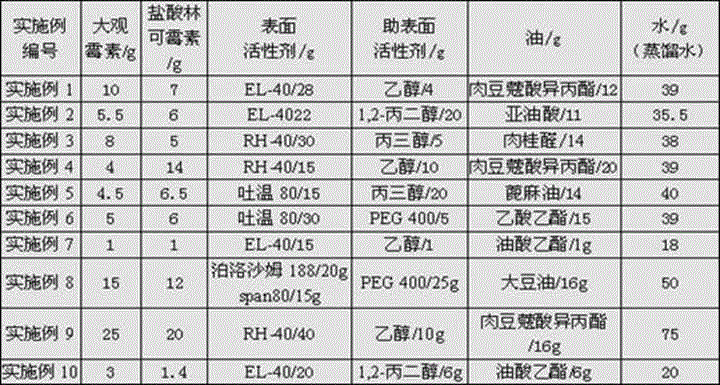
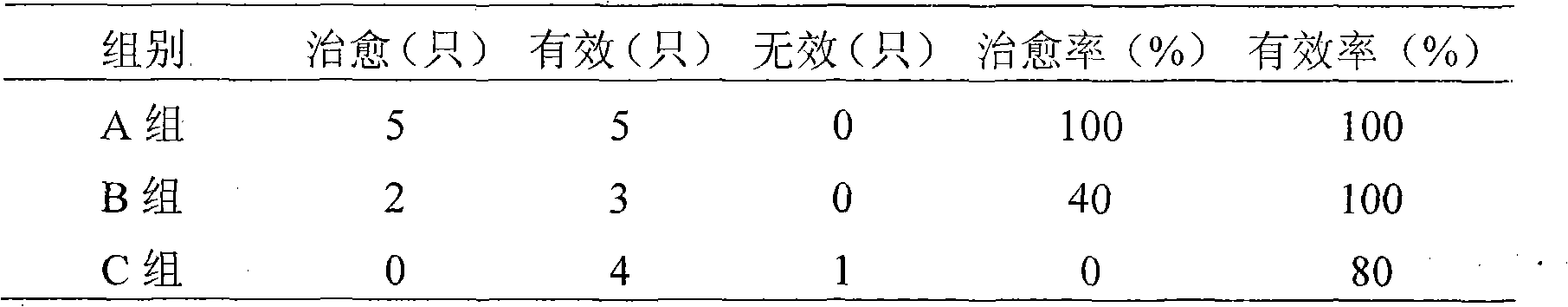
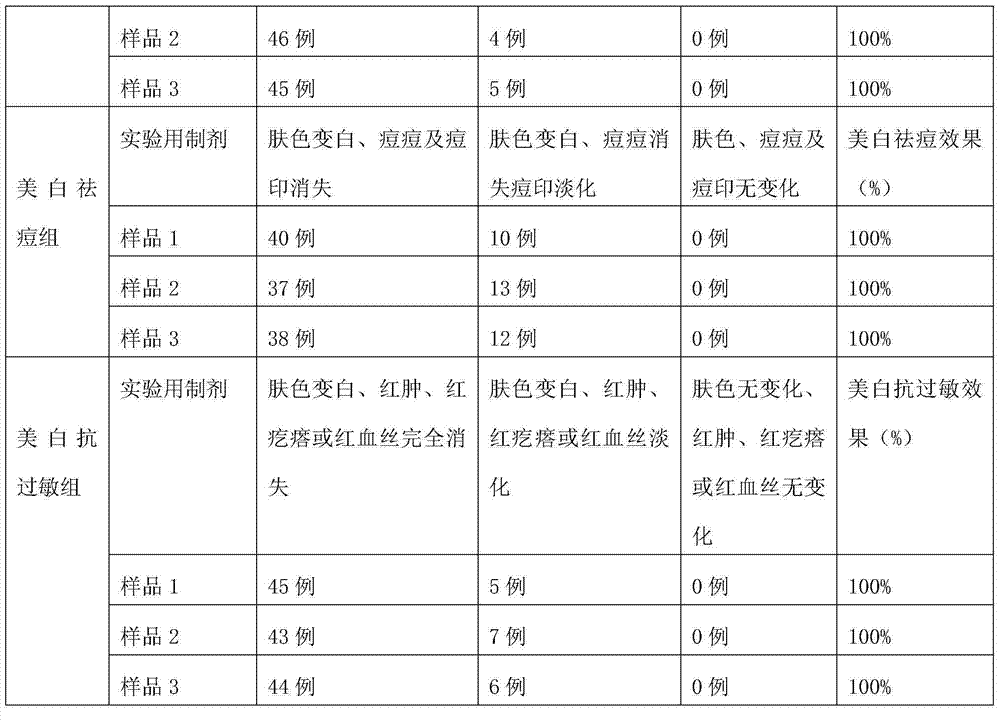
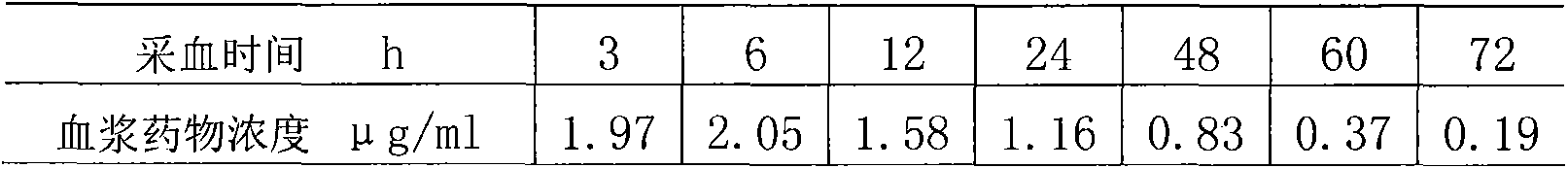Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "Lincomycin Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt form of lincomycin, a lincosamide antibiotic originally identified in actinomycete Streptomyces lincolnensis with activity against gram-positive cocci and anaerobic bacteria.
