Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Naphazoline Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt form of naphazoline, an imidazole derivative and a direct-acting sympathomimetic amine with vasoconstrictive properties. Upon ocular administration, naphazoline hydrochloride exerts its effect by acting on alpha-adrenergic receptors in the arterioles of the conjunctiva to produce vasoconstriction, resulting in decreased conjunctival congestion and diminished itching, irritation and redness.
Method and composition for treatment of skin conditions
InactiveUS20050222101A1Improve effectivenessPromote absorptionBiocideAnimal repellantsPhenylephrine hclPoison oak
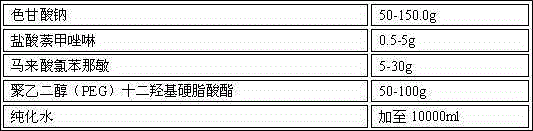
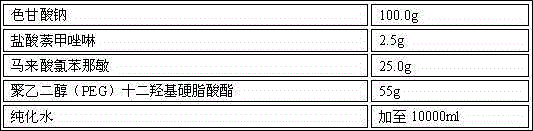
A method for treating dermatological conditions as eczema, seborrheic dermatitis, psoriasis and allergic skin reactions such as hives, skin irritations, poison ivy, poison oak, and the like. The method involves applying to the affected area of the skin a few drops of a combination of pheniramine maleate and either naphazoline HCL or phenylephrine HCL and then applying to the same affected area a topical corticosteroid cream, ointment or lotion. The preferred ratio is approximately one drop of solution for every approximately 0.1 grams of topical corticosteroid cream applied to each patch of skin affected with any of the dermatological conditions.
Owner:HUTTERER JEFFREY
Eye medicine for preventing and treating asthenopia and allergy
InactiveCN1839834AOvercome the disadvantages of taking single medicineCompatibility is reasonableSenses disorderAnhydride/acid/halide active ingredientsMentholAllergy
Disclosed is a compound preparation for treating and preventing eye strain and allergy, which comprises (by weight percent) taurine 0.1-99.9%, naphazoline hydrochloride 0.01-99.9%, vitamin B6 0.01-99.9%, chlorpheniramine maleate 0.01-99.9%, and right amount of auxiliary therapeutic medicaments such as menthol, sodium hyaluronate, glycerine and medicinal findings such as preservative agent and buffering agent.
Owner:崔彬
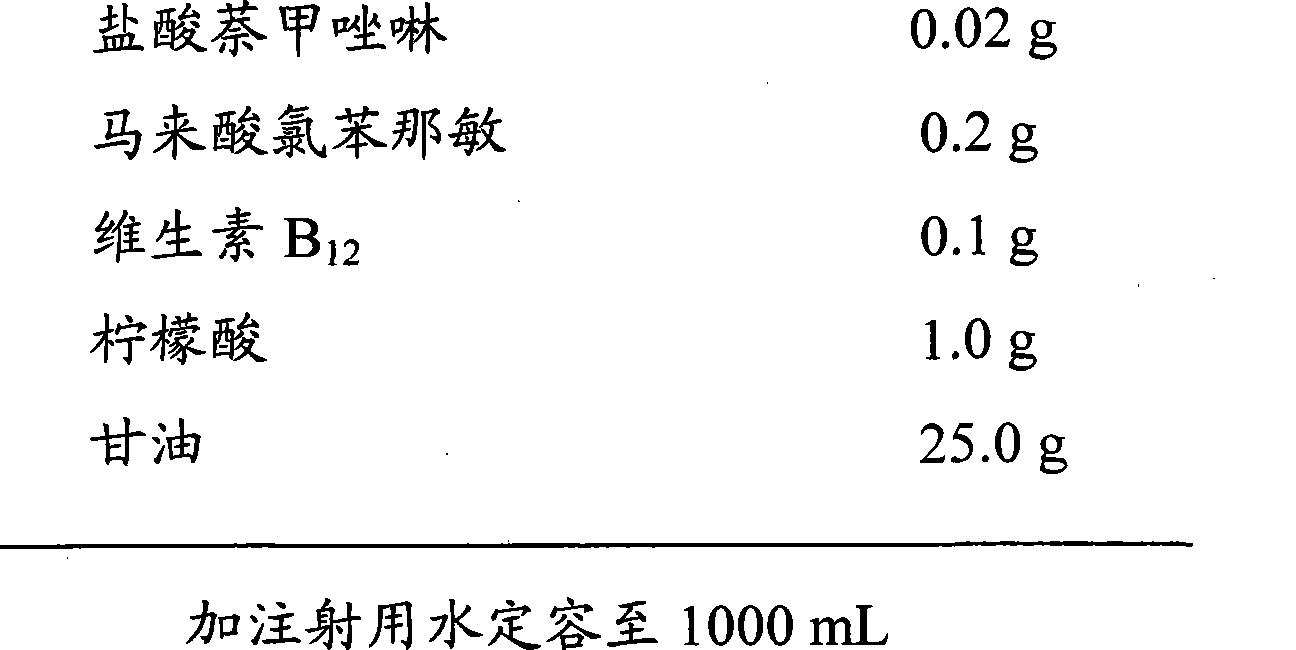
Naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops without bacteria inhibitor and preparation method thereof
InactiveCN101455636AAvoid side effectsAvoid potential dangerOrganic active ingredientsSenses disorderChlorphenamine maleateVitamin B12
The present invention discloses a naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops which do not contain bacteriostat and a preparing method thereof, wherein the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops comprise naphazoline hydrochloride, chlorphenamine maleate, vitamin B12, pH modifying agent, isoosmotic agent, stabilizing agent, thickening agent, etc. The preparing method adopts an aseptic manipulation filling technique. Furthermore the eye drops of the invention adopt a disposable single-dose independent packaging. The sterilized performance of product is guaranteed. The product is safer, more reliable, easier and more sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Eyedrops
