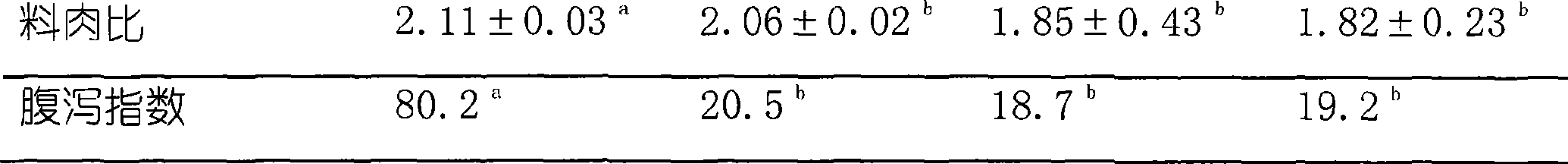Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Million Units" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blocking buffer for encapsulated plate
The invention provides a blocking buffer for encapsulated plate, which consists of 0.5 to 10g / L of protein substances, 0.5 to 5g / L of sugar substances, 0.5 to 10g / L of amino acid substances, 0.2 to 2g / L of surfactant, 50 to 5 million unit / L of gentamicin sulphate, 0.2 to 2g / L of Proclin 300 and 0.02mol / L of phosphate buffer with the pH of 7.4. The encapsulated plate using the blocking buffer of the invention can not only block off biological active substances, but can also maintain the activity of the biological active substances for a longer time when the encapsulated plate departs from low temperature environment, thus the problem in relevant technology that the encapsulated plate is liable to lose activity when departing from lower temperature storage environment can be settled. In addition, the blocking buffer of the invention is easy in formulation, free from the impact on sensitivity, low in cost and simple in operation and has no environmental pollution and no toxicity and harm to human body.
Owner:刘萍 +1
Novel process for producing sodium heparin
The invention provides a novel process for producing sodium heparin, which comprises the following steps of enzymolysis, gum exchange adsorption, resin washing, heparin eluting, alcohol precipitation,precipitation once more, dehydration drying, crushing, mixing and packaging. The process has mature flow, convenient operation, no emission of waste intestinal dregs and overproof wastewater, complete enzymolysis reaction, short reaction time of 30 hours, low production energy consumption, low reaction temperature of between 70 and 80 DEG C, heparin extracting ratio over 90 percent, high unit yield of the heparin, only need of 1,700 pieces of small intestines of pigs for each 100 million units of heparin, low production cost, increase of economic benefit over 30 percent per 100 million units,no emission of dirty intestinal dregs and overproof wastewater, and suitability for large-scale production.
Owner:SICHUAN TIANCHENG BIOCHEM TECH
Biological cell freezing antifreeze agent
InactiveCN101220346AImprove survival rateSolve the worldwide problem of low survival rateDead animal preservationTissue cultureFiltrationGlycerol
The invention discloses a biological cell freezing antifreeze, and 100ml of the cell freezing antifreeze consists of the following raw materials: 0.025 to 0.3g of kelp polysaccharide, 2.0 to 3.5g of glucose, 0.5 to 1.5g of sodium citrate, 60 to 80ml of distilled water, 10 to 20ml of albumin, 5 to 18ml of glycerol and 0.05 to 0.15 million units of penicillin. The preparation method is that: the glucose and the sodium citrate are dissolved in the distilled water to prepare a base liquid; the albumin is added, the mixture is mixed evenly, and the penicillin is added to prepare a I liquid; the glycerol is added in the I liquid to prepare a II liquid; the kelp polysaccharide is added in the II liquid for even mixing; the pH value is adjusted to 6 to 7.5, then the filtration and sterilization are carried out, and the mixture is cooled until reaching the room temperature and then arranged in a refrigerator of 2 to 5 DEG C for standby. The cell freezing antifreeze is applicable to the cryopreservation of mammalian semen, somatic cells, oocytes, early embryos, organs and tissues, in particular to the cryopreservation of the semen of various mammals.
Owner:NORTHWEST A & F UNIV
Prepn. process for freezing dry powder of bromelain
InactiveCN1834239APromote absorptionAnti-inflammatoryPowder deliveryPeptide/protein ingredientsLow activityFreeze-drying
This invention discloses a method for manufacturing pineapple proteinase freeze-dried powders, which can solve the problem of low activity faced by conventional pineapple proteinase. The method comprises the steps of: washing pineapple stems with water, pressing to obtain juice, filtering, adsorbing the filtrate with zinc oxide, desorbing, ultrafiltering, concentrating and freeze-drying to obtain pineapple proteinase freeze-dried powders, whose enzyme activity can reach 600 million units per gram.
Owner:王美岭 +1
Fat-soluble vitamin nano microemulsion oral liquid and preparation method thereof
ActiveCN102100304ASolve the problem of low bioavailabilityAnimal feeding stuffAccessory food factorsVitamin E AcetateVitamin b6
The invention discloses a fat-soluble vitamin nano microemulsion oral liquid which comprises 2-4g of vegetable oil, 4-6g of vitamin E acetate, 3-3.6 million units of vitamin D3, 2-4g of vitamin K3, 8-12ml of Tween-80, 3-5g of RH 40, 5-7g of AEO-9 (fatty alcohol-polyoxyethylene ether-9), 0.3-0.7g of folic acid, 13-17g of calcium pantothenate, 1-2g of antioxidant, 0.8-1.4g of vitamin C, 8-11 million units of vitamin A, 32-36g of nicotinamide, 2.5-4.5g of vitamin B2, 8-12g of glycine, 1.8-2.4g of vitamin B1, 40-80mg of biotin, 0.8-1.2g of vitamin B6 and 8-12mg of vitamin B12. The invention also discloses a preparation method of the fat-soluble vitamin nano microemulsion oral liquid.
Owner:SHANDONG SINDER TECH
Injection for preventing and treating porcine viral diseases and preparation method thereof
InactiveCN102078598ARaise the level of responseEfficient removalAntibacterial agentsOrganic active ingredientsAntioxidantInterferon alpha
The invention belongs to the technical field of veterinary medicine, specifically to an injection for preventing and treating porcine viral diseases and a preparation method thereof. Every 1000ml of the injection contains 1 to 100g of thymopoietin, 1 to 10*10 <8> IU of interferon, 10 to 50 million units of gentamycin sulfate, 0.1 to 2g of antioxidant, 0.2 to 0.4g of protective agent, 50 to 100ml of buffer, 20 to 80ml of osmotic pressure adjusting agent, a pH adjusting agent and the balance of water for injection. The preparation in the invention is wide in application, fast in curative effect, little in medicine dosage, low in cost, and difficult to bring germ contamination. The injection reduces the stress reaction caused by capturing the animal for many times in injecting, and overcomes the shortcoming that the water needle made of thymopoietin and interferon is unstable.