Treatment method of antibiotic fermenting bacterial residues
ActiveCN104212840ATake advantage ofReduce pollutionMicroorganism based processesFermentationResource utilizationSmall footprint
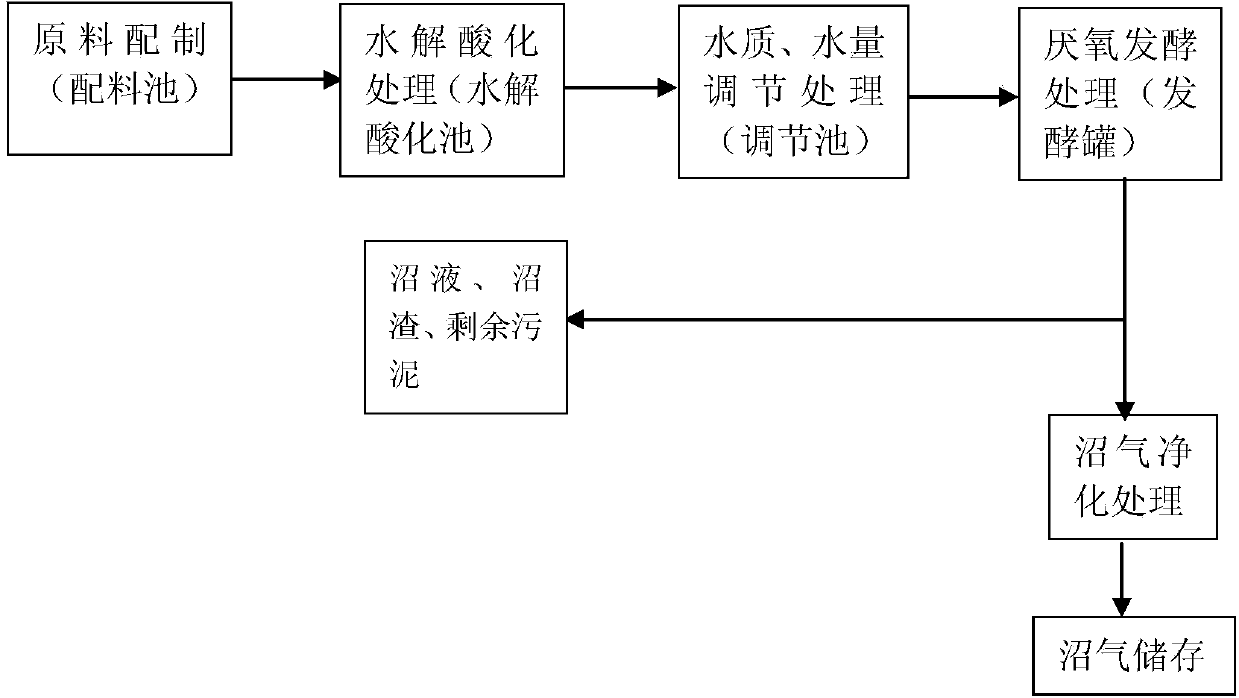
The invention discloses a treatment method of antibiotic fermenting bacterial residues. The treatment method comprises the following steps: uniformly mixing the fermenting bacterial residues of lincomycin hydrochloride and gentamicin with water, and preparing a suspension; performing anaerobic fermentation treatment on the suspension. The treatment method is an efficient fermentation treatment process of the fermenting bacterial residues of lincomycin hydrochloride and gentamicin and belongs to the technical field of resource utilization of the bacterial residues. By adopting the method disclosed by the invention, the toxicity, the harm and an inhibition effect of residual antibiotics to fermenting bacteria in an anaerobic fermentation process of the traditional antibiotic bacterial residues are overcome, the problems of blockage, short flow and the like which are produced when a traditional filler is fed are simultaneously avoided, the biomass of a hydrolysis acidification pool and an L-fermentation tank is ensured, the gas production is improved and the method further has the advantages of low treatment cost, easiness in control of operation process, high load of a reactor matched with the method, small occupied area and the like.
Owner:ZHENGZHOU UNIV
Process for synthesizing clindamycin hydrochloride
InactiveCN101891778ASimple processPractical applicationSugar derivativesSugar derivatives preparationChemical reactionHydrolysis
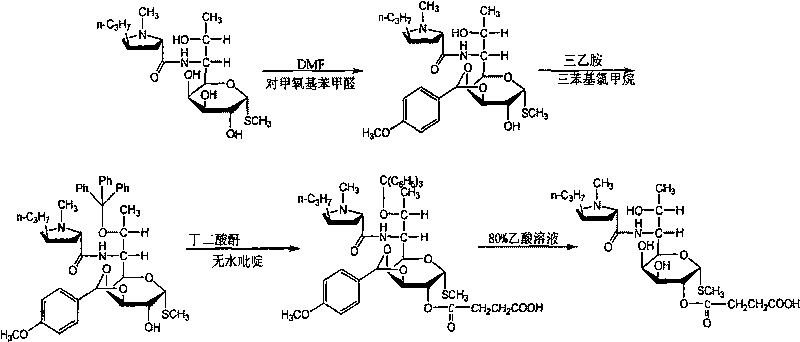
The invention relates to a process for synthesizing new clindamycin hydrochloride. The process comprises the following steps of: 1) finishing chlorination reaction by using lincomycin hydrochloride as a basic raw material and using low-C halogenated hydrocarbon as a solvent; 2) finishing hydrolysis reaction of sodium hydroxide in an aqueous phase by using a product obtained in the step 1), and demixing the solution to obtain clindamycin free alkali; and 3) in a solvent system of acetone, performing salt forming reaction on the clindamycin free alkali obtained in the step 2) and hydrochloric acid, and crystallizing the reaction product to obtain the clindamycin hydrochloride. The invention has the advantages that: 1, the process is simple; 2, the process reduces one-step chemical reaction on chemical unit reaction; 3, the process reduces one raw material and one intermediate on materials and intermediate links; and 4, the yield of the product is greatly improved, the epimer clint content of impurities is reduced by 80 percent, the process has high yield, and the yield is improved by over 5 percent compared with a four-step method.