InactiveCN1623549ATo promote metabolismEliminate fatigueOrganic active ingredientsSenses disorderChlorphenamine maleateAlcohol
Owner:赵文达
Sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray
ActiveCN103751188AGuaranteed pHAvoid side effectsOrganic active ingredientsAntipyreticPreservativeNasal spray
The invention provides a sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray for treating allergic rhinitis. The sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray comprises sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, and pharmaceutically acceptable excipients, wherein the pharmaceutically acceptable excipients are selected from a buffering agent, an osmotic pressure regulator, a preservative, an absorption enhancer, a pH (Power of Hydrogen) regulator and the like. According to the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray, the active ingredients can be quickly absorbed through mucosa of nasal cavity and then acts quickly; the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray has the advantages of high bioavailability, outstanding compliance, and convenience in carrying and use, etc.
Owner:SHANDONG TIANSHUN PHARMA
Method for measuring content of triamcinolone acetonide chloromycetin solution
InactiveCN101196495AQuantitative analysis methods are sensitiveQuantitative analysis method is fastComponent separationTesting medicinal preparationsAcetic acidBULK ACTIVE INGREDIENT
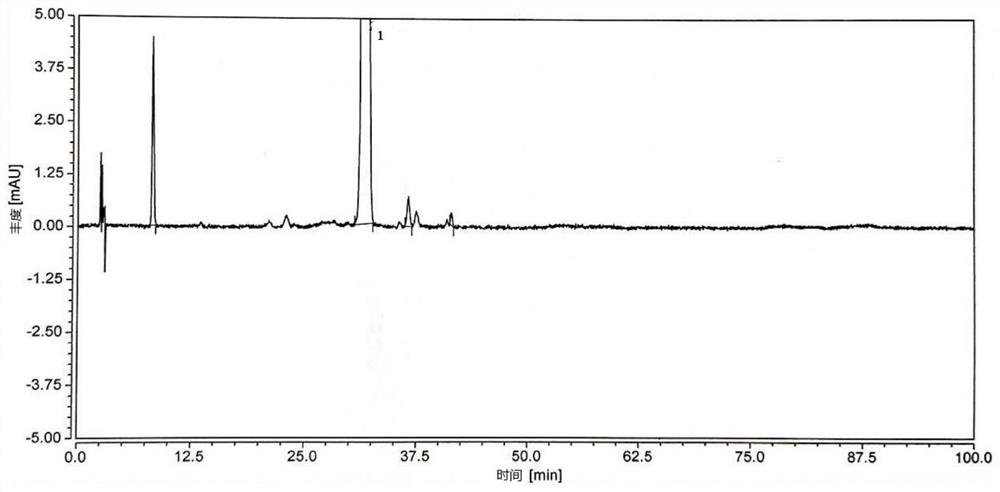
The invention provides a method for measuring the high efficiency liquid chromatography by the content of triamcinolone acetonide chloramphenicol solution, which can simultaneously measuring the content of three active ingredients comprising acetic acid triamcinolone acetonide, chloramphenicol and naphazoline hydrochloride. The method is characterized in high accuracy, good specificity, high sensitivity, easy operation and low cost. Compared with the prior art, the content measuring method provided in the invention not only increases the method for controlling the content of the naphazoline hydrochloridem but also brings a better effect of each component separation and more sensitive and accurate measuring which can avoid the complicated and time-consuming operation, less specificity and no content measuring of the naphazoline hydrochloride prescribed in the active standard, and guarantees the advancement and accuracy of the quality standard, thus further effectively ensure the safe and effective use of the medicine for the people.