Owner:河南亚卫动物药业有限公司
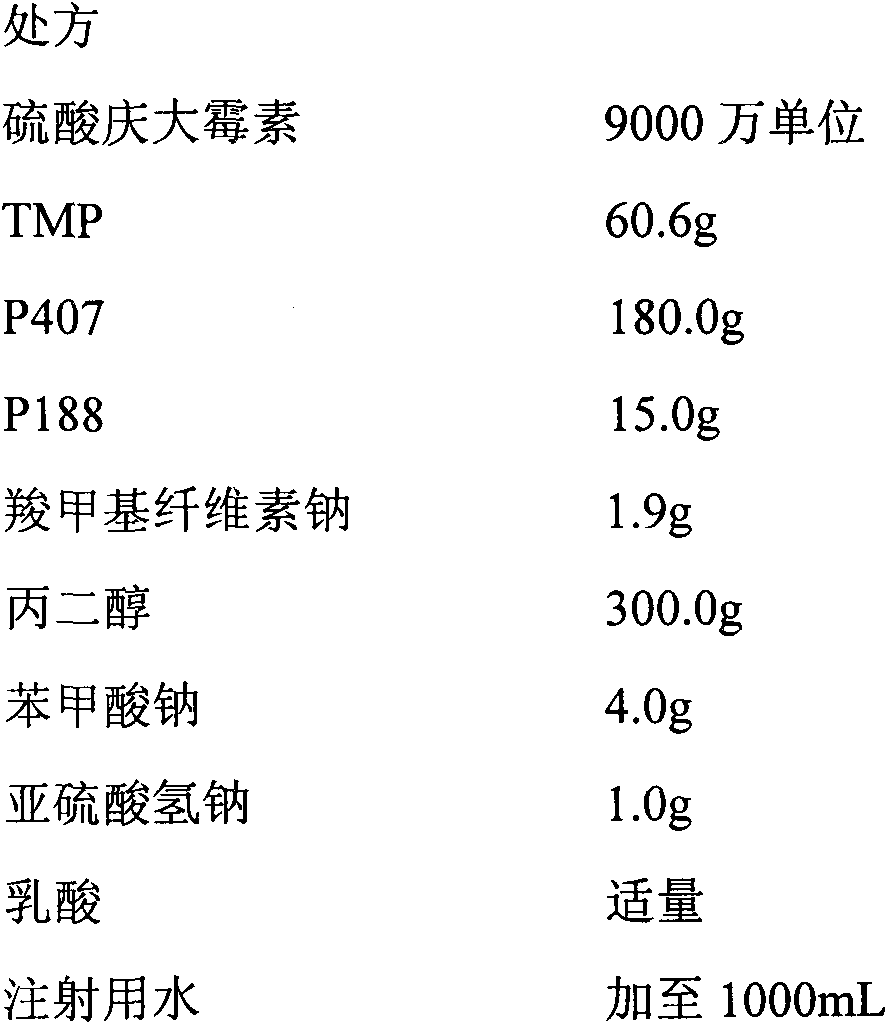
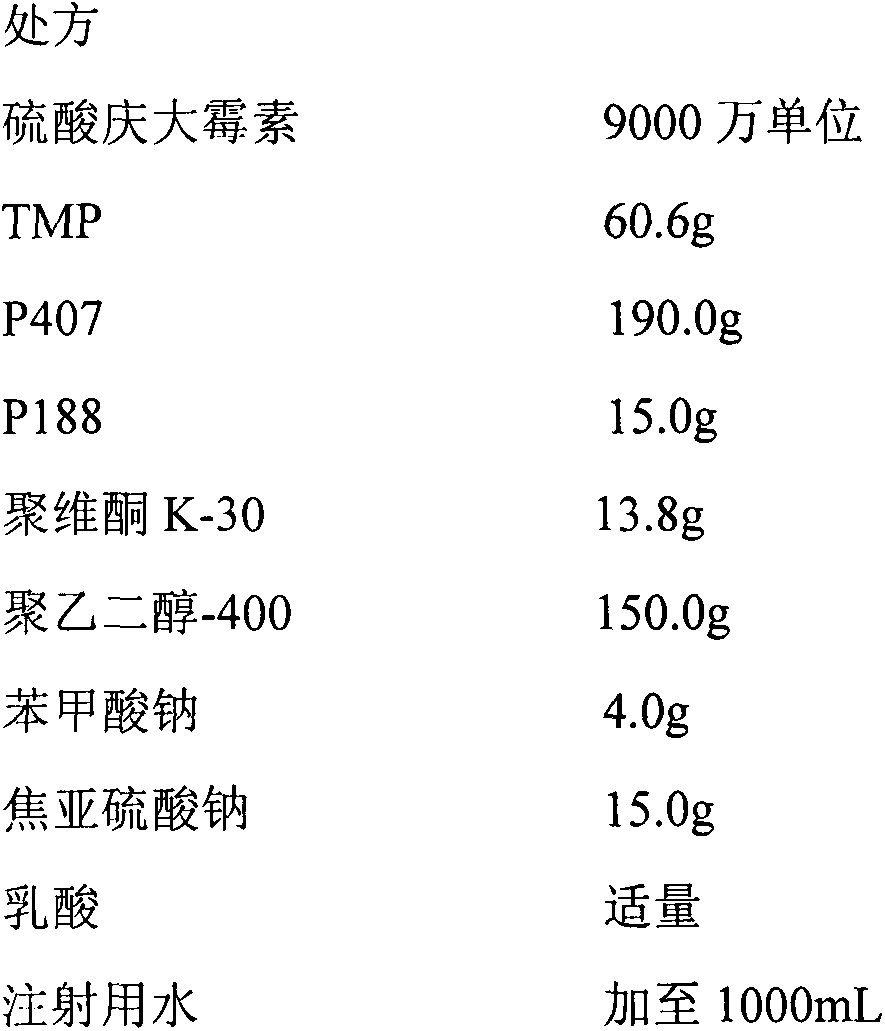
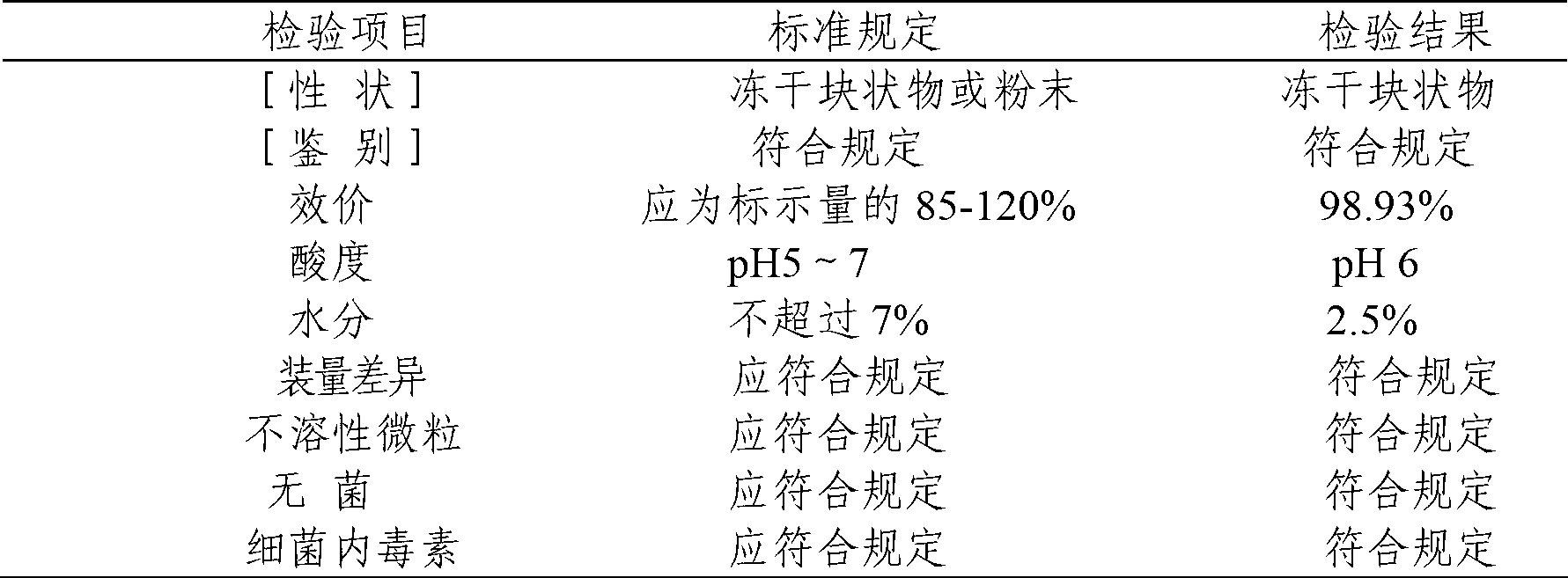
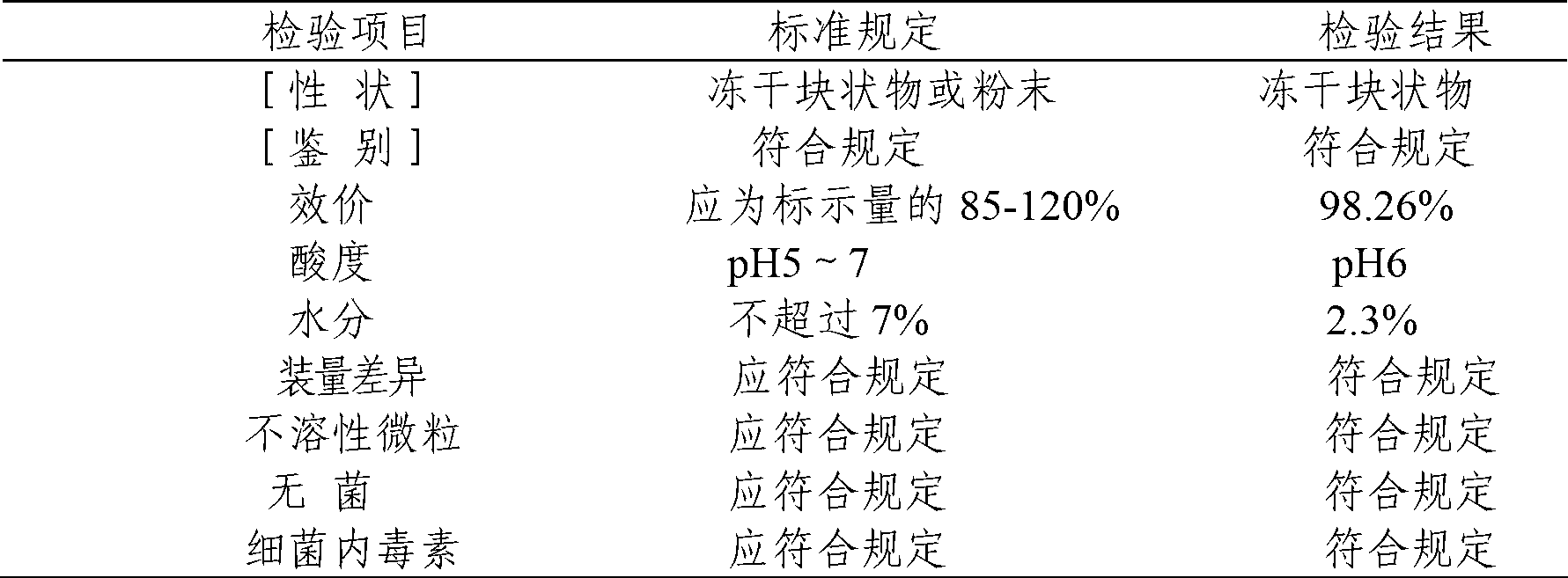
Compound gentamicin sulphate in-situ gel for injection and preparation method thereof
InactiveCN103340882ASlow drug releaseImprove complianceAntibacterial agentsOrganic active ingredientsDiseaseSide effect
The invention belongs to the field of animal pharmaceutical preparations, and relates to a compound gentamicin sulphat in-situ gel preparation for injection and a preparation method thereof. The in-situ gel preparation disclosed by the invention comprises the following components in parts by weight: 9.0 parts of gentamicin (9 million units), 6.0 parts of TMP (Trimethoprim), 15.5-30.5 parts of P407, 0.5-10.5 parts of P188, 30.0-90.5 parts of water, 0.01-10.0 parts of polymer blocker, 5.0-45.0 parts of solubilising stabilizer, 0.01-3.0 parts of preservative, 0.01-5.0 parts of antioxygen and a proper amount of pH adjustor. The in-situ gel preparation disclosed by the invention is freely moveable liquid at room temperature and forms semi-solid gel after drug delivery through intramuscular injection or subcutaneous injection; drug delivery is only carried out once during a treatment course; the in-situ gel preparation has the advantages of steady property, long drug delivery duration, exact curative effect, strong animal adaptation, no toxic and side effects and adverse reaction and the like, and is simple to operate and convenient for drug delivery; the in-situ gel preparation disclosed by the invention has broad-spectrum antibacterial activity and can be used as a medicine for preventing and treating diseases of pigs, sheep, dogs, cats, cattle and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
