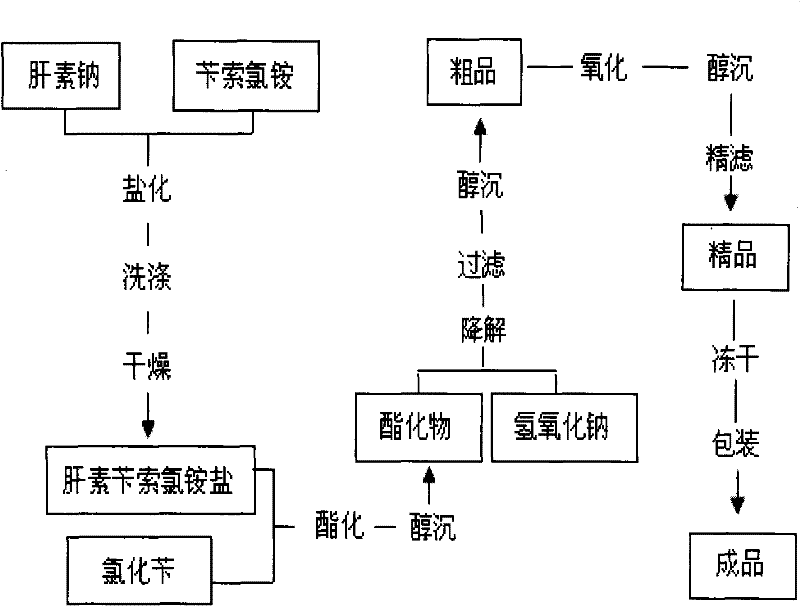
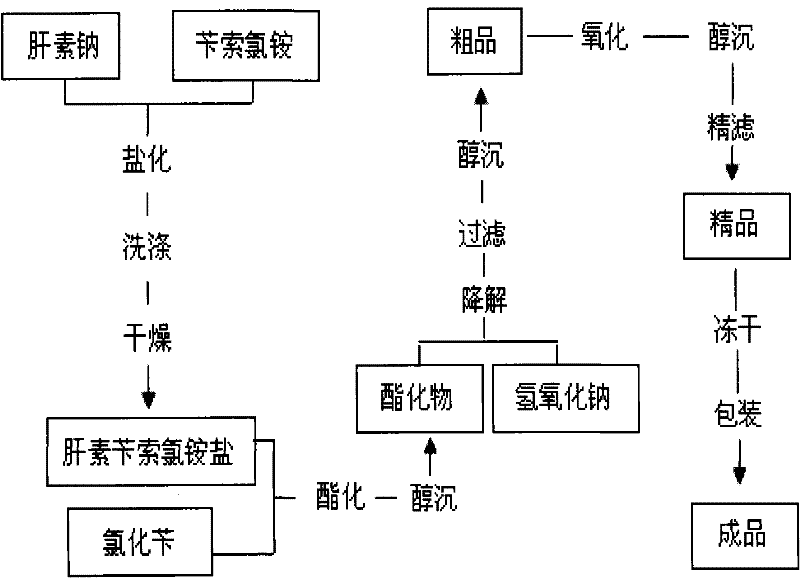
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminating the side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Production method for purifying enoxaparin sodium
InactiveCN1850865AWon't breakEfficient separationOther chemical processesBenzyl chlorideSodium heparin
The invention relates to a purifying manufacture method for Yino sodium heparin that adopts long chain quaternary ammonium salt salinization heparin, after taking benzyl chloride esterification, gaining the raw Yino heparin raw product. After taking decoloration by active carbon and macropore adsorptive resin, the sodium heparin product could be gained. The invention is easy to realize industrializing producing.
Owner:HANGZHOU JIUYUAN GENE ENG
Growth factor-loading silk fibroin/collagen bracket material and preparation method thereof
InactiveCN104667349ASolve the problem of too short half-life in vivoEffective induction of growthProsthesisPolymer scienceNanoparticle
The invention discloses a growth factor-loading silk fibroin / collagen bracket material and a preparation method thereof. The material is prepared from the raw materials of collagens, growth factors, silk fibroin, polylysine (PLL) and heparin sodium. A loose layer fibroin / collagen film and a compact layer fibroin / collagen film form a double-layered structure, and growth factor-loading PLL-heparin sodium nanoparticles are compounded in the middle of the double-layered structure. The preparation method comprises the following steps: preparing the loose layer fibroin / collagen film and the compact layer fibroin / collagen film; preparing the growth factor-loading nanoparticles, compounding the fibroin / collagen film and the nanoparticles and the like. The growth factor-loading silk fibroin / collagen bracket material disclosed by the invention plays a directional differential slow release role on the loaded growth factors and can fully maintain the activity of the growth factors and effectively prolong the action time of the growth factors, so that the material is a good guided tissue regeneration material for prosthodontic treatment of impaired and damaged tissues.