Owner:安军永
Composite aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops without bacteria inhibitor and preparation method thereof
InactiveCN101455634AAvoid side effectsAvoid potential dangerSenses disorderPharmaceutical delivery mechanismMedicineDipotassium Glycyrrhizate
The present invention discloses compound aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops which do not contain bacteriostat and a preparing method thereof, wherein the compound aspartate, vitamin B6 and dipotassium glycyrrhetate eye drops comprises aspartic acid, vitamin B6, dipotassium glycyrrhetate, naphazoline hydrochloride, neostigmine methysulfate, chlorpheniramine maleate, pH modifying agent, isoosmotic agent, stabilizing agent, thickening agent, algefacient, etc. The preparing method adopts an aseptic manipulation filling technique. Furthermore the eye drops of the invention adopt a disposable single-dose independent packaging. The sterilized performance of product is guaranteed. The product is safer, more reliable, easier and more sanitary.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Contact lens care solution
InactiveCN104906619AImprove wettabilityIncrease moistureChemicalsDisodium EdetateVitamin B6 synthesis
The invention discloses a contact lens care solution, which comprises the following components: 100-200 parts of trisodium citrate dihydrate, 30-80 parts of sodium chloride, 50-100 parts of boric acid, 60-120 parts of sorbitol, 30-80 parts of aminomethyl propanol, 30-100 parts of edetate disodium, 50-130 parts of a wetting agent, 50-150 parts of a wetting matrix, 15-35 parts of vitamin B6, 30-50 parts of sodium carboxymethylcellulose, 0.1-0.3 part of polyquaternary ammonium salt-1, 0.01-0.03 part of myristamidopropyl dimethylamine, 0.01-0.03 part of polyhexamethylene biguanide and 0.01-0.03 part of naphazoline hydrochloride, wherein the wetting agent is any several kinds of taurine, panthenol and potassium L-aspartate; and the wetting matrix is a poly(oxyethylene) / poly(oxybutylene) copolymer and a polyoxyethylene / polyoxypropylene copolymer. The contact lens care solution has a good wetting effect; meanwhile, lipid and protein deposits on a contact lens can be effectively removed; and harmful microorganisms can be effectively killed.
Owner:蔡婷婷
Contact lens care solution
InactiveCN105561369AHas anti-congestant propertiesEasy to prepareSenses disorderChemicalsActive enzymeSide effect
The invention discloses a contact lens care solution, and relates to the technical field of care solutions. The preparation method of the contact lens care solution comprises the steps that sodium citrate, sodium pyrrolidonecarboxylate, sodium chloride and deionized water are heated according to the proportion in a formula by 45 DEG C, even mixing is conducted for dissolution, polyhexamethylene guanidine, alga active enzymes, N-[3-(dimethylamino)propyl]myristamide and naphazoline hydrochloride are slowly added at the speed of 70-85 revolutions / min, chrysanthemum flower extract, vitamin B6 and fructus lycii essence are added then, evenly mixing is conducted for 5-10 min, after the mixture is placed at room temperature for 10-12 h, the solution is loaded into a sterile eye drop bottle, inspection is conducted, and the contact lens care solution is put in storage. The contact lens solution comprises multiple natural ingredients, toxic and side effects on human bodies do not exist, on the premise that the good moisturizing and antibacterial effects are guaranteed, asthenopia is relieved, eye nourishing is achieved, wearing comfort is kept, and lipid and protein sediments on contact lenses can be effectively removed; due to the fact that naphazoline hydrochloride is added, the care solution has the effect of resisting hyperemia of the eyes.
Owner:KUIBO SHANGHAI IND CO LTD
Detection method for naphazoline hydrochloride and related substances thereof
InactiveCN107389826AEffective quality controlEfficient separationComponent separationPhosphoric acidMonopotassium phosphate
The invention relates to the technical field of chemical drug analysis methods, and particularly relates to a detection method for naphazoline hydrochloride and related substances thereof. The method is characterized by comprising the steps of carrying out gradient elution on a high-performance liquid chromatograph, wherein the chromatographic conditions are as follows: a chromatographic column is an octadecylsilane chemically bonded silica chromatographic column, a sodium octane sulfonate solution is taken as a mobile phase A, acetonitrile is taken as a mobile phase B, the detection wavelength is 275-285nm, the column temperature is 20-40 DEG C and the flow velocity is 0.9-1.1mL / min; and carrying out gradient elution, wherein the preparation method of the mobile phone A comprises the steps of taking sodium octane sulfonate and monopotassium phosphate, dissolving and diluting the sodium octane sulfonate and the monopotassium phosphate to 1000mL by using water; and adding 5mL of trimethylamine and adjusting the pH value to be 2.6-3.0 by using a phosphoric acid. The method is capable of effectively separating the naphazoline hydrochloride and the related substances thereof, and is good in separation degree, thereby effectively controlling the quality of the naphazoline hydrochloride.