Medicine for treating coryza and its preparation method
InactiveCN101380328AAvoid surgical sequelaeRespiratory disorderHeterocyclic compound active ingredientsMillion UnitsPenicillin K
A drug for treating rhinitis and a preparation method thereof relate to an improved drug for treating the rhinitis. The invention provides the drug for treating the rhinitis and the preparation method thereof. The drug contains 1.6 million units of penicillin sodium salt, 6-9mg of hydrocortisone and 4-7mg of dexamethasone sodium phosphate according to weight proportion. The preparation method is characterized by comprising the following steps: (1) 0.8 million units of the penicillin sodium salt and 6-9mg of the hydrocortisone are mixed and dissolved; (2) 0.8 million units of the penicillin sodium salt and 4-7mg of the dexamethasone sodium phosphate are mixed and dissolved; and (3) the two mixed solutions are mixed to prepare the drug for treating the rhinitis.
Owner:李想
Prepn. process for freezing dry powder of bromelain
InactiveCN100368537CPromote absorptionAnti-inflammatoryPowder deliveryPeptide/protein ingredientsLow activityFreeze-drying
This invention discloses a method for manufacturing pineapple proteinase freeze-dried powders, which can solve the problem of low activity faced by conventional pineapple proteinase. The method comprises the steps of: washing pineapple stems with water, pressing to obtain juice, filtering, adsorbing the filtrate with zinc oxide, desorbing, ultrafiltering, concentrating and freeze-drying to obtain pineapple proteinase freeze-dried powders, whose enzyme activity can reach 600 million units per gram.