Owner:FUZHOU UNIV
Method for separating and purify dermatansulfate and low-molecular heparan sulfate from sodium heparan product
ActiveCN1850864AStable separationImprove solubilityOrganic active ingredientsDermatan sulfateSodium heparin
The invention relates to a method to separate and purify dermatan sulfate and low molecule heparan sulfate from sodium heparin by-product. It uses the by-product of producing sodium heparin as raw material taking fractional precipitation through alcohol to gain raw dermatan sulfate; uses nitrite and nitrous acid ester compounding as oxidant to degrade the heparan sulfate into low molecule heparan sulfate and taking fractional precipitation through alcohol to separate high purity dermatan sulfate and low molecule heparin sulfate.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Method for quickly precipitating and separating oversulfated chondroitin sulfate in sodium heparin
The present invention discloses a method for quickly precipitating and separating oversulfated chondroitin sulfate in sodium heparin. The method adopts an ion exchange method to remove a mass of impurities combined with a hydrogen peroxide oxidation method, which greatly improves the color and the potency of the sodium heparin, quickly precipitates and separates the oversulfated chondroitin sulfate in the sodium heparin by alcohol, which ensures the yield coefficient of the sodium heparin more than 85 percent and the potency more than 180u / mg, and each index totally satisfies the USP and EP standards. The present invention is more remarkable than the reported acetone extraction method; because the solvability of the oversulfated chondroitin sulfate and sodium heparin is very low to ethanol and acetone and the like, the separating effect of the organic solvent extraction method is bad, specifically, the separating effect is more unconspicuous when the oversulfated chondroitin sulfate in sodium heparin is higher. The quick precipitation method is designed according to the feature that the absolute molecular weight of the oversulfated chondroitin is greater than the sodium heparin and the differences of structure and the like. The method is featured with simple operation and notable separating effect.
Owner:江苏麦德森制药有限公司
Reagent, method and kit for measuring small-and-dense lipoprotein
The invention discloses a reagent, a method and a kit for measuring small-and-dense lipoprotein. The reagent comprises first agents and second agents, the first agents include 1-500 mg / L reagent A, 10-300 U / mL sodium heparin, 0.1-90 mmol / L divalent metal ion, 0.5-2 KU / L cholesterol esterase, 1-3.5 KU / L cholesterol oxidase, 100-300 KU / L catalase, 0.1-10 mmol / L 4-amino antipyrine, 0.05-6% KU / L surfactant A, and the second reagents include 0.2-10 KU / L peroxidase, 0.3-20 mmol / L Trinder's chromogen compound, 0.01-0.3% sodium azide and 0.05-12% surfactant B. The reagent can be used for specifically detecting sdLDL. By using the method, sdLDL can be detected specifically. The kit is simple and convenient to operate.
Owner:上海微鸿企业管理有限公司
Process for producing low molecular sodium heparin
The invention discloses a process for producing low molecular sodium heparin by using raw sodium heparin as a raw material, which comprises the steps of: producing a sodium heparin solution; degradingthe solution; neutralizing the solution; collecting a precipitation; producing low molecular raw sodium heparin; producing low molecular fine sodium heparin and the like. The low molecular sodium heparin has the characteristics of rich sources of the raw material, high yield, stable and reliable quality, high purity, low cost, long curative effect, no toxic or side effect, simple process, convenient operation and no 'three wastes' discharge, has the effects of coagulation resistance, thrombosis resistance, tumor resistance, inflammation resistance, allergy resistance and the like and the efficiency of regulating blood lipid, has significant curative effect and wide application range, can be produced into injections, capsules, ointments and the like, is convenient for massive production, and is widely used for producing coagulation resistant low molecular sodium heparin.