Owner:山东绅联药业股份有限公司
Rat pulmonary cell fibrosis model
InactiveCN107115343AAvoid damageReduce exogenous damageOrganic active ingredientsRespiratory disorderNasal cavityTracheotomy
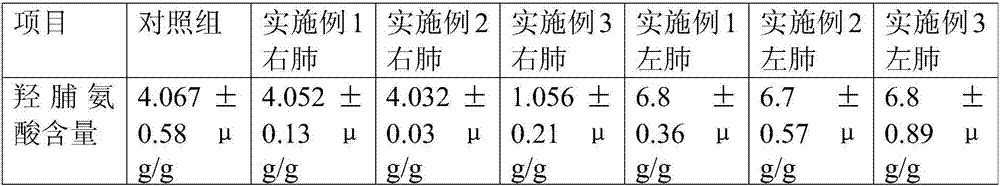
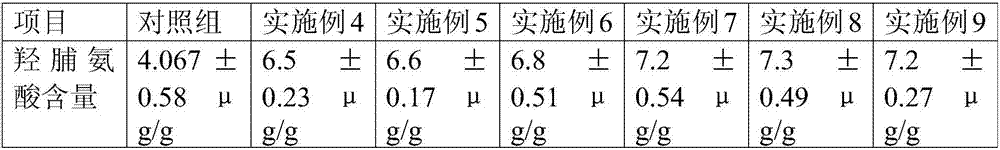
The invention discloses a rat pulmonary cell fibrosis model. For a medication administration group, a medicine is administrated in an intranasal administration mode with an administration gun, the medicine comprises amiodarone and naphazoline hydrochloride, the amiodarone is adopted as an inducing agent for establishing the rat pulmonary cell fibrosis model, and the content of hdroxyproline is tested through high-resolution CT (Computed Tomography) observation. According to the rat pulmonary cell fibrosis model disclosed by the invention, the amiodarone is injected in a noninvasive intranasal administration mode to establish a lung fibrosis animal model, the operation is convenient, the success rate is high, small damage can be made to rats in the administration process, exogenous damage caused by tracheotomy of the rats is reduced, the death rate of the rats in the administration process is small, and medicines can be fed into tracheas completely.
Owner:WENZHOU PEOPLES HOSPITAL
A bioadhesive nasal gel product of compound rupatadine and its preparation method
ActiveCN106265664BRapid relief of various symptomsRelieve various symptomsOrganic active ingredientsAntipyreticMetizolineXylometazoline hydrochloride
The invention discloses a compound rupatadine bioadhesion nasal gel product and a preparation method. The compound rupatadine bioadhesion nasal gel product is characterized by being prepared from, 10-100 parts of rupatadine and salts thereof, 5-20 parts of xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, 1-4 parts of furacilin, 300-5000 parts of polymer gel materials, 2-200 parts of a biological adhesive, 180-200 parts of a buffer salt, 800-1600 parts of a wetting agent and 20000 parts of sterile water for injection. The wetting agent is dissolved into 2 / 3 of the sterile water for injection to obtain a solution A; the polymer gel material is stirred and added into the solution A to obtain a gel system B; the biological adhesive is dispersed into the gel system B to obtain a gel system C; the rupatadine and salts thereof, the xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, the furacilin and the buffer salt are successively added into 1 / 3 of the sterile water for injection, and sonication is performed to obtain a solution D; the solution D is dispersed into the gel system C, and stirring is performed to obtain the final product.
Owner:重庆市人民医院
Aspartic vitamin eye drops and preparation method thereof
ActiveCN101129392ASimple preparation processImprove yieldSenses disorderPharmaceutical delivery mechanismMentholBenzalkonium chloride
The invention discloses a compound asparticitin gutta and making method, which comprises the following parts: aspartic acid, vitamin B6, glycyrrhizin dipotassium, naphazoline hydrochloride, neostigmine methylsulfate, maleic chlorobenzenenamin, polysorbate 80, sodium chloride, menthol, benzachloridamine, sodium hydroxide solution and injecting water. The invention paves basis for scaling manufacturing of the compound asparticitin gutta further, which improves the productivity with stable content of maleic chlorobenzenenamin.
Owner:江西闪亮制药有限公司
Cool and refreshing contact lens care solution
InactiveCN106929199AMoist for a long timeRelieve fatigueCationic surface-active compoundsNon-ionic surface-active compoundsDisodium EdetateNaphazoline Hydrochloride
The invention discloses a cool and refreshing contact lens care solution. The care solution comprises the following components in parts by weight: 6-10 parts of menthol, 4-6 parts of an aloe extract product, 10-20 parts of sodium chloride, 15-25 parts of glycerin, 20-30 parts of polyethylene glycol, 8-12 parts of hydroxyethyl cellulose, 1-3 parts of poloxamer, 3-5 parts of polyquaternium, 6-8 parts of disodium edetate, 20-30 parts of ethylene diamine tetraacetic acid, 15-25 parts of boric acid, 15-25 parts of borax, 10-20 parts of taurine, 0.03-0.05 parts of naphazoline hydrochloride, 0.03-0.05 parts of myristoyl propyl dimethylamine, 0.3-0.5 parts of vitamin B6, and 200-400 parts of deionized water. The care solution can be used for effectively sterilizing and disinfecting, and cleaning protein deposition on the surface of the eyeglass; the care solution contains natural mint components, and is cool and refreshing as well as comfortable with fragrant smell; the care solution can be used for effectively alleviating fatigue of eyes, and keeping long time moistening of eyeglasses; the care solution is safe and mild without stimulation.