Owner:王美岭 +1
Liquid multi-dimensional formula, and preparation process and application thereof
InactiveCN102008489ASimple preparation processNo side effectsHydroxy compound active ingredientsMetabolism disorderVitamin CGram
The invention relates to a liquid multi-dimensional formula, and a preparation process and application thereof. Every 1L of formula contains 50 million units of vitamin A, 100 grams of vitamin C, 0.5 million units of vitamin D3, 5 grams of vitamin E, excipient I, excipient II, 70 grams of 35 percent NaOH and deionized water for fixing the volume to 1 liter. The preparation process comprises the following steps of: 1) preparing solution I; 2) preparing solution II; 3) preparing solution of composite vitamin: adding liquid in a tank B into a tank A with stirring, and fully stirring the liquid till uniform mixing; 4) adjusting the pH value of the composite vitamin solution and fixing the volume; 5) packing the composite vitamin solution; and 6) storing: storing the composite vitamin solution in a dark and light shading place. The preparation process is simple, and the product is environmentally-friendly and has no side effect.
Owner:王志军
Aprotinin freeze-dried powder and preparation method thereof
InactiveCN103054816AImprove product qualityGuaranteed stabilityPowder deliveryPeptide/protein ingredientsArginineSodium phosphates
The invention provides an aprotinin freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection is composed of the following substances: 28-556 million units of aprotinin, 10-20g arginine, 5-20g human serum albumin, and 100g mannitol. In aprotinin freeze-dried powder injection production process of the present invention, rginine and human serum albumin are selected to be excipients which mainly play the roles of protecting the active ingredient aprotinin; and mannitol is selected to be a freeze-drying excipient to enable the shape-forming by freeze drying. A 5% phosphoric acid solution or a 5% sodium phosphate solution is selected as a pH adjusting agent which enables the pH of a prepared liquid medicine to be maintained consistent with the pH of the liquid medicine which is redissolved after the powder is freeze dried, so that the product quality is more stable. According to the invention, an improved freeze-drying process is adopted, thus shortening the freeze-drying time and helping to improve product quality and save energy and reduce cost.
Owner:蚌埠丰原涂山制药有限公司
Japanese bigleaf magnolia leaf cutting propagation method
ActiveCN104641921AShort reproductive cycleSimple and fast operationPlant cultivationCultivating equipmentsVitamin CBud
The invention discloses a Japanese bigleaf magnolia leaf cutting propagation method, which comprises the following steps: (1) preparation of cutting matrix and rooting agent solution, namely mixing perlite, vermiculite and yellow sand to prepare the cutting matrix, and adding the following raw materials by weight per 1kg: 50-200mg of vitamin C, 50-200mg of vitamin B2, 50-200mg of vitamin B6, 80-160 million units of gentamicin, 200-300mg of naphthalene acetic acid, and 200-300mg of indolebutyric acid, to prepare a rooting agent solution; (2) cutting propagation of leaves, namely shearing Japanese bigleaf magnolia leaves and soaking into the rooting agent solution, washing with clear water, and inserting the leaves into the cutting matrix; (3) fertilizer spraying of the leaves, namely spraying a rooting accelerator into the leaves once a week; and (4) transplanting, namely when new buds grow from leaves and at least two leaves grow, transplanting into a field. The Japanese bigleaf magnolia leaf cutting propagation method is short in propagation period, fast in growth propagation, and few in propagation materials, and has the time-saving and effort-saving effects.
Owner:RIZHAO HENGJIUFENG AGRI TECH CO LTD
Chymotrypsin composition freeze-dried powder and preparation method thereof
ActiveCN102860988AFull and uniform appearanceGood resolubilityPowder deliverySenses disorderPorositySolubility
The invention relates to a chymotrypsin preparation, particularly relates to chymotrypsin composition freeze-dried powder and a preparation method thereof. The hymotrypsin composition freeze-dried powder comprises chymotrypsin, dextran 20 and sorbitol, wherein the ratio of chymotrypsin to dextran 20 to sorbitol is 1 million units: 1-1.8g: 0.05-0.6g. The average porosity of the hymotrypsin composition freeze-dried powder is 85-98%. The invention further relates to a preparation method of the hymotrypsin composition freeze-dried powder. The hymotrypsin composition freeze-dried powder disclosed herein has the advantages of full and uniform appearance, high porosity, good re-solubility, high stability, simple prescription, low proportion of pharmaceutic adjuvants, low side effect to human body, safety and reliability. According to the invention, the preparation technology of the hymotrypsin composition freeze-dried powder is further improved, the temperature, liquid volume, and freeze-drying process are improved, and sterilization is conducted twice.
Owner:HAINAN HERUI PHARMA
Composition capable of reducing baby pig stress
InactiveCN101455272AReduce stressImprove digestibilityAnimal feeding stuffAccessory food factorsPectinaseAmylase
The present invention relates to a composition capable of reducing piglet irritability. Each kg of the composition contains, 1-4 million units of warm amylase, 5-50 million units of neutral protease, 10-80 million units of pectinase, 60-60000 million units of xylanase, 50-6000 million units of cellulase, and 10-200g of oligosaccharide.
Owner:TIANJIN RINGPU BIO TECH
Pesticide for eradicating fruit silver blight disease
The invention discloses a medicament for the treatment of fruit tree silver leaf, wherein the formulation of the medicament includes 72% streptomycin sulfate (10 million units) 3-4%, kanamycin sulphate (5 million units) 5-8%, 4% kasugamycin 11-14%, 20% Validamycin (5 million units) 25-30%, water 30%, nutrient solution 2116 2%, leftover bits and pieces of gourmet 18%.
Owner:李广进
Chemiluminiscent substrate solution and procalcitonin detection kit utilizing same
ActiveCN105606597AAchieving High Sensitivity DetectionHigh luminous intensityChemiluminescene/bioluminescenceLuminous intensityPolyvinyl alcohol
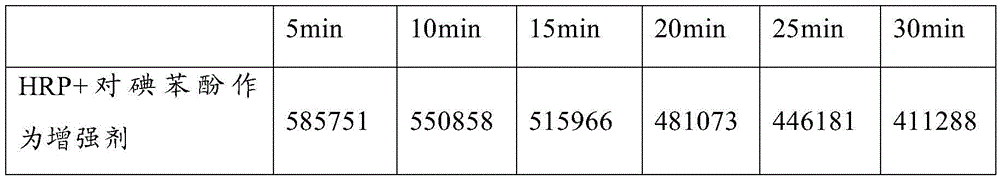
The invention discloses a chemiluminescent substrate solution and a procalcitonin detection kit utilizing the same. The chemiluminescent substrate solution is prepared from 0.8-1.5 g / L of luminol or its derivatives, 0.05-0.6 g / L2 of amino-4-nitro phenol, 0.01-0.05 mg / L of 9,9-dihexyl-2,7-dibromofluorene, 3-6 g / L of polyvinyl alcohol, 6-20 g / L of polyvinylpyrrolidone, 3-4.5 g / L of polyethylene glycol 600, 100-300 million units / L of gentamycin sulfate, 0.1-1.0 g / L of carbamide peroxide and 0.1 mol / L of a Tris-HCl buffer solution with the pH of 9.0. The chemiluminescent substrate solution is a single solution, can not only achieve the HRP enzyme catalyzed chemiluminescence reaction and can also effectively improve the luminous intensity and decrease a luminous background to achieve high-sensitivity detection.