Owner:SICHUAN TIANCHENG BIOCHEM TECH
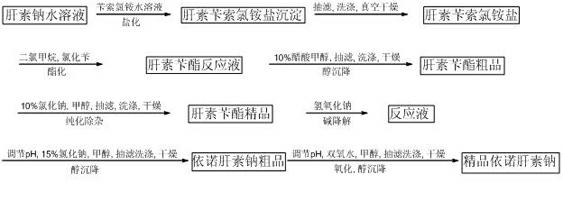
Enoxaparin sodium and production purification method thereof
InactiveCN102585037AReduce manufacturing costImprove product qualityBlood disorderExtracellular fluid disorderPurification methodsBenzyl chloride
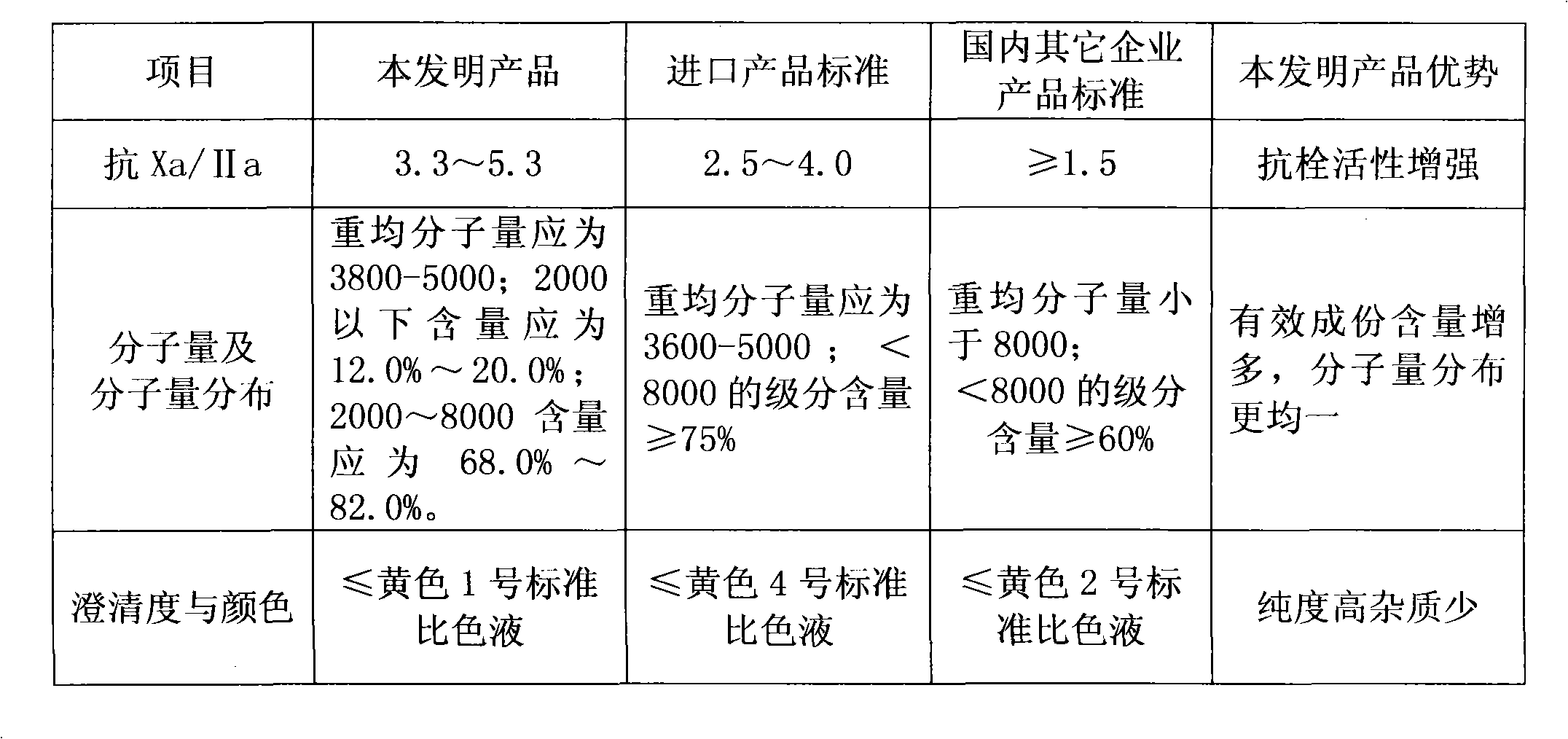
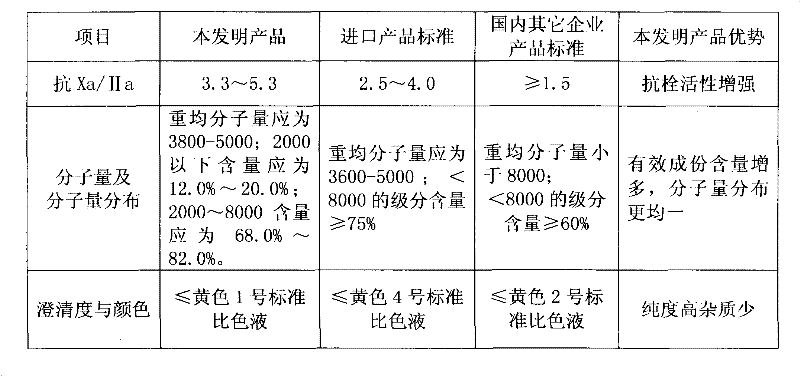
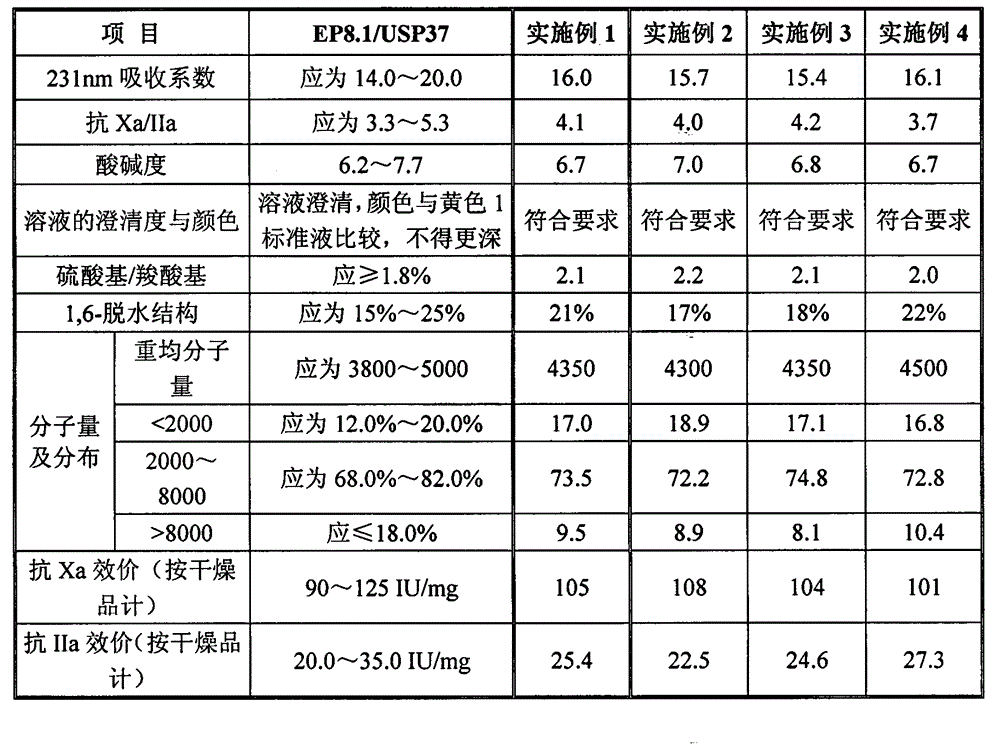
The invention discloses enoxaparin sodium. The average molecular weight of the enoxaparin sodium is between 3500-5500 dalton, thrombolytic biological activity is 100-125IU / mg, and the ratio value of resistance to Xa and IIa resistance is between 3.3-5.3. A production purification method of the enoxaparin sodium comprises preparation of heparin benzyl chloride ammonium salt, preparation of heparin benzyl ester, purification of heparin benzyl ester, preparation of enoxaparin sodium and purification of enoxaparin sodium. By means of the enoxaparin sodium, the molecular weight and distribution range of a product are controlled, and the quality of a fine enoxaparin sodium product meets the quality standard of the european pharmacopoeia. The enoxaparin sodium adopts a crude product of heparin sodium as an initial raw material and can effectively reduce production cost. The refined heparin benzyl ester stabilizes the final quality of the product. Purification difficulties are simplified, the coloring problem in the production is effectively solved, and the product quality is improved.
Owner:麦科罗夫(南通)生物制药有限公司
Novel process for producing sodium heparin