Owner:QINGDAO ZHITONG SIHAI FURNITURE DESIGN RES & DEV CO LTD
Nasal drop for treatment of acute and chronic rhinitis, sinusitis and allergic rhinitis and preparation method thereof
PendingCN108771677AReduce swelling and congestionReduce exudateOrganic active ingredientsPharmaceutical delivery mechanismSinusitisDisease
The invention relates to a nasal drop for treatment of acute and chronic rhinitis, sinusitis and allergic rhinitis and a preparation method thereof. The nasal drop comprises neomycin sulfate, chlorpheniramine maleate, naphazoline hydrochloride, auxiliary ingredients and solvents. Specifically, per 1000ml of the nasal drops comprise 0.975-5 million units of neomycin sulfate, 0.3-1.0g of chloropheniramine maleate and 0.3-1.0g of naphthozoline hydrochloride. The nasal drop provided by the invention has anti-bacterial, anti-inflammation and anti-allergy effects, at the same time can constrict blood vessels and improve the nasal obstruction symptom caused by rhinitis, is atraumatic, and has the characteristics of easy carrying, convenient use, quick action, shortening of the course of disease,good patient compliance, and high cure rate, and has good efficacy on acute and chronic rhinitis, sinusitis and allergic rhinitis and the like. The preparation method of the nasal drop provided by theinvention has the advantages of simple and feasible processing technology and good repeatability, and is convenient for industrial production.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Eye drop prepared from both traditional Chinese medicines and western medicines
InactiveCN105193850AQuick resultsLittle side effectsSenses disorderHydroxy compound active ingredientsWestern medicineSide effect
The invention discloses an eye drop prepared from both traditional Chinese medicines and western medicines, and relates to technical field of agents for relieving eyestrain. The eye drop is characterized by comprising the constituents of liquid pearl, borneol, vitamin B6, taurine, aspartate, chloromycetin, naphazoline hydrochloride and dipotassium glycyrrhizinate. The eye drop integrates the characteristics of the traditional Chinese medicines and the western medicines, combines both the traditional Chinese medicines and the western medicines, and has the characteristics of being quick in effect and little in side effect.
Owner:何建军
Nasal spray for treating allergic rhinitis
ActiveCN103893174AGuaranteed pHAvoid side effectsOrganic active ingredientsAerosol deliveryNasal cavityNonallergic rhinitis
The invention provides nasal spray for treating allergic rhinitis. The nasal spray comprises cromolyn sodium, naphazoline hydrochloride, chlorpheniramine maleate and a pharmaceutically acceptable excipient selected from a buffering agent, an osmotic pressure regulator, an absorption enhancer, a pH regulator and the like. Active ingredients in the preparation can take effect rapidly by being rapidly adsorbed through mucosa of nasal cavity. The nasal spray has the advantages of high bioavailability, high compliance, convenience in carrying and use, and the like.
Owner:SHANDONG TIANSHUN PHARMA
Aspartic vitamin eye drops and preparation method thereof
ActiveCN100586440CSimple preparation processImprove yieldSenses disorderPharmaceutical delivery mechanismMentholBenzalkonium chloride
The invention discloses a compound asparticitin gutta and making method, which comprises the following parts: aspartic acid, vitamin B6, glycyrrhizin dipotassium, naphazoline hydrochloride, neostigmine methylsulfate, maleic chlorobenzenenamin, polysorbate 80, sodium chloride, menthol, benzachloridamine, sodium hydroxide solution and injecting water. The invention paves basis for scaling manufacturing of the compound asparticitin gutta further, which improves the productivity with stable content of maleic chlorobenzenenamin.
Owner:江西闪亮制药有限公司
Compound rupatadine bioadhesion nasal gel product and preparation method
ActiveCN106265664AGood bioadhesionStrong organizational affinityOrganic active ingredientsAntipyreticNitrofurazoneMedicine
The invention discloses a compound rupatadine bioadhesion nasal gel product and a preparation method. The compound rupatadine bioadhesion nasal gel product is characterized by being prepared from, 10-100 parts of rupatadine and salts thereof, 5-20 parts of xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, 1-4 parts of furacilin, 300-5000 parts of polymer gel materials, 2-200 parts of a biological adhesive, 180-200 parts of a buffer salt, 800-1600 parts of a wetting agent and 20000 parts of sterile water for injection. The wetting agent is dissolved into 2 / 3 of the sterile water for injection to obtain a solution A; the polymer gel material is stirred and added into the solution A to obtain a gel system B; the biological adhesive is dispersed into the gel system B to obtain a gel system C; the rupatadine and salts thereof, the xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, the furacilin and the buffer salt are successively added into 1 / 3 of the sterile water for injection, and sonication is performed to obtain a solution D; the solution D is dispersed into the gel system C, and stirring is performed to obtain the final product.
Owner:重庆市人民医院
Compound nose drops and its prepn.