Owner:CUSABIO TECH LLC
Oral milk coagulant for treating infant thrush
InactiveCN103638362AImprove bad tasteUnprofessional solutionOrganic active ingredientsAntimycoticsSodium bicarbonateSide effect
The invention discloses an oral milk coagulant for treating infant thrush. The oral milk coagulant is prepared from the following components by weight: 150 million units of 10-20 million / ml nystatin, 8ml of medical 5% sodium bicarbonate, 0.5g of vitamin C, 250mg of vitamin B, 2ml of cod-liver oil, 10ml of liquid pectin, 1g of lactose and 0-0.2ml of traditional Chinese medicine extract. Compared with the prior art, the oral milk coagulant has the effects of simpleness in use, easiness for coating, lasting pasting effect of medicines at an infected part, long action time, rapid effect and high bioavailability; by adopting the oral milk coagulant, the problems of unprofessional operation of family numbers of patients in drug separation, waste of traditional Chinese medicines during a drug blending process and difficulty in drug application; clinical trials prove that the oral milk coagulant disclosed by the invention has a significant effect in treating infant thrush and has no toxic and side effects through the selection of proper components in proper proportion.
Owner:曲文超
Preparations for treating mastitis of cow and preparation thereof
InactiveCN101209343AInhibitory activitySlow degradationPeptide/protein ingredientsSexual disorderSuberedamine BVitamin C
The invention relates to a preparation for the treatment of dairy cow mastitis and the preparation method thereof, the preparation is a water or physiological saline solution containing nisin, vitamin C and EDTA-Na2; each milliliter of water or physiological saline solution contains 0.05 to 0.125 million units of nisin, 4 to 10 milligrams of vitamin C and 16 to 40 milligrams of EDTA-Na2. The nisin, vitamin C and EDTA-Na2 sterile powder with the proportion are mixed and packaged, and the sterile powder is dissolved in water or physiological saline before use. The experiments prove that the medicament can inhibit the activity of protease in milk after the EDTA-Na2 is added, so as to slow the degradation speed of nisin in milk, prolong the action time of the drug and prolong the efficacy for the treatment of dairy cow mastitis.
Owner:ZHEJIANG UNIV
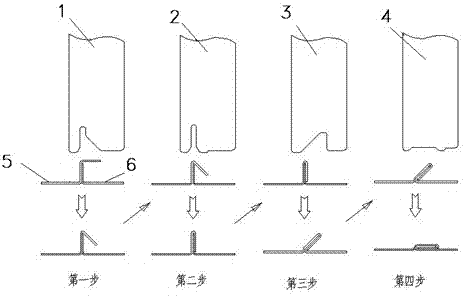
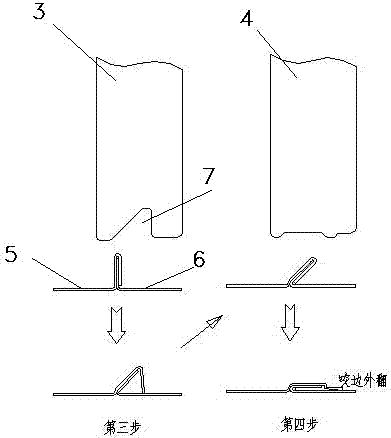
Edge-rolling occluding cutter forming process
The invention discloses an edge-rolling occluding cutter forming process which totally comprises four steps each of which is finished by a matched forming cutter. The forming process specifically comprises the following steps of: 1, bending the flange at the end cover of a dish-washing machine liner for 45 degrees; 2, rolling the flanges of the end cover of the dish-washing machine liner and the cylindrical body of the dish-washing machine liner into a whole body in a vertical state; 3, rolling the vertical edges which are rolled into a whole body to form a 45-degree inclined angle, thereby being beneficial to the final forming; and 4, compacting the flanges to finally finish the occluding forming step. The edge-rolling occluding cutter forming process is characterized in that the straight face of the edge of the third forming cutter used in the step 3 in the forming process is an inclined plane having an angle of 3-45 degrees with a vertical line. The forming process has the beneficial effects that the percent of pass of liner products of dish-washing machines reaches 100%; and in economic benefit, given that the dish-washing machine yield of one dish-washing machine manufacturerin China is 1 million units per year at present and the defective rate is reduced by 3% by adoption of the forming process provided by the invention, 30 thousand units of defective products can be reduced for one manufacturer every year because of the adoption of the new process, and the economic loss is reduced by 9 million yuan per year if the cost of one unit is 300 yuan.
Owner:704TH RES INST OF CHINA SHIPBUILDING IND CORP
Grosvenor momordica proteinase and its extraction method
InactiveCN1766099AImprove the level of comprehensive utilizationStrong hydrolytic activityHydrolasesMomordicaFreeze-drying
The invention discloses a protease of Momordica grosvenori Swingle as well as its extract method, which is prepared by: with fresh fruit, extracting juice, filtering, ultrafiltrating or salting-out chromatography, depositing with organic solvent, vacuum freezing or spray drying, and obtaining the coarse powder; dissolving the powder with distilled water for column chromatography, dialyzing to desalt, freeze drying, and obtaining the objective product. This product has strong hydrolytic activation to casein; the activity of coarse enzyme can achieve 1.5 million units / g and about two times to coarse papain. This invention adds one plant protease resource, makes our rotease resource do not depend on only pawpaw and pineapple, and has important meaning to enzymology research, protease food development, and other opposite fields.
Owner:GUANGXI NORMAL UNIV
Administration method and administration device for experimental animal brain
InactiveCN104274255ASolve the problem of relatively large physical damageEasy injectionVeterinary instrumentsHeterocyclic compound active ingredientsDrugs solutionPenicillin
The invention discloses an administration method for an experimental animal brain; by adopting a method for burying a catheter into the experimental animal brain, injection to the experimental animal brain is more convenient. According to the administration method, a special successive administration device is adopted, and a mouse or a rat is taken as an experimental animal, and the administration method comprises the following steps: 1, narcotizing the experimental animal with 30 percent of chloral hydrate, fixing the experimental animal on a mouse brain locator, and determining the position of a paracele; 2, burying an administration catheter into the paracele of the experimental animal according to the step, and fixing the catheter to the head of the experimental animal by dental cement when cerebrospinal fluid overflows from the catheter; 3, in 4 days after the catheter is buried successfully, performing successive intramuscular injection every day with 0.2ml of 0.8 million units of penicillin sodium, and diminishing inflammation of the experimental animal; 4, performing administration: sucking up a right amount of to-be-experimented drug solution by a 10mul microinjector, and partially inserting the syringe needle of a microsyringe into the catheter buried into the experimental animal brain, wherein the insertion depth is 0.5-2.0cm, and successive administration can be performed on the mice brain from the catheter.