The invention provides a novel process for producing sodium heparin, which comprises the following steps of enzymolysis, gum exchange adsorption, resin washing, heparin eluting, alcohol precipitation,precipitation once more, dehydration drying, crushing, mixing and packaging. The process has mature flow, convenient operation, no emission of waste intestinal dregs and overproof wastewater, complete enzymolysis reaction, short reaction time of 30 hours, low production energy consumption, low reaction temperature of between 70 and 80 DEG C, heparin extracting ratio over 90 percent, high unit yield of the heparin, only need of 1,700 pieces of small intestines of pigs for each 100 million units of heparin, low production cost, increase of economic benefit over 30 percent per 100 million units,no emission of dirty intestinal dregs and overproof wastewater, and suitability for large-scale production.
Owner:SICHUAN TIANCHENG BIOCHEM TECH
Method for extracting sodium heparin and co-producing amino acid
The invention discloses a method for extracting sodium heparin and co-producing amino acid, comprising the following steps: adding refined salt accounting for 1-1.8 weight percent of an intestinal mucosa into small intestinal mucosa; regulating the pH value of a solution to 9.0-9.2; adding fresh porcine pancreas to carry out zymolysis and filtering; carrying out resin absorption, elution and deposition; and drying to obtain the sodium heparin crude product; adding parenzyme into a filtering liquid, keeping the temperature to carry out the zymolysis and filtering to obtain an amino acid solution. The method for extracting sodium heparin and co-producing amino acid co-produces the amino acid solution on the basis of extracting the sodium heparin, thereby improving the economic effect, reducing the waste liquid and lightening the environment pollution.