InactiveCN1336175AReduce edemaAdjust immune functionOrganic active ingredientsRespiratory disorderCurative effectTherapeutic effect
The invented naristillae is composed of naphazoline hydrochloride nose drop 3-8 ml. contianing 0.1% naphazoline hydrochloride, lincomycin hydrochloride solution 2-6 ml. containing 0.6-1.8 gm licomycin hydrochloride, prednisolone aectate solution 1-5 ml. containing prednisolone acetate 25-125 mg. Advantages include: apparent therapeutic effect quick action, easy to use it, no recurrence etc.
Owner:杨俊爱
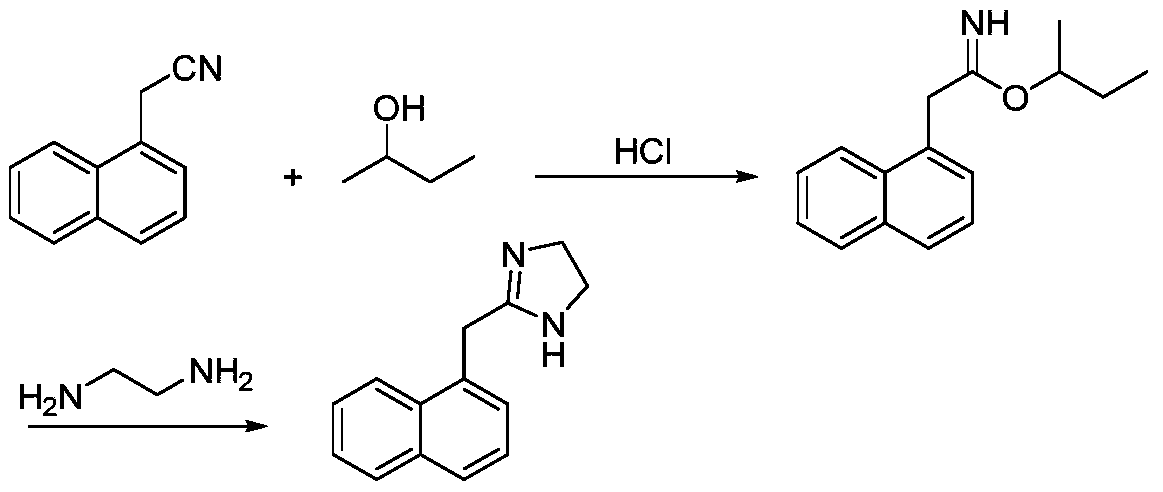
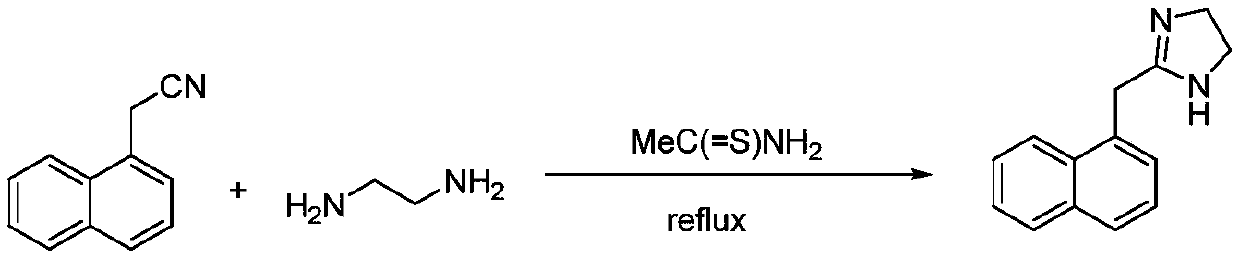
Preparation method of naphazoline hydrochloride
The invention discloses a preparation method of naphazoline hydrochloride. A specific sulfur-containing catalyst CS2, Na2S or thiourea is used for synthesizing naphazoline, and a naphazoline hydrochloride product with high yield, high purity and good powder property is obtained through simple salification and recrystallization processes.
Owner:GUANGDONG XIANQIANG PHARMA +2
Pharmaceutical composition for treating rhinitis
ActiveCN103751189APromote absorptionNo damageOrganic active ingredientsRespiratory disorderBioavailabilityBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition for treating rhinitis. The pharmaceutical composition contains sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate. The composite is a spraying agent, active ingredients in the preparation can realize fast onset of action by rapid absorption through nasal mucosa, and the composition has the advantages of high bioavailability, good compliance, convenience in carrying and use and the like.