Owner:ZHANGJIAGANG XINYI CHEM
Enzyme-linked immunosorbent assay for measuring content of lincomycin hydrochloride in food
InactiveCN101726590AModify molecular structureHigh sensitivityColor/spectral properties measurementsRelative standard deviationAntibiotic Y
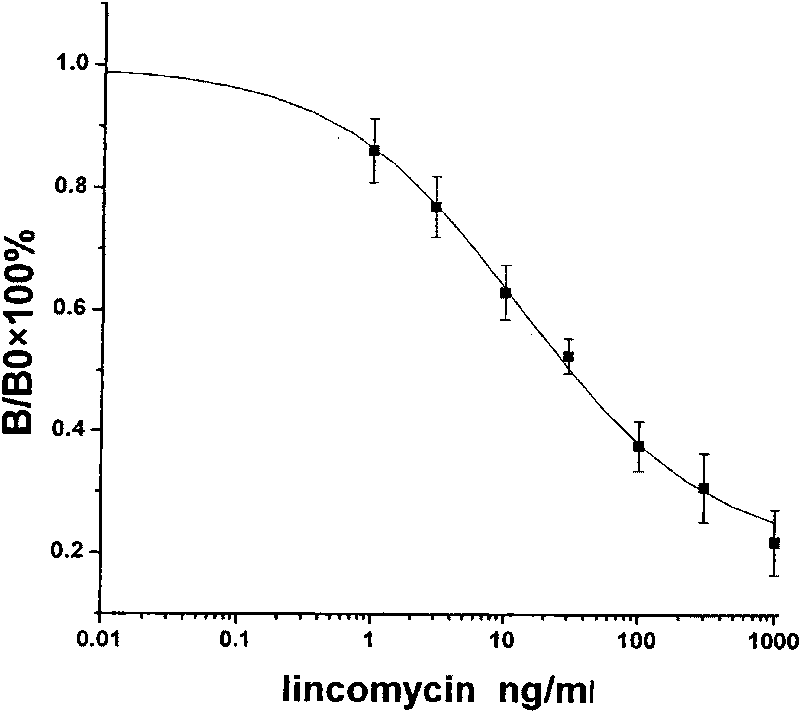
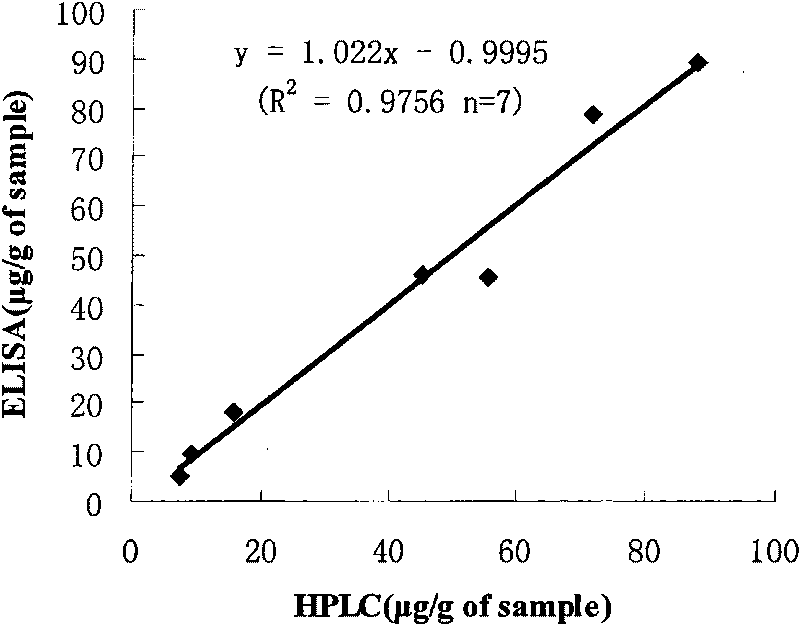
The invention discloses an enzyme-linked immunosorbent assay (EISA) for measuring the content of lincomycin hydrochloride in food. The method is characterized in that a novel hapten modification method is used to synthesize a modifier of lincomycin hydrochloride, and the modifier is linked to the protein to prepare an immunogen and an envelope antigen. A polyclonal antibody of rabbit anti-lincomycin hydrochloride is acquired by an immune animal. The antibody is used for the immunoassay of lincomycin hydrochloride; the concentration range of a standard curve is 1-1000ng / mL, IC50 is 23.7-29.3ng / mL, the cross reactivity of the antibody and the lincomycin hydrochloride is 18.9%, and the antibody and other seven popular antibiotics or hormones hardly have cross reaction. Six kinds of market-sold foods are extracted by using 0.01mol / L HCL after being labeled; an extract is properly diluted and measured by ELISA, the labeling recovery rate is 76.6-117.6%, and the relative standard deviation is 1.7-34.6%. The seven labeled samples are analyzed by using the HPLC and the ELISA methods which have better pertinence (R2=0.9756 and n=7). The EISA established in the invention has reliability, high sensitivity and strong specificity and can be used for detecting the content of lincomycin hydrochloride in food.
Owner:SICHUAN UNIV
Lincomycin hydrochloride extraction method
ActiveCN105237593AEfficient enrichmentReduce the amount of extractionSugar derivativesSugar derivatives preparationActivated carbonLincomycin Hydrochloride
The invention relates to a lincomycin hydrochloride extraction method which comprises the following process steps: performing acidification pre-treatment on lincomycin fermentation broth, dual aqueous phase extraction, butanol extraction, hydrochloric acid reextraction, activated carbon decoloring, acetone crystallization, and drying, thereby obtaining a solid product lincomycin hydrochloride. Due to the adopted dual aqueous phase extraction technique for extract lincomycin hydrochloride, lincomycin can be effectively enriched, the use amount of butanol can be reduced, the solvent consumption can be reduced, dual aqueous phase raw materials used in the method are free of environment pollution, lincomycin can be produced in a large scale, economic and environment-friendly requirements can be met, and positive contribution can be made.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
Treating agent for lincomycin hydrochloride production waste water and its preparation method and usage method
InactiveCN1865175AEasy to useSimple and fast operationTreatment with aerobic and anaerobic processesMultistage water/sewage treatmentSolventEnergy conservation
This invention relates to a waste water treatment agent for treating the waste water during the jiemycin hydrochloride production and the preparation method and process of using thereof. This treatment agent is made of formaldehyde, dicyandiamide, catalyst, cationic starch, water and assisting solvent raw material. The COD of the waste water can be decreased below 300mg / L after treated by this treatment agent and can meet the national standard of discharging. It overcomes the disadvantage that the total discharging pollutant does not decrease by diluting the high-concentrated jiemycin organic waste water in the current manufacturing enterprise. Besides, this treatment agent is easy soluble and dispersing in waste water, thus has a high usage efficiency and good economical benefits. In the meantime, the mass amount marsh gas and organic deposition substances produced during the treatment process can be used as fuel and farm manure respectively, which can obtain a good effect of comprehensive utilization, energy saving and cost decreasing.
Owner:南阳市鹿城生化研究所
Lincomycin and spectinomycin compound oil suspension injection and preparation method and application thereof
ActiveCN102048748ALess irritatingEasy to administerAntibacterial agentsOrganic active ingredientsAntioxidantBiochemical engineering
The invention discloses a lincomycin and spectinomycin compound oil suspension injection and a preparation method and application thereof. The injection comprises a mixed auxiliary material solvent, lincomycin hydrochloride and spectinomycin hydrochloride. The mixed auxiliary material solvent system comprises oil or ester for injection, suspending agent, emulsifier, wetting agent and antioxidant. The preparation method comprises adding suspending agent into oil or ester for injection to obtain gel solvent system, sequentially adding emulsifier, wetting agent and antioxidant, adding lincomycin hydrochloride and spectinomycin hydrochloride, and grinding with a colloidal mill. The invention can effectively improve the stability of spectinomycin and reduce the irritation on animal body during usage, and has simple preparation process, strong operability and high value of popularization.