Owner:牛玉娥
Process using intestinal casing waste liquid as raw material for production of sodium heparin and intestinal membrane protein
The invention discloses a process using intestinal casing waste liquid as a raw material for production of sodium heparin and intestinal membrane protein, and the process is characterized in that, the preparation process comprises the following steps: the intestinal casing waste liquid is taken as the raw material for enzymolysis, resin adsorption and filtration to respectively obtain resin and filtrate; the resin is washed, eluted, precipitated and dried to obtain the sodium heparin, and the filtrate is concentrated and dried to obtain the intestinal membrane protein. The process comprehensively uses the intestinal casing waste liquid, is simple, not only greatly improves the economic benefit, but also helps to improve the environment.
Owner:GUANGYUAN HAITIAN IND
Separation purification method of refined heparin sodium
The invention discloses a separation purification method of refined heparin sodium. The method comprises the steps of: (1) salt hydrolysis; (2) adsorption and elution; (3) ethanol precipitation; (4) first oxidation; (5) secondary oxidation; and (6) precipitation. The method provided by the invention purifies crude heparin sodium by a two-step oxidation, not only reduces the production cost, and has no need for special equipment, also the prepared refined heparin sodium product has good purity and high recovery rate, and the quality and activity of heparin sodium are ensured.
Owner:ANHUI IND & TRADE VOCATIONAL TECHN COLLEGE
Method for extracting heparin sodium from small intestine of pig and co-producing protein powder
ActiveCN103665192AIncrease added valueExtraction rate is not affectedFermentationPROTEIN S HEERLENMedicine
The invention relates to a method for extracting heparin sodium, and particularly relates to a method for extracting heparin sodium from a small intestine of a pig and co-producing protein powder. The method comprises steps of (1) carrying out enzymolysis and salt hydration; (2) separating protein powder out; and (3) extracting heparin sodium: adsorbing, eluting, precipitating and drying. The method improves the yield of heparin sodium, furthermore, the equipment for co-production has higher usage rate than single producing equipment, saves resources, and can lower the production cost of heparin sodium and protein powder. The small intestine of the pig is fully used and is improved in additional value, furthermore, the pollution of waste liquid and waste residue containing protein to environment is reduced, and the waste liquid and waste residue are turned into wealth.
Owner:SHIFANG LELI FUYANG BIOLOGY CO LTD
Production method for purifying enoxaparin sodium
InactiveCN100436483CWon't breakEfficient separationOther chemical processesBenzyl chlorideSodium heparin
The invention relates to a purifying manufacture method for Yino sodium heparin that adopts long chain quaternary ammonium salt salinization heparin, after taking benzyl chloride esterification, gaining the raw Yino heparin raw product. After taking decoloration by active carbon and macropore adsorptive resin, the sodium heparin product could be gained. The invention is easy to realize industrializing producing.
Owner:HANGZHOU JIUYUAN GENE ENG
Method for separating and cultivating rat hepatocytes
InactiveCN101538552AExcellent adhesionA large amountTissue cultureBovine serum albuminCell separation
The invention provides a method for separating and cultivating rat hepatocytes. A rat is anaesthetized by Nembutal and is given sodium heparin for anticoagulation; portal catheterization is carried out; two-step reperfusion is carried out for perfusion; a digestive mature liver is taken off; hepatocytes are collected in Hanks liquid containing 1 percent of bovine serum albumin (BSA); hepatocyte suspension is screened by a screen stencil; after the filtrate is centrifugated and deposited, the deposit is collected in Leibovitz's-15(l-15) complete medium; the density of hepatocyte is adjusted to 3 to 6 *10<5> / ml and inoculated on a culture plate coated with rat tail collagen in the environment of temperature of 37 DEG C and 5 percent of CO2; and unattached and dead hepatocytes are removed by changing medium after 4 hours of inoculation, and the hepatocytes can be used for experiment after 20 hours of cultivation. The method is easy, simple and stable, has low cost and high efficiency, can obtain high-activity hepatocytes, is easy to attach, and can be used for pharmacological and toxicity experiments and popularized in common laboratories.
Owner:YANGZHOU UNIV
Process for using intestine casing to extract sodium heparin
ActiveCN102633907AImprove solubilityEasy to separate and purifyIntestinal structureTransmitted power
The invention relates to a process for using an intestine casing to extract sodium heparin. The process provided by the invention mainly comprises the following steps: material processing, enzymatic extraction, adsorption, desorption, precipitation and oven-drying. The innovative points provided by the invention comprise: mixed liquor of mucous membrane of small intestine and feed liquid is firstly put in a vibrating enzymolysis machine; protease is added in the vibrating enzymolysis machine for vibrating enzymolysis; the vibration frequency and the vibration time of the vibrating enzymolysis machine are 250HZ and 20-35min, respectively; then the mixed liquor is put in an ultrasound machine for further enzymolysis; and the ultrasound machine has an ultrasonic wave transmitting power at the range of 240-270W, a total ultrasonic wave transmitting duration time at the range of 7-9min and a single ultrasonic wave transmitting duration time at the range of 10-12S. According to the invention, the extraction process is adopted to extract sodium heparin, so as to increase the extraction volume by 18-23% compared with a single enzymatic method, increase the extraction volume by 13-16% and save the extraction time by 30-72min compared with a single ultrasonic wave method. The process provided by the invention has high extraction ratio and reduces the production cycle.