Owner:SHANDONG TIANSHUN PHARMA
Ophthalmic drug composition, and preparation method and application thereof
ActiveCN109453151ALittle side effectsGood treatment effectSenses disorderAnhydride/acid/halide active ingredientsVitamin B12Therapeutic effect
The invention relates to the field of ophthalmic preparations, in particular to an ophthalmic drug composition, and a preparation method and application thereof. The composition per 1000 ml is prepared from 0.05 to 0.15 g of naphazoline hydrochloride, 3.2 to 5.8 g of taurine, 0.1 to 0.3 g of pH adjuster, 0.05 to 0.15 g of osmotic pressure regulator, 0.01 to 0.05 g of metal ion complexing agent, 0.2 to 0.5 g of vitamin B12 and 0.1 to 0.3 g of chlorpheniramine. Taurine, naphazoline hydrochloride, vitamin B12 and chlorpheniramine together perform synergistic interaction to improve the treatment effect of the ophthalmic drug composition on conjunctivitis, achieve a good treatment effect on hyperemia, edema, and the like caused by inflammation, and also can significantly reduce the side effectsof the composition, reduce the stimulation of the composition on the eye, and enhance the experience of a patient in medication.
Owner:湖北远大天天明制药有限公司
A nasal spray for treating allergic rhinitis
ActiveCN103893174BSmall toxicityQuick resultsOrganic active ingredientsAerosol deliveryNasal cavityNose
The invention provides nasal spray for treating allergic rhinitis. The nasal spray comprises cromolyn sodium, naphazoline hydrochloride, chlorpheniramine maleate and a pharmaceutically acceptable excipient selected from a buffering agent, an osmotic pressure regulator, an absorption enhancer, a pH regulator and the like. Active ingredients in the preparation can take effect rapidly by being rapidly adsorbed through mucosa of nasal cavity. The nasal spray has the advantages of high bioavailability, high compliance, convenience in carrying and use, and the like.
Owner:SHANDONG TIANSHUN PHARMA
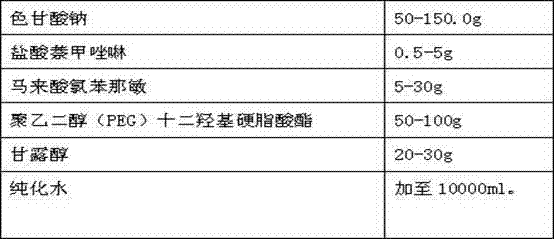
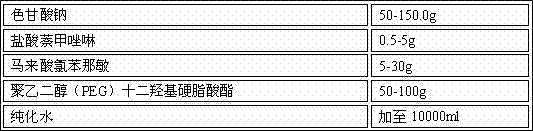
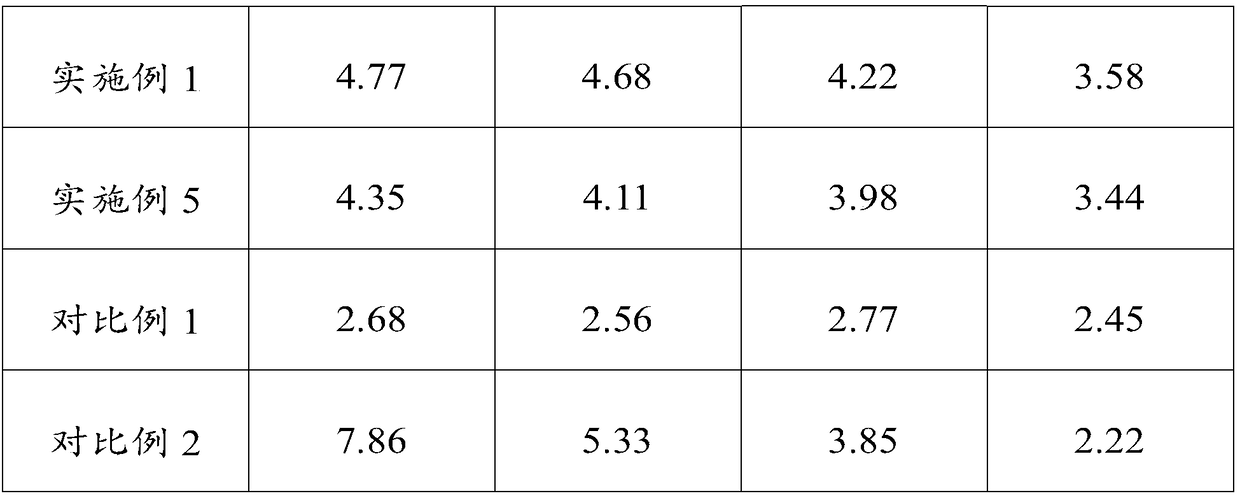
Separation and detection methods for related substances in naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops
InactiveCN112540135AEnsure safetyQuality assuranceComponent separationVitamin B12Pharmaceutical Substances
The invention relates to separation and detection methods for related substances in naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops, and belongs to the field of pharmaceutical analysis. The separation method comprises the following steps of: injecting the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops into a high performance liquid chromatograph, and carrying out separation under the following conditions: a chromatographic column is a C8 or C18 chromatographic column; the mobile phase comprises a mobile phase A and a mobile phase B, themobile phase A is disodium hydrogen phosphate, and the mobile phase B is an alcohol compound and / or acetonitrile; and the elution time and the volume content of the mobile phase B in the elution timeare as follows: 0-25 min, 18%-20%; 25-70 min, 20%-45%; 70-80 min, and 45%; 80-85 min, and 45%-18%. The separation method can be used for separating related substances corresponding to three effectivecomponents in the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops. The detection method comprises the following steps of: taking the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops as a test solution, preparing reference solutions, respectively carrying out chromatographic detection on the test solution and the reference solutions, and calculating the content of each related substance according to a recorded chromatogram. The detection method can be used for rapidly and accurately detecting the contents of related substances correspondingto the three effective components in the naphazoline hydrochloride, chlorphenamine maleate and vitamin B12 eye drops.