Owner:SOUTH CHINA AGRI UNIV
Lincomycin hydrochloride freeze-dried powder injection for livestock injection and preparation method thereof
InactiveCN102000036AAvoid degradationLoose textureAntibacterial agentsOrganic active ingredientsMicropore FilterSterile environment
The invention discloses lincomycin hydrochloride freeze-dried powder injection for livestock injection and a preparation method thereof and belongs to the technical field of veterinary medicines. The lincomycin hydrochloride freeze-dried powder injection comprises the following components: 3 to 30 percent of lincomycin hydrochloride, 0.1 to 90 percent of excipient and 0.1 to 90 percent of additive. The preparation method comprises: adding lincomycin hydrochloride and prescription amount of excipient in water for injection in a sterile environment under a dark condition and fully stirring for dissolution; adding the additive to regulate the pH value to between 4.0 and 6.5; performing ultrafiltration, adding medicinal active carbon, stirring, absorbing and removing a heat source; filtering by 0.45-micrometer micropore filtering film, and fixing volume; filtering by a 0.22-micrometer micropore filter film terminal for sterilization; and charging with nitrogen, filling separately, freeze drying, capping, sealing and storing. The product of the invention is based on a simple formula, has safe quality and high stability, can be prepared by a simple process at low cost and is easy to store and transport.
Owner:商丘市康森动物药品研究所
Spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and preparation method of spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection
InactiveCN106420635AGood quality and stabilitySignificant effectPowder deliveryOrganic active ingredientsSpectinomycin HydrochlorideFreeze-drying
The invention relates to a spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and a preparation method of the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection, and solves the problems of high cost of industrial production and low drug stability in the prior art. The preparation method comprises the steps of firstly preparing a freeze-dried propping agent and antioxidant mixed solution, then preparing a to-be-freeze-dried sample of spectinomycin hydrochloride and lincomycin hydrochloride, and finally preparing the white loose block or powder spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection in a freeze drying box. According to the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and the preparation method of the spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection, the drug stability and the bioavailability are high, and the industrial production is realized.
Owner:LESHAN RECONDEX BIOPHARM CO LTD +1
Whitening, acne removal and freckle removal cosmetology medicine
ActiveCN103479661AGet rid of acneImprove acneDermatological disorderHeterocyclic compound active ingredientsHypopigmentationJuvenile acne
The invention discloses a whitening, acne removal and freckle removal cosmetology medicine which is prepared by the following raw materials in parts by weight: 2-5 parts of chloramphenicol injection, 5-7 parts of akafen powder, 14-16 parts of lincomycin hydrochloride gel and 6-8 parts of compound ketoconazole cream. The chloramphenicol injection, the akafen powder, the lincomycin hydrochloride gel and the compound ketoconazole cream are put into a container according to the weight parts, then are uniformly mixed and stirred and are finally bottled and packaged. The whitening, acne removal and freckle removal cosmetology medicine can effectively remove skin acnes and whelk and solve the problems of hyperpigmentation and the like which are caused by chloasma, freckles, physical machinery or chemical burn and is low in cost, easy to manufacture and convenient to use.
Owner:刘彩群
Compound sodium sulfadimidine injection liquid for pig and preparation method thereof
InactiveCN101606945ADelay drug resistanceIncreased sensitivityAntibacterial agentsOrganic active ingredientsDoxofyllineTrimethoprim
The invention discloses a compound sodium sulfadimidine injection liquid for a pig and a preparation method thereof, aiming at providing a compound kanamycin sulfate injection liquid which has fast effect for treating toxoplasmosis of the pig, addresses both the symptoms and root causes, reduces the time and dosage of used drug and has convenient drug usage, and a preparation method with simple technique and easy implementation. Each 100L injection liquid comprises: 5 to 20 sodium sulfadimidine, 1 to 10kg of lincomycin hydrochloride, 5 to 20kg of doxofylline, 1 to 4kg of trimethoprim, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 40kg of propylene glycol, 10kg of 95% alcohol and the balance of water for injection. The injection liquid has fast effect for toxoplasmosis of the pig, addresses both the symptoms and root causes, can effectively treat and prevent secondary infection of bacterium, reduces drug resistance of pathogenic microorganism and leads the pig to accelerate restoring. Simultaneously, the compound sodium sulfadimidine injection liquid is an injection liquid and has convenient use.
Owner:TIANJIN SHENGJI GRP CO LTD
Compound enrofloxacin injection for animals and preparation thereof
InactiveCN101301291AIncrease weightDelay drug resistanceOrganic active ingredientsAntiinfectivesDoxofyllineAntimicrobial drug
The invention discloses a compound enrofloxacin injection for animals and a preparation method thereof, which aims to provide the enrofloxacin injection for the animals and the preparation method thereof, wherein, the enrofloxacin injection for the animals can treat swine enzootic pneumonia, is quick to take effect, decreases drug resistance, and can treat symptoms and root causes; and the preparation method has a simple process and is easy to realize. The injection comprises the following compositions by weight portion: 0.5 to 5kg of enrofloxacin, 1 to 10kg of lincomycin hydrochloride, 0.01 to 0.05kg of sodium hydroxide, 0.1 to 2kg of doxofylline, 0.2kg of sodium sulfite, 0.01kg of EDTA-2Na, 30kg of propanediol, and the balance being water for injection. The injection adopts a compound preparation of an antimicrobial drug and antibiotics; due to the combined use of the enrofloxacin and the lincomycin hydrochloride as well as the doxofylline, the injection is quick to take effect to the swine enzootic pneumonia, is effective and highly efficient, reduces the probability of the drug resistance, can apparently relieve clinical symptoms caused by the swine enzootic pneumonia, can treat symptoms and root causes, and increase the weight of pigs.