Owner:RUGAO YONGXING CASING
Method for preparing enoxaparin sodium
The invention discloses a method for preparing enoxaparin sodium, comprising the steps of salinizing, drying, esterfying, alcohol precipitating, oxidizing, alcohol precipitating, fine filtering and freeze drying. In the method provided by the invention, a hydrophilic liquid phase reaction, a hydrophobic liquid phase reaction and a solid phase reaction are adopted, so that macromolecule sodium heparin is degraded into micromolecule sodium heparin with a specific structure, and the molecular weights of products and molecular weight distribution ranges are controlled, thus anti-FIIa activity resulting in bleeding risk is greatly reduced, the anti-FXa activity is relatively improved, and the product effectiveness and safety advantages are obvious. The enoxaparin sodium can be used for effectively preventing venous thromboembolism and pulmonary embolism, can be used for thrombosis before and after operations of orthopedic surgery and neurosurgery, and can be used for greatly reducing apoplexy risk, more effectively reducing death, cardiac failure and recurrent angina of patients suffering from unstable coronary artery syndromes, reducing hypertriglyceridemia and effectively eliminatingthe side effects of haemorrhage, osteoporosis and induced thrombocytopenia after long-term use of common unfractionated heparin sodium and derivates of common unfractionated heparin sodium.
Owner:HEBEI CHANGSHAN BIOCHEM PHARMA
Method for producing enoxaparin sodium by using crude sodium heparin products
ActiveCN104558252AGuarantee product qualityFree from multiple oxidationDepolymerizationFreeze-drying
The invention discloses a method for producing enoxaparin sodium by using crude sodium heparin products. The method is implemented by taking crude sodium heparin products as a raw material through the steps of pretreating the crude sodium heparin products by using a salt hydrolysis process; sequentially carrying out oxidation, ion exchange resin adsorption, washing and elution on the obtained object; sequentially carrying out ultrafiltration and freeze-drying on the obtained product so as to obtain a fine sodium heparin product; and sequentially carrying out salifying, esterification, depolymerization, oxidation, alcoholic precipitation, and freeze-drying treatment on the fine sodium heparin product, so that enoxaparin sodium is obtained. The method disclosed by the invention has the advantages that the quality of enoxaparin sodium is controlled from the aspects of source and process, the production cycle is short, the energy consumption of production is low, and the quality of products is high, therefore, the method is suitable for large-scale industrial production.
Owner:NORTH CHINA PHARMA HUAKUN HEBEI BIOTECH
Synthesis method of 6-O-carboxymethyl chitosan sulfuric sulfation product
The invention discloses a synthesis method of a 6-O-carboxymethyl chitosan sulfation product. The method comprises the following steps: (1) chitin alkaline treatment of chitin; (2) carboxymethylation of C6-O site of chitin; (3) deacetylation reaction of 6-O-carboxymethyl chitin; and (4) sulfation of 6-O-carboxymethyl chitosan. The synthesis method disclosed by the invention is a bran-new method for selectively replacing and controlling the replacement rate by using chitin. The synthesis product of the method provides N-site -SO3H with main anticoagulant activity and introduces -COOH, so that a lot of -COOH and SO3H which have negative electricity in the molecular structure are regularly distributed, and an anticoagulant effect of heparinoid is generated by the synergistic effect. High molecular polysaccharide is a heparinoid drug which is selectively modified by chitin via a safe reagent, so that reagent pollution of bulk drugs is reduced, the virus contamination risk of heparin biological extraction is avoided, the safety performance of the drug in the clinical experiment is more excellent in theory as compared with heparin sodium, so the 6-O-carboxymethyl chitosan sulfation product provided by the invention is expected to serve as a cheap direct thrombin inhibitor to replace heparin sodium anti coagulation drugs.
Owner:SHENZHEN BRIGHT WAY NOVEL BIO MATERIALS TECH CO LTD
Heparin compound, and its preparing method and use
InactiveCN1580080AImprove efficacyDoes not affect testingOrganic active ingredientsBlood disorderSolubilityZinc hydroxide
The invention refers to a heparin compound and its preparation method and application. The heparin compound is a heparin zinc-potassium double salt demonstrated by the formula of R(ZnOH)aKb. The R represents the heparin radical and a is between 3 and 5, b between 1 and 3. The heparin compound can be made through the following preparation method: dissolve the sodium heparin into the water and contact with the cinnamene cation exchange resin to get the heparin solution; add an amount of zinc hydroxide and potassium hydroxide to the heparin solution, whisk and dry it and get the mentioned solid heparin compound. The heparin compound can be used as the anticoagulant agent as well as the main material of the anti thrombus medicine. The heparin zinc-potassium as the anticoagulant agent meets not only the requirements of not affecting the components' balance and stability of the blood samples to be tested but also the requirement of the solubility in the blood. When it is chosen as the main resource of the anti thrombus medicine, the heparin zinc-potassium releases slowly, has long effect of medicine and no toxicity and is good for the zinc-adding of the human body.