Owner:SHENYANG PHARMA UNIVERSITY
Double-layer polyethylene composite tubular product with ultrahigh molecular weight
InactiveCN102242834AExtended service lifeReasonable structural designRigid pipesSpiral woundMillion Units
The invention discloses a double-layer polyethylene composite tubular product with ultrahigh molecular weight, belonging to the technical field of tubular products. The tubular product comprises an inner wall, a metal layer and an outer wall; the metal layer consists of a steel strip layer and a steel wire layer; the steel strip layer is arranged along the direction parallel to the tubular product axial lead equidistantly, the steel strip thickness is 0.5-3 millimeters, and the space between the steel strips is 10-20millimeters; an ultrahigh molecular weight polyethylene hot melt adhesive is coated on the steel strip layer with the coating layer thickness of 0.7-3.0millimeters; the steel wire layer is of a structure that the packed steel strip layer is spiral wound at the direction vertical to the tubular product axial lead, wherein the steel wire is a spring steel wire, and the diameter of the steel wire is 0.8-2.5millimeters; and the materials of the inner wall and the outer wall are polyethylene with molecular weight more than 2.5 million units. The tubular product provided by the invention has the advantages of reasonable structural design, high abrasion and corrosion resistance performances and high bearing capability, the optimal pressure can reach 16MPa, and is the double-layer polyethylene composite tubular product with ultrahigh molecular weight which has wide application wide and long service life.
Owner:JIANGSU SHENSHI NEW MATERIAL TECH
Method for preparing polymyxin B sulfate for injection
ActiveCN108309945AReduce the use of security risksImprove stabilityAntibacterial agentsPowder deliveryMillion UnitsInjections water
The invention relates to a method for preparing polymyxin B sulfate for injection. The auxiliary material of the prepared polymyxin B sulfate for injection is injection water only, and the specification of the polymyxin B sulfate for injection is 0.5 million units. The polymyxin B sulfate for injection, prepared through the method, has significantly improved quality and stability, and has significantly reduced insoluble particle quantity.
Owner:湖北美林药业有限公司
Special conditioning and nourishing film for use at genital of woman
ActiveCN102309750BWith detoxificationHas producedOrganic active ingredientsCosmetic preparationsAdditive ingredientArginine
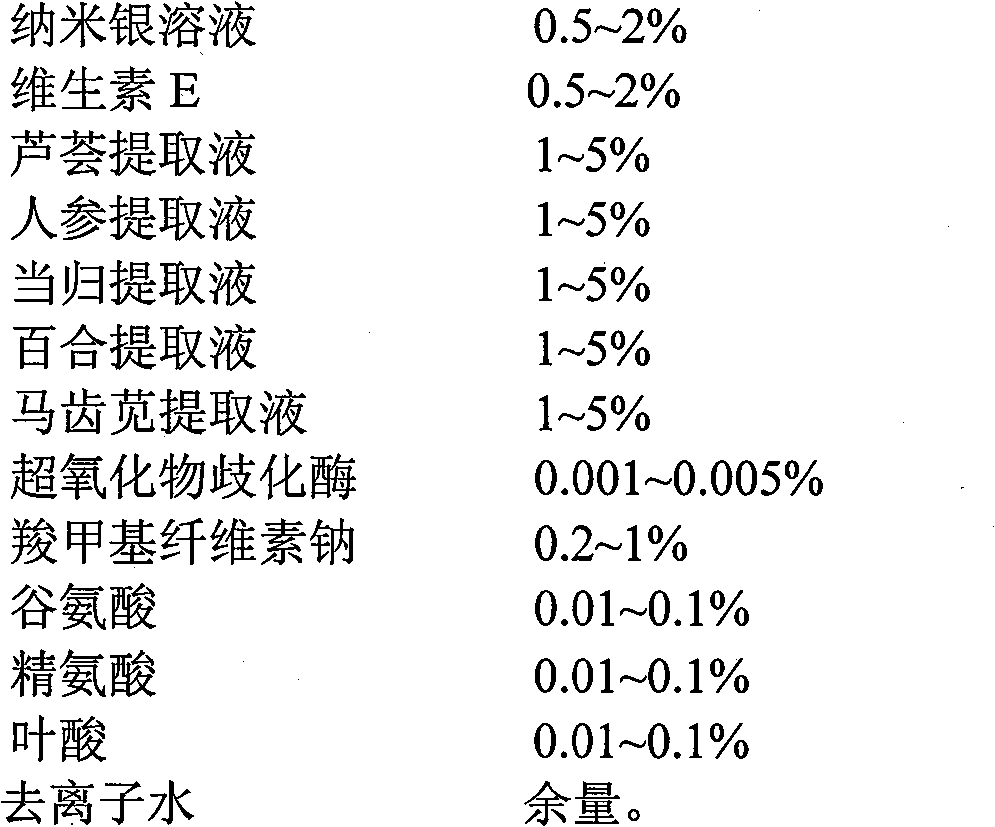
The invention provides a special conditioning and nourishing film for use at a genital of a woman. The special conditioning and nourishing film is a product formed by uniformly infiltrating conditioning and nourishing liquid on a non-woven fabric film and packaging the non-woven fabric film, wherein the conditioning and nourishing liquid is prepared from the following ingredients in percentage by weight: 0.5-2% of colorless nano silver solution (with the concentration of 1,000 ppm), 0.5-2% of vitamin E, 1-5% of aloe extract (with 3% solid content), 1-5% of ginseng extract (2.5% solid content), 1-5% angelica extract (3% solid content), 1-5% of lily extract (2.5% solid content), 1-10% of purslane extract (30% solid content), 0.001-0.005% of lauric acid-modified superoxide dismutase (the number of active cells is 4 million units / g), 0.2-1% of sodium carboxymethylcellulose, 0.01-0.1% of glutamic acid, 0.01-0.1% of arginine, 0.01-0.1% of folic acid and the remaining of deionized water; and the non-woven fabric film is 50-100g / m<2> in gram weight, and is prepared from the following materials in percentage by weight: 50-99.9% of viscose fiber and 0.1-50% of Dacron. The special conditioning and nourishing film provided by the invention has the functions of killing bacteria, diminishing inflammation, contracting vagina, whitening the genital, increasing skin elasticity of the genital and the like.
Owner:中健生命科技(深圳)有限公司
Heparin sodium extracting method
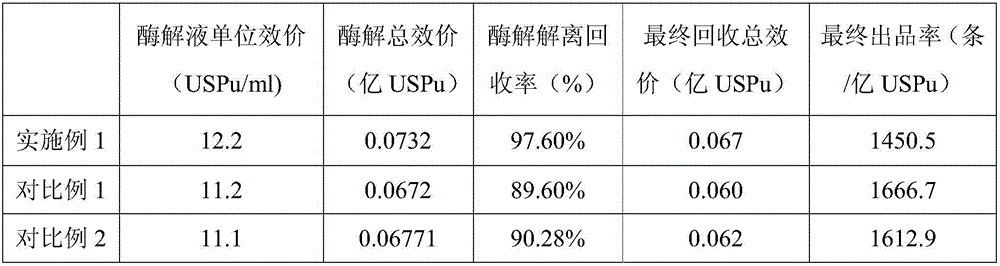
The invention relates to the technical field of deep processing of agricultural and sideline products, and specifically relates to a heparin sodium extracting method. The heparin sodium extracting method comprises the following steps: performing first enzymolysis under the salinity of 4.0-4.5 degrees and the temperature of 38+ / -2 DEG C; then performing secondary enzymolysis under the salinity of 3.0-3.5 degrees and the temperature of 55+ / -1 DEG C; and then sequentially carrying out protein denaturation, absorbing, washing, eluting, dewatering and desalting, and drying after the two enzymolysis. According to the heparin sodium extracting method, the temperature of 38+ / -2 DEG C and the salinity of 4.0-4.5 degrees are creatively selected for the first enzymolysis, and the temperature of 55+ / -1 DEG C and the salinity of 3.0-3.5 degrees are selected for the second enzymolysis, so that the heparin sodium yield reaches 1490-1510 items per hundred million units; compared with a traditional first enzymolysis method and a secondary enzymolysis method disclosed in the prior art, the heparin sodium extracting method has the advantages that the enzymolysis dissociation recovering rate is increased, so that the heparin sodium yield is increased.