Owner:TIANJIN SHENGJI GRP CO LTD
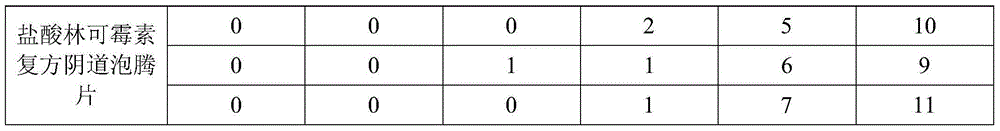
Lincomycin hydrochloride compound vaginal effervescent tablets and preparation method thereof
InactiveCN104906066ARelief the painRelieves symptoms of itchingPharmaceutical delivery mechanismAmine active ingredientsAdditive ingredientTopical treatment
The invention discloses lincomycin hydrochloride compound vaginal effervescent tablets and a preparation method thereof. The effervescent tablets comprise the following western medicine effective ingredients in parts by weight: 4-6 parts of lincomycin hydrochloride and 1-3 parts of domiphen bromide; and the effervescent tablets comprise the following raw medicinal materials of traditional Chinese medicine active ingredients in parts by weight: 20-25 parts of densefruit pittany root-bark, 22-27 parts of tuber fleeceflower root, 18-25 parts of Chinese waxgourd seed, 30-35 parts of argy wormwood leaf, 21-25 parts of herb of red-knees, 18-24 parts of plantain seed, 17-20 parts of villous amomum fruit, 25-28 parts of largehead atractylodes rhizome, 22-26 parts of Chinese angelica (stir-fried with wine), and 20-25 parts of medicinal auxiliary materials. The preparation method of the effervescent tablets comprises the steps of preparing materials, extracting active ingredients of the traditional Chinese medicines, performing granulation, and tabletting. The lincomycin hydrochloride compound vaginal effervescent tablets disclosed by the invention effectively overcome adverse reactions caused by oral administration of lincomycin hydrochloride, directly act on affected parts by adopting an external method for local treatment of vaginitis, take effect quickly, are good in curative effect, and are convenient to use.
Owner:QINGDAO HAIZHIXING BIOLOGICAL SCI & TECH
Preparation method of lincomycin hydrochloride injection
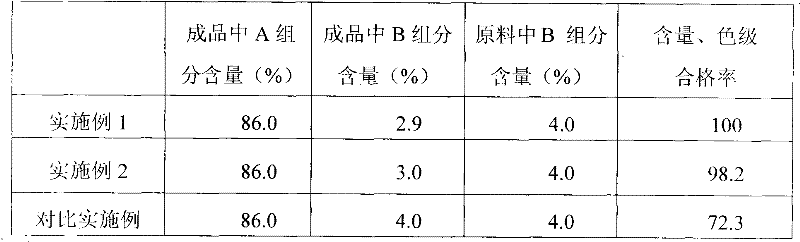
InactiveCN103830172AReduce contentQuality improvementAntibacterial agentsOrganic active ingredientsSodium bicarbonateEthylenediamine
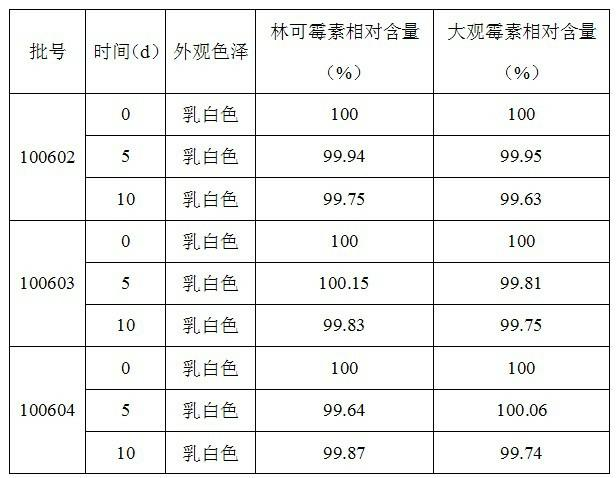
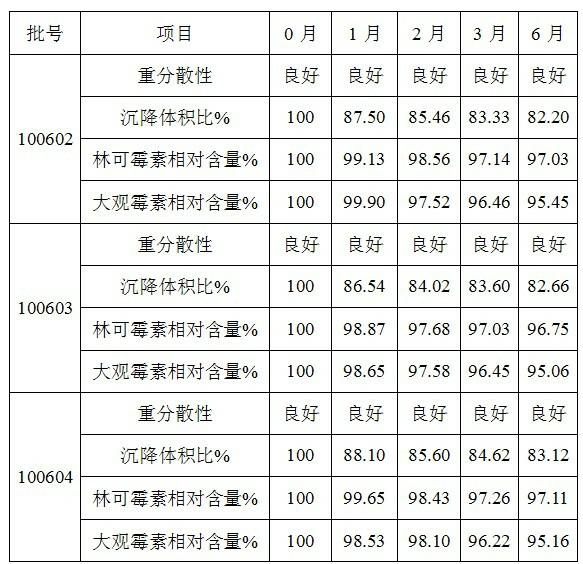
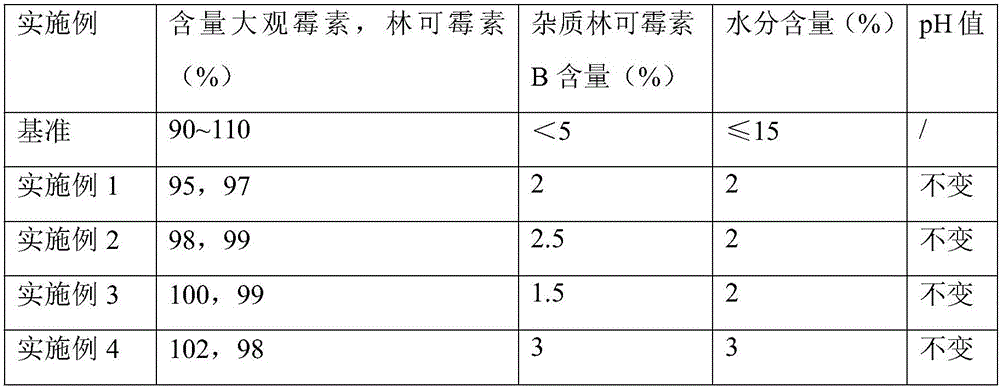
The invention provides a preparation method of lincomycin hydrochloride injection, which comprises the following steps: (1) fetching 20,000ml of injection water, 6kg of lincomycin hydrochloride raw powder, 8g of EDTA (ethylenediamine tetraacetic acid)-2Na, 172g of anhydrous sodium bicarbonate and appropriate hydrochloric acid and anhydrous sodium bicarbonate for later use; (2) soaking a reaction kettle, a conveying pipeline and a constant-temperature water supply system with EDTA, and flushing for later use; (3) leading the stored injection water into a water storage tank, and connecting the water storage tank with a water outlet pipe through a pipeline; (4) injecting the injection water into the reaction kettle through the water outlet pipe, adding the lincomycin hydrochloride raw powder, and dissolving; (5) adding the anhydrous sodium bicarbonate into the solution after ultrafiltration to adjust the pH value, adding EDTA-2Na, sufficiently stirring, and then measuring the content; (6) if the liquid medicine is qualified, filling in a hundred-class environment to obtain the lincomycin hydrochloride injection. According to the method provided by the invention, the content of a lincomycin component B is effectively reduced in order to improve the quality of the lincomycin hydrochloride injection and reduce the preparation cost.