Owner:汕头市金丰医疗器械科技有限公司
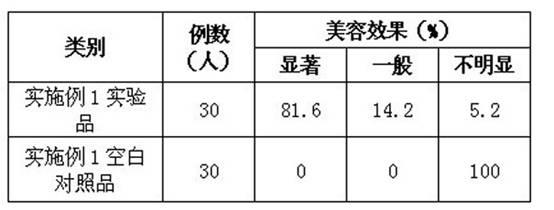
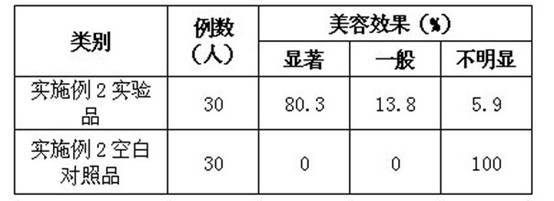
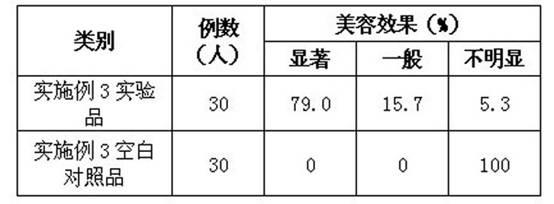
Method for preparing relaxing and face-cleansing cosmetics and application
ActiveCN102379838ACosmetic preparationsAntipyreticRecombinant Human Keratinocyte Growth FactorVitamin C
The invention relates to a method for preparing relaxing and face-cleansing cosmetics and application. The cosmetics comprises the following components in percentage by mass: 0.00001 to 0.001 percent of recombinant human keratinocyte growth factors, 0.00001 to 0.001 percent of recombinant human interleukin-1 receptor antagonist, 0.1 to 0.5 percent of low-molecular sodium heparin, 5 to 25 percent of stabilizer, 0.02 to 0.3 percent of hyaluronic acid, 0.5 to 8 percent of vitamin C sodium phosphate, 0.5 to 5 percent of methyl propanediol, 0.3 to 0.7 percent of 1,2 hexanediol, 0.5 to 3.5 percent of lactobacillus / mung bean fermentation liquor, 0.5 to 5 percent of dissolved protease and the balance of water. The relaxing and face-cleansing cosmetics are applied to sensitive skin, can be stored for a long time, and is suitable for long-term use.
Owner:广州泰润合投资有限公司
Method for extracting sodium heparin crude product
The invention discloses a method for extracting a sodium heparin crude product. The method comprise the following steps:: adding alkali to fresh intestinal mucosa water, keeping temperature and discharging material, and filtering by using a nylon mesh cloth of 90-120 meshes; carrying out a secondary extraction on filter residues and combining filtering liquids in the two steps of filtering; heating the combined filtering liquids and adding fresh porcine pancreas to react for 1 to 2 hours; putting resins into the filtering liquids to elute; adding alcohol for static deposition in the filtered elution liquid; extracting the deposit and placing in a Busher funnel to vaccumize; and dewatering and drying to obtain the heparin crude product. Combining an enzymolysis method with a salt method, the invention has the advantages of low concentration of the waste liquid and easy post-treatment. Because the process adopts the two-step decomposition of the salt method and the enzymolysis method, the sodium heparin dissociates completely with higher yield.