Owner:湖北远大天天明制药有限公司
Eyedrop recipe and manufacturing method thereof
InactiveCN111568925AEasy to prepareNo side effectsOrganic active ingredientsSenses disorderEye drynessEthylic acid
The invention discloses an eyedrop recipe which comprises the following raw materials in parts by weight: 0.5 to 0.9 part of chloramphenicol, 0.2 to 0.3 part of naphazoline hydrochloride, 0.3 to 0.8 part of triamcinolone acetonide acetate and 7000 to 8000 parts of Hertz functional water. The recipe is prepared from the following raw materials in parts by weight: 0.8 part of chloramphenicol, 0.25 part of naphazoline hydrochloride, 0.5 part of triamcinolone acetonide acetate and 7500 parts of Hertz functional water. A preparation method of an eyedrop using the recipe comprises the following steps of firstly mixing the chloramphenicol and the naphazoline hydrochloride according to the recipe quantities; adding the Hertz functional water; performing uniform stirring; then, adding the triamcinolone acetonide acetate; performing sufficient and uniform mixing; charging the materials into a high-temperature sterilized bottle; and performing sealing for use. The eyedrop using the recipe provided by the invention can be used for various kinds of eye discomfort, and has the special effects on vitreous opacity, slight cataract, keratitis, pinkeye, welding wounded eye diseases, dry or itching eye syndromes and the like.
Owner:于莉
Compound nose drops and its prepn.
InactiveCN1130202CClinical application safetyOrganic active ingredientsRespiratory disorderTherapeutic effectLincomycin Hydrochloride
The invented naristillae is composed of naphazoline hydrochloride nose drop 3-8 ml. contianing 0.1% naphazoline hydrochloride, lincomycin hydrochloride solution 2-6 ml. containing 0.6-1.8 gm licomycin hydrochloride, prednisolone aectate solution 1-5 ml. containing prednisolone acetate 25-125 mg. Advantages include: apparent therapeutic effect quick action, easy to use it, no recurrence etc.
Owner:杨俊爱
A kind of medicinal composition for treating rhinitis
ActiveCN103751189BQuick resultsLittle side effectsOrganic active ingredientsRespiratory disorderChlorobenzeneSodium cromoglicate
The invention provides a pharmaceutical composition for treating rhinitis, which comprises sodium cromolyn, naphazoline hydrochloride and chlorpheniramine maleate. The composition is a spray, and the active ingredient in the preparation can be quickly absorbed through the nasal mucosa to take effect quickly, and has the advantages of high bioavailability, good compliance, convenient carrying and use, and the like.
Owner:SHANDONG TIANSHUN PHARMA
New medicinal application of naphazoline hydrochloride
PendingCN114652717APromote proliferationOrganic active ingredientsSkeletal/connective tissue cellsPharmaceutical drugPharmaceutical medicine
The invention discloses a medicinal application of naphazoline. The application refers to application of naphazoline or pharmaceutically acceptable salts thereof in preparation of drugs for promoting myocardial cell proliferation. Experiments prove that naphazoline can promote myocardial cell proliferation of rats. Therefore, naphazoline can be used in the related fields of preparation of drugs for promoting myocardial cell proliferation and drugs for treating or preventing heart diseases, and provides a new drug and treatment thought for treating or preventing heart diseases such as myocardial infarction.
Owner:SYNOGENBIOPHARMACO LTD NANJING CHINA +1
A kind of Naphthalene Naphthalene Nasal Spray
ActiveCN103751188BSmall toxicityQuick resultsOrganic active ingredientsAntipyreticNasal cavityBULK ACTIVE INGREDIENT
The invention provides a sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray for treating allergic rhinitis. The sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray comprises sodium cromoglycate, naphazoline hydrochloride, chlorpheniramine maleate, and pharmaceutically acceptable excipients, wherein the pharmaceutically acceptable excipients are selected from a buffering agent, an osmotic pressure regulator, a preservative, an absorption enhancer, a pH (Power of Hydrogen) regulator and the like. According to the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray, the active ingredients can be quickly absorbed through mucosa of nasal cavity and then acts quickly; the sodium cromoglycate, naphazoline hydrochloride and chlorpheniramine maleate nasal spray has the advantages of high bioavailability, outstanding compliance, and convenience in carrying and use, etc.
Owner:SHANDONG TIANSHUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com