Owner:ANHUI HONGYE PHARMA
Preparation method of lincomycin hydrochloride injection
ActiveCN102475677AReduce contentQuality improvementAntibacterial agentsOrganic active ingredientsSodium bicarbonateActivated carbon
The invention discloses a preparation method of a lincomycin hydrochloride injection. The preparation method comprises the following steps of: (a) heating water for injection to 55-65 DEG C and dissolving raw powder of lincomycin hydrochloride; (b) regulating the pH of anhydrous sodium bicarbonate to be neutral, adding EDTA-2Na (ethylenediaminetetraacetic acid-disodium), fully and uniformly stirring, then removing pyrogen with 0.02% of activated carbon for injection and determining the content; and (c) filtering through a microporous filtering film, filling under a class-100 environment and circulating steam at the temperature of 100-105 DEG C for 15 minutes for sterilizing twice. According to the preparation method disclosed by the invention, a lincomycin B component can be degraded in the preparation process of the injection by improving the methods for regulating the temperature of liquid medicine, performing secondary sterilization and the like in the preparation process, so that the content of the lincomycin B component in the injection is reduced, the quality of a product is improved, and thus high cost caused by particular extraction and purification against raw materials is also avoided.
Owner:NORTH CHINA PHARMA COMPANY
Compound niacin norfloxacin injection for animals and preparation thereof
InactiveCN101297813ADelay drug resistanceReduce excess residueAntibacterial agentsDigestive systemDiseaseSulfite salt
The invention discloses a veterinary compound norfloxacin nicotinate injection and a preparation method thereof, which aims at providing the veterinary compound norfloxacin nicotinate injection that has rapid onset of action for the treatment for white diarrhea of piglets and can prevent the infection and the secondary infection and treat both manifestation and root cause of disease, and the preparation method that has simple technology and easy realization. In the veterinary compound norfloxacin nicotinate injection, each 100l injection is prepared by the following components: 1 to 8kg of the norfloxacin nicotinate, 2 to 10kg of lincomycin hydrochloride, 0.05 to 0.5kg of atropine sulfate, 0.2kg of sodium sulfite, 0.01kg of EDTA-2Na and the rest of water for injection. By adopting a compound preparation which combines anti-bacterial drugs and antibiotics for usage, and the combining usage of the norfloxacin nicotinate, the lincomycin hydrochloride and the atropine sulfate, the injection of the invention has rapid onset of action for white diarrhea of piglets, effectivity and high efficiency and can ease the stress reactions of the affected animals, significantly ease the clinical symptoms caused by the white diarrhea of piglets, treat both manifestation and root cause of disease, prevent the secondary infection and increase the weight of the pigs.
Owner:TIANJIN SHENGJI GRP CO LTD
Method and device for preparing lincomycin hydrochloride
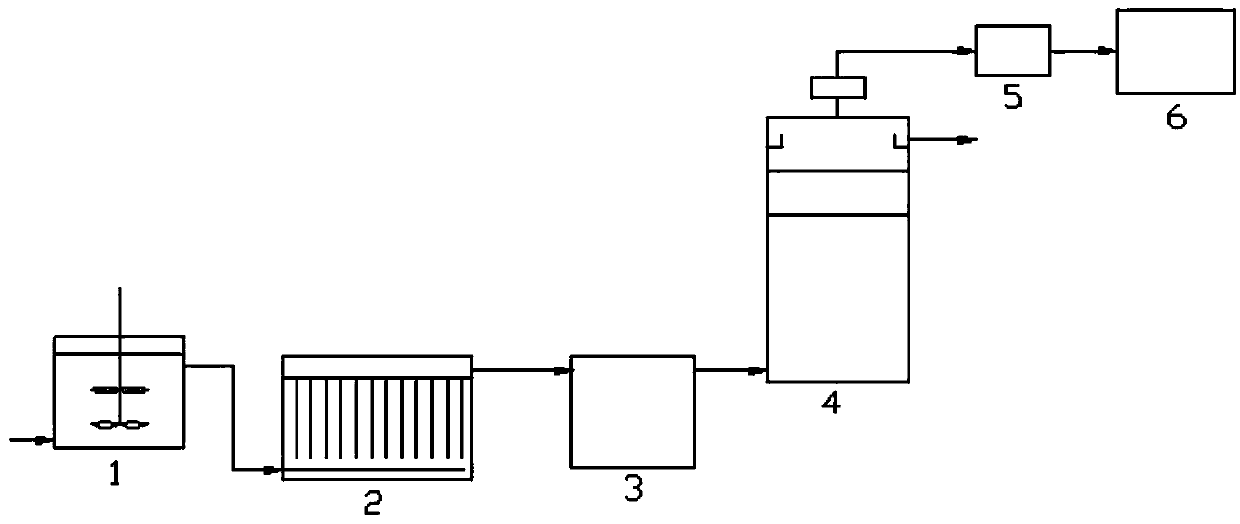
ActiveCN101624411AEfficient separationQuality improvementSugar derivativesAntiinfectives2-OctanolLincomycin Hydrochloride
The invention relates to a method and a device for preparing lincomycin hydrochloride. On the basis of the prior lincomycin hydrochloride production, repurifying processing procedure is added to prepare lincomycin hydrochloride with lincomycin B component less than or equal to 0.1 percent. The method is characterized by comprising the following steps: lincomycin hydrochloride with B component less than or equal to 1.0 percent is dissolved to have thetconcentration of 80000-100000 microgramme / ml, the pH value is adjusted to 10-11, extraction is carried out in extraction tower with 1 / 2-1 / 3 (weight ratio) of 2-octanol, the extraction temperature is 20-30 DEG C, the pH value of extraction liquid is adjusted to 2-4 by hydrochloric acid for back extraction, and acetone is used for crystallization. By adopting the method and the extraction tower device, the concentration of B component in lincomycin hydrochloride can be effectively reduced to be less than or equal to 0.1 percent, so that the quality of lincomycin hydrochloride is improved, and the yield is increased by 5-7 percent.