Owner:HENAN ZHONGPIN FOOD IND +1
Nasal drop for treatment of acute and chronic rhinitis, sinusitis and allergic rhinitis and preparation method thereof
PendingCN108771677AReduce swelling and congestionReduce exudateOrganic active ingredientsPharmaceutical delivery mechanismSinusitisDisease
The invention relates to a nasal drop for treatment of acute and chronic rhinitis, sinusitis and allergic rhinitis and a preparation method thereof. The nasal drop comprises neomycin sulfate, chlorpheniramine maleate, naphazoline hydrochloride, auxiliary ingredients and solvents. Specifically, per 1000ml of the nasal drops comprise 0.975-5 million units of neomycin sulfate, 0.3-1.0g of chloropheniramine maleate and 0.3-1.0g of naphthozoline hydrochloride. The nasal drop provided by the invention has anti-bacterial, anti-inflammation and anti-allergy effects, at the same time can constrict blood vessels and improve the nasal obstruction symptom caused by rhinitis, is atraumatic, and has the characteristics of easy carrying, convenient use, quick action, shortening of the course of disease,good patient compliance, and high cure rate, and has good efficacy on acute and chronic rhinitis, sinusitis and allergic rhinitis and the like. The preparation method of the nasal drop provided by theinvention has the advantages of simple and feasible processing technology and good repeatability, and is convenient for industrial production.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Ulinastatin injection and preparation method for same
ActiveCN105535951AReduce dosageReduce manufacturing costPeptide/protein ingredientsAntipyreticActivated carbonHydrogen phosphate
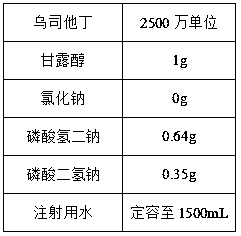
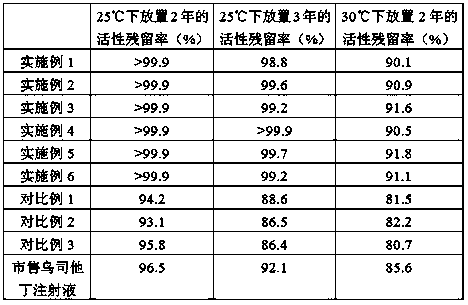
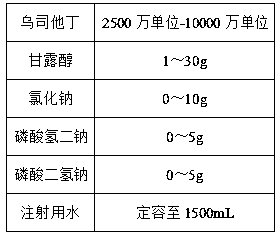
The invention relates to ulinastatin injection and a preparation method for the same. A formula of every 1,000 tubes of ulinastatin injection comprises 2,500 to 10,000 million units of ulinastatin, 1 to 30 grams of mannitol, 0 to 10 grams of sodium chloride, 0 to 5 grams of sodium hydrogen phosphate, 0 to 5 grams of monosodium phosphate and water for injection. The preparation method for the ulinastatin injection comprises the following steps of preparing an auxiliary solution of 2 to 4 DEG C, adding 1 to 5 grams of activated carbon with an average particle size of 6 to 9 microns to perform low-temperature adsorption treatment on the prepared auxiliary solution for 12 to 20 minutes at 2 to 4 DEG C, and dissolving ulinastatin with the treated auxiliary solution. The ulinastatin injection prepared by the preparation method according to the formula is relatively lower in adverse reaction rate and relatively higher in stability.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
Special fermentation microbial agent for treating industrial solid waste raw materials
The invention discloses a special fermentation microbial agent for treating industrial solid waste raw materials. Five microbial floras including an aerobic gram-positive actinomycetes flora, an aerobic saccharomycetes flora, an anaerobic lactic acid bacterium flora, an aerobic and anaerobic photosynthetic bacterium flora and an anaerobic filamentous bacterium flora are compounded with one anotherto obtain the special fermentation microbial agent. The content of the aerobic gram-positive actinomycetes flora is higher than or equal to 50%, and the content of effective active bacteria in the aerobic gram-positive actinomycetes flora is higher than or equal to 0.35 hundred million units / gram; the content of total nutrients in the special fermentation microbial agent is higher than or equal to 5.1%, the volume weight of the special fermentation microbial agent is 0.51-0.59 g / cm<3>, the water content of the special fermentation microbial agent is 20-30%, and the pH (potential of hydrogen)of the special fermentation microbial agent is 6.0-7.0. The special fermentation microbial agent has the advantages of high heating speed, temperature rise and adaptability, simplicity and conveniencein preparation and the like. Besides, good service effects can be realized by the special fermentation microbial agent, and the special fermentation microbial agent is particularly suitable for treating the industrial solid waste raw materials for substrate production enterprises.