Owner:赵娟珍
Clexane and preparation method thereof
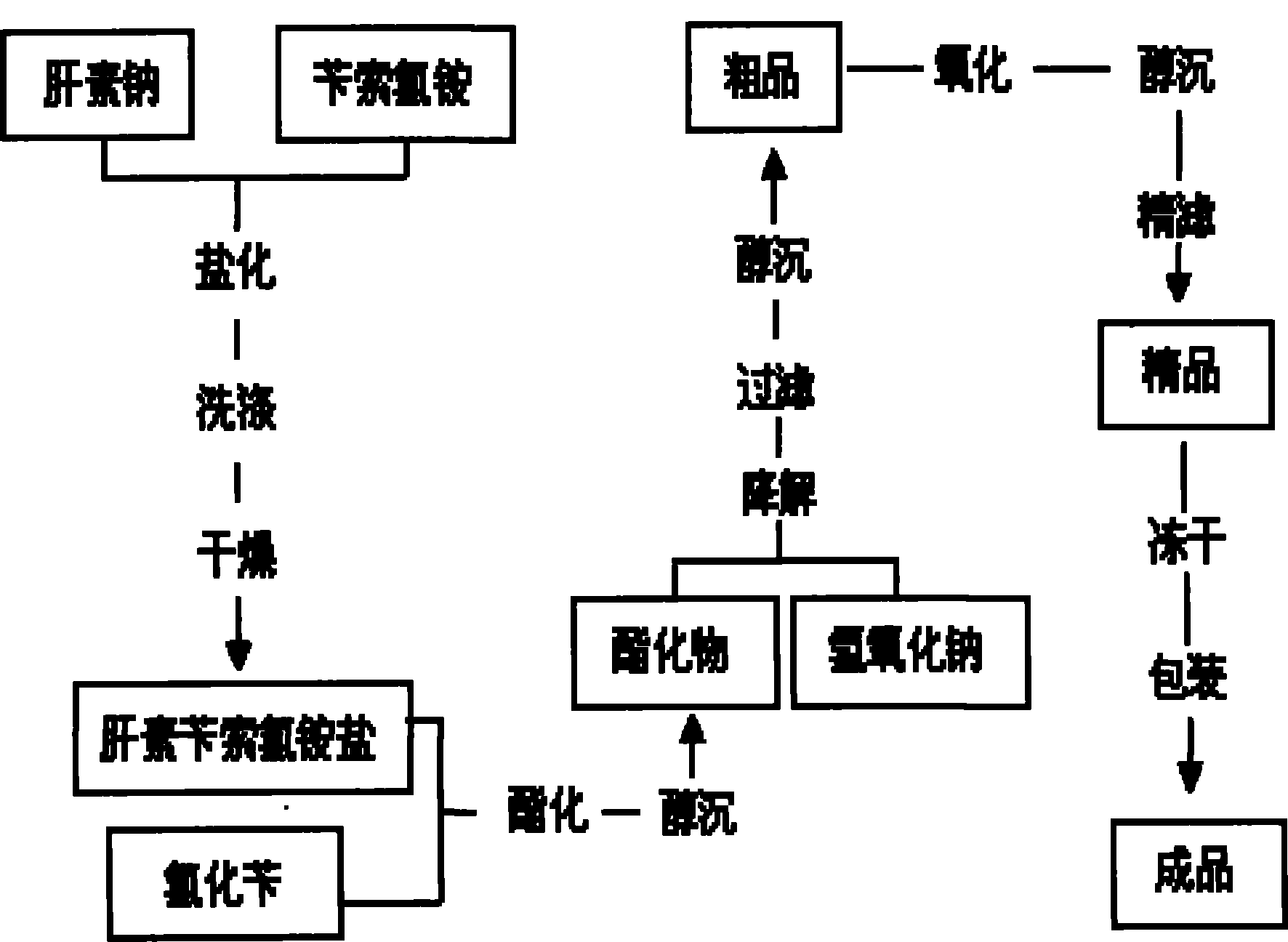
ActiveCN100582123CReduce manufacturing costImprove product qualityOrganic active ingredientsBlood disorderSodium acetateBenzyl chloride
The present invention is enoxaparin and its preparation process, and features that the preparation process includes the following steps: dissolving sodium heparin in water, dissolving benzethonium chloride in water, mixing these two kinds of solution to react, vacuum suction filtering, washing the solid with water and stoving to obtain quaternary ammonium salt of heparin; dissolving the salt in dichloromethane, adding benzyl chloride to react, adding methanol solution of sodium acetate to produce precipitate, suction filtering, washing the solid with methanol and stoving to obtain heparin benzyl ester; dissolving heparin benzyl ester in 0.1N water solution of sodium hydroxide, adding sodium chloride, membrane filtering after dissolving, adding methanol to separate out precipitate, suction filtering, washing and stoving solid to obtain coarse enoxaparin sodium product; and purifying to obtain refined enoxaparin sodium product. The present invention has low production cost and high product quality.
Owner:JIANGSU ALAND NOURISHMENT
Coating method for activated carbon adsorbent for blood perfusion
InactiveCN109621912AGood blood compatibilityImprove anticoagulant performanceOther blood circulation devicesOther chemical processesCrosslinked chitosanActivated carbon
The invention provides a coating method for an activated carbon adsorbent for blood perfusion. Firstly, coating film is formed on the surface of activated carbon with chitosan, then crosslinking is executed with epichlorohydrin to increase the strength of the film, and finally heparin sodium having an anticoagulant effect is grafted to the surface of the film. The crosslinked chitosan film can improve the blood compatibility of the activated carbon and decrease particle falling of the activated carbon; the heparin sodium having an excellent anticoagulant property is grafted on the film so thatthe blood coagulation phenomenon occurring when blood is in contact with the surface of the activated carbon can be decreased, and the safety of a perfusion device is improved.
Owner:重庆希尔康血液净化器材研发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com