Owner:XINYU PHARM CO LTD
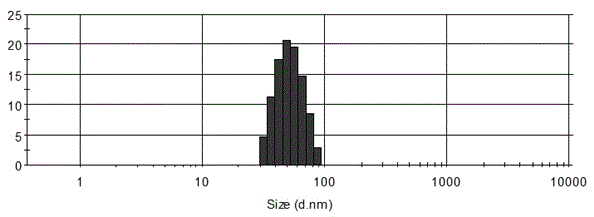
Lincomycin-spectinomycin compound nano-emulsion
InactiveCN104306389AReduce interfacial tensionIncrease oily waterAntibacterial agentsOrganic active ingredientsBiotechnologyActive agent
The invention provides lincomycin-spectinomycin compound nano-emulsion, and belongs to the technical field of pharmacy. The compound nano-emulsion is prepared from the following components in parts by weight: 1-20 parts of lincomycin hydrochloride, 1-25 parts of spectinomycin, 15-40 parts of surfactant, 1-25 parts of co-surfactant, 1-16 parts of oil and 18-75 parts of water. The compound nano-emulsion is a novel preparation which is combined with characteristics of lincomycin hydrochloride and lincomycin hydrochloride, and has the advantages of uniform grain diameter distribution, good fluidity, high stability, good permeability and good bioavailability; the compound nano-emulsion can be quickly phagocytosed by reticuloendothelial cells after being taken orally, drugs can quickly take effects, and constant plasma concentration and pharmacological effect can be maintained, the bioavailability can be improved, drug effect can be enhanced, and using amount and taking times of the medicine can be reduced.
Owner:HENAN SOAR VETERINARY PHARMA
Acidic enrofloxacin injection and preparation method thereof
InactiveCN102091087AControl asthma infectionReduce morbidityAntibacterial agentsOrganic active ingredientsAcetic acidLincomycin Hydrochloride
The invention relates to an acidic enrofloxacin injection and a preparation method thereof. The acidic enrofloxacin injection comprises the following components: 5g of enrofloxacin, 1.25g of acetic acid, 1ml of benzyl alcohol, 0.5-5 percent of lincomycin hydrochloride and 100ml of injection water. The weights of all components can be changed according to the accurate proportion of the materials and the solution. The preparation method of the injection comprises the following steps of: taking 5g of enrofloxacin to be added into 80-90ml of injection water, stirring, heating to 50-60 DEG C, cooling, adding 1.25g of acetic acid and 1ml of benzyl alcohol, stirring uniformly, then adding 0.5-5g of lincomycin hydrochloride, stirring until dissolving, finally adding the injection water until reaching 100ml, canning, and sterilizing at high pressure.
Owner:上海恒丰强生物技术有限公司
Injection for curing diarrhea of pig and preparation thereof
InactiveCN101297793AQuick treatmentIncreased sensitivityOrganic active ingredientsDigestive systemIntestinal structureAntimicrobial drug
The invention discloses an injection for the treatment for swine dysentery and a preparation method thereof, which aims at providing the injection for the treatment for swine dysentery that has rapid onset of action for the treatment for swine dysentery and can reduce the times and the dosage of drug administration and reduce the residual drugs which exceed the standards, and the preparation method that has simple process and easy realization. Each 100l injection contains: 0.5 to 10kg of mequindox, 1 to 10kg of lincomycin hydrochloride, 0.2kg of sodium bisulfite, 1.5 to 30kg of sodium salicylate, 0.01Kg of EDTA-2Na, 0.2kg of atropine sulfate and the rest of water for injection. The invention is a compound preparation which combines antimicrobial drugs and antibiotics for usage, the mequindox and the lincomycin hydrochloride are combined for usage, thus adopting double effects, having the dual-killing ability of serpulina hyodysenteriae, reducing the probability of generating the drug resistance of the bacteria and increasing the sensitivity of the serpulina hyodysenteriae; in addition, the atropine sulfate can inhibit the secretion of intestinal digestive glands, so the injection can reduce the water content in the swine intestine during the prevalence period and have rapid onset of action for the treatment for swine dysentery.
Owner:TIANJIN SHENGJI GRP CO LTD
Compound preparation for treating acute mastitis of milk cow
InactiveCN102114175AEffective fastQuick cureOrganic active ingredientsSexual disorderLincomycin HydrochlorideHibiscus
The invention relates to a compound preparation for treating acute mastitis of a milk cow and the preparation method thereof. The composition mainly comprises lincomycin hydrochloride, houttuynia, honeysuckle flower, weeping forsythia fruit, dandelion, Chinese angelica root, luffa, ricepaper plant pith, cotton rose hibiscus leaves, chekiang fritillary bulb, cosolvent, preservative and proper amount of water for injection. The composition provided by the invention has the advantages of quick action and small irritation, is not easy to generate drug tolerance, and is used for treating both principal and secondary aspect of disease. The prepared compound preparation is injection which is convenient for application and easy for operation and can be easily accepted by farmers.
Owner:TIANJIN RINGPU BIO TECH
Injection for treating paratyphoid of dogs and preparation method thereof
InactiveCN101590012AInfection controlReduce chance of drug resistanceAntibacterial agentsDigestive systemGlucocorticoidSulfamonomethoxine
The invention discloses an injection for treating the paratyphoid of dogs and a preparation method thereof, which aim to provide an injection which has quick effect and can reduce the drug resistant probability of pathogenic bacteria, treat both symptoms and root causes and quicken the recovery, and a preparation method which has simple process and is easy to realize. Per 100L of the injection comprises 2-15kg of sulfamonomethoxine sodium, 1-10kg of lincomycin hydrochloride, 0.05-0.3kg of atropine sulfate, 0.06kg of dexamethasone sodium phosphate, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na and the balance of water. The injection adopts antibacterial drugs, antibiotics, glucocorticoids for diminishing inflammation and detoxifying and atropine sulfate for controlling the diarrhea of the dogs and reducing dehydration, and a compound preparation prepared by reasonably proportioning the dosages of the components has the function of double anti-bacteria, can reduce the drug resistant probability of salmonella typhimuria, control the infection of other gram negative bacteria and positive bacteria and accelerate the recovery and has high cure rate.
Owner:TIANJIN SHENGJI GRP CO LTD
Oil injection containing antibacterial agents/polyethylene glycol drug-loading particles
InactiveCN103721263APromote absorptionRetain water swellingAntibacterial agentsPharmaceutical delivery mechanismCelluloseDoxycycline hydrochloride
The invention discloses an oil injection containing antibacterial agents / solid polyethylene glycol drug-loading particles. The oil injection is prepared by consisting of the drug-loading particles through antibacterial agents and solid polyethylene glycol and suspending the drug-loading particles in an oil medium. The antibacterial agents comprise enrofloxacin, danofloxacin mesylate, Marbofloxacin, mequindox, tilmicosin, tylosin, oxytetracycline hydrochloride, ceftiofur hydrochloride, cefquinome sulfate, lincomycin hydrochloride, florfenicol, erythrocin and doxycycline hydrochloride; preferentially the polyethylene glycol with the molecular weight of more than 6000 is used for preparing the preparation; more preferentially, any one of isopropyl myristate, soybean oil for injection, corn oil and tea-seed oil is used for preparing the preparation. Hydroxypropyl methyl cellulose or high-substituted hydroxy propyl cellulose can be added into the preparation.