Owner:江苏兴农基质科技有限公司
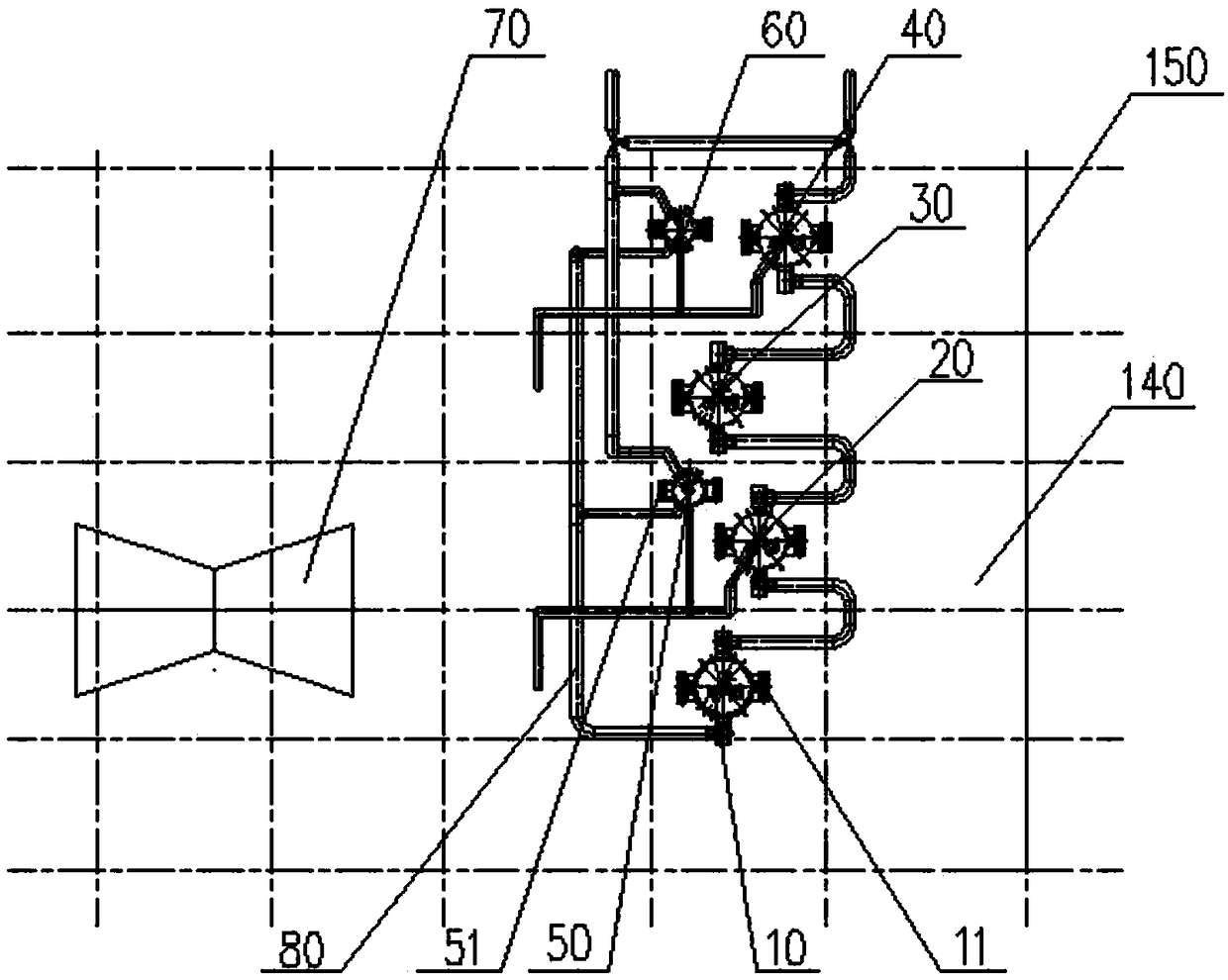
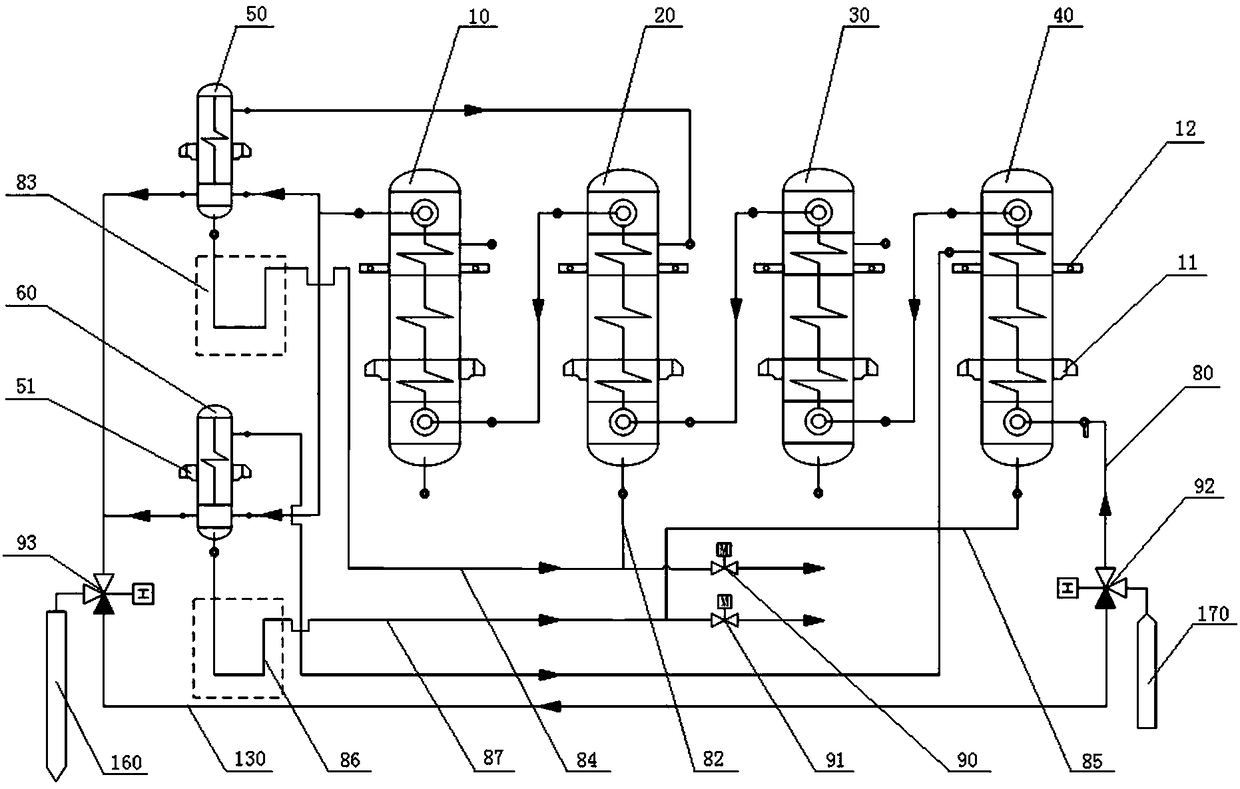
Secondary reheating million unit high-pressure heater system structure and application method thereof
The invention discloses a secondary reheating million unit high-pressure heater system structure. The structure comprises a steam turbine, a high-pressure heater set and an external steam cooler set;the high-pressure heater set comprises a first high-pressure heater, a second high-pressure heater, a third high-pressure heater and a fourth high-pressure heater; single-row coiler vertical high-pressure heaters are adopted; the first high-pressure heater, the second high-pressure heater and the third high-pressure heater are arranged in a zero-meter layer of a steam machine room; the fourth high-pressure heater is arranged in an operation layer of a deaeration room; the external steam cooler set comprises a second external steam cooler and a fourth external steam cooler; U-shaped tube vertical heaters are adopted; the second external steam cooler is arranged in a middle layer of the steam machine room; and the fourth external steam cooler is arranged in the operation layer of the deaeration room. Meanwhile, the invention discloses an application method of the secondary reheating million unit high-pressure heater system structure. The structure has the characteristics of capability ofreducing the manufacturing cost of the steam machine room, simplification of the system, reduction of the engineering quantity, improvement of the unit economy, acceleration of the unit start time and the like.
Owner:CEEC JIANGSU ELECTRIC POWER DESIGN INST +1
Preparation method for producing heparin sodium by animal small intestines
The invention discloses a preparation method for producing heparin sodium by animal small intestines. The preparation method is characterized in that the preparation of the heparin sodium is completed through steps of intestinal mucosa preparation, enzymolysis, adsorption, elution, sedimentation and desalination and drying. The preparation method disclosed by the invention has the advantages that the yield of the heparin sodium is effectively increased by reasonably regulating the preparation method, and the loss of activity of the heparin sodium is reduced; the prepared heparin sodium is more stable in quality; only 1400 to 1500 animal small intestines are needed by producing 100 million units of heparin sodium by adopting the preparation method disclosed by the invention, so that the production cost is greatly reduced.
Owner:NANTONG RENSHOU FOOD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com