Owner:王玉万
Temperature-sensitive type gel for curing endometritis of livestock and preparation method for temperature-sensitive type gel
InactiveCN105030660AControl release speedIncrease surface tensionAerosol deliveryOintment deliverySulfamonomethoxineTherapeutic effect
The invention belongs to the technical field of veterinary drug preparations, in particular to temperature-sensitive type gel for curing endometritis of livestock and a preparation method for the temperature-sensitive type gel. The temperature-sensitive type gel comprises the following main drug: one of 5 to 10 percent of ofloxacin, 1 to 2 percent of sodium new houtluyfonate, 1 to 2 percent of sodium houttuyfonate, 5 to 20 percent of sulfadiazine sodium, 5 to 20 percent of sulfamonomethoxine sodium and 10 to 20 percent of lincomycin hydrochloride, the following auxiliary materials: 10 to 30 percent of gel matrix, 1 to 2 percent of release rate regulator, 0.1 to 0.5 percent of antioxygen and 0.5 percent of acetic acid, and balance of water for injection in weight percentage. By fully utilizing the characteristics of good flowability, long resistance time, slow drug release and lasting and stable drug effect of the temperature-sensitive type gel disclosed by the invention, the endometritis of the female livestock is effectively cured, and the curative effect is thorough; meanwhile, the labor strength of a cultivation worker is reduced; the temperature-sensitive type gel is more convenient in therapeutic use.
Owner:张永奎
Medicament composite and injecta for treating swine streptococcosis
InactiveCN102247399AImprove permeabilityPromote recoveryAntibacterial agentsOrganic active ingredientsAntiendomysial antibodiesAntibiotic drug
The invention discloses a medicament composite for treating swine streptococcosis, which is mainly composed of the following medicament components in parts by weight: 5-10 parts of Lincomycin hydrochloride, 1-5 parts of Gentamycin sulfate, 15-25 parts of analginum, 2-8 parts of inosine and 0-0.5 part of antioxygen. In the medicament composite for treating swine streptococcosis, analginum and inosine are added on the basis of taking the Lincomycin hydrochloride and the Gentamycin sulfate as the main antibiotics, so that the minimum inhibitory concentration (MIC) of the medicment composite can be obviously lowered; meanwhile, the analginum has the action on allaying fever, easing pain and resisting inflammation, high heat and fever caused by mixture of swine streptococcosis and other pathogenic microorganisms and secondary infection can be quickly and effectively solved, and temperature quickly returns to normal; the analginum also has the action on alleviating inflammation; the inosine can improve the activity of various enzymes; and an organism is stimulated to generate an antibody to strengthen organism immunity. The drug composite disclosed by the invention is suitable for the situations that swine streptococcosis and mixture are subjected to secondary infection.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Acne removing preparation
InactiveCN103536664AAcne Removal QuicklyAcne breakoutOrganic active ingredientsDermatological disorderSide effectKetoconazole
The invention provides an acne removing preparation with no side effect and good curative effect. The preparation comprises the following components in percentage by weight: 28-56 percent of ketoconazole and clobetasol propionate cream, 20-28 percent of lincomycin hydrochloride gel, 8-20 percent moroxydine hydrochloride, 4-10 percent of metronidazole and 10-40 percent of traditional Chinese medicinal extract, wherein the traditional Chinese medicinal extract comprises extract of white peony root, white poria, bighead atractylodes rhizome and angelica root. The acne removing preparation is effectively combined with traditional Chinese medicines and western medicines, can be used for quickly removing pox and acne, and has the effect of whitening.
Owner:朱晓明 +1
Externally applied preparation for treating whelks
InactiveCN101716184AEasy to useShort course of treatmentTetracycline active ingredientsAlgae medical ingredientsSide effectTobramycin
The invention relates to an externally applied preparation for treating whelks. The externally applied preparation is prepared by dissolving one or several medicaments of erythrocin, aureomycin, chloramphenicol, lincomycin hydrochloride, tobramycin, natamycin, norfloxacin, rifampicin, cyclosporine, gentamicin and neomycin into a polysaccharide aqueous solution. An obtained solution contains 0.005-1 wt% of medicaments and 0.01-30 wt% of glucan, xyloglucan, mycose, sea-tangle polysaccharides, tamarind gum, pectin or other Chinese medicinal herb polysaccharides, or a proper quantity of glucose, sodium chloride and potassium chloride are added to the aqueous solution to prepare a plaster preparation. The externally applied preparation has quick effect and definite curative effect on the whelks or acne and has small stimulation to the skin, extremely light skin decrustation degree and no obvious toxic or side effects. The process of the preparation can guarantee the chemical stability of each component in a production process.
Owner:HUAZHONG NORMAL UNIV
Compound Bupleurum injection for curing common cold of dog and preparation thereof
InactiveCN101297826AControl secondary infectionFast recoveryAntibacterial agentsOrganic active ingredientsDiseaseSulfite salt
The invention discloses a compound bupleurum injection for the treatment for canine influenza and a preparation method thereof, which aims at providing the compound bupleurum injection that can control the canine secondary infection at the same time of treating the canine influenza, have rapid onset of action, reduce the canine suffering and accelerate the canine healing speed, and the preparation method that has simple technology and easy realization. Each 100l of the injection contains: bupleurum extract liquid which is prepared by 50 to 200kg of bupleurum, 1 to 10kg of lincomycin hydrochloride, 0.05 to 1kg of chlorpheniramine, 0.2kg of sodium sulfite, 0.01kg of EDTA-2Na, 0.5kg of Tween-80 and the rest of water for injection. The injection of the invention adopts a compound preparation which combines the traditional Chinese medicine and the western medicine, the bupleurum and the chlorpheniramine can control the clinical symptoms caused by canine influenza and reduce the suffering caused by the canine influenza, the lincomycin hydrochloride can control the bacterial infection, the three are combined together for the usage, thus having rapid onset of action for the canine influenza and controlling the canine secondary infection, significantly ease the clinical symptoms caused by the canine influenza, treat both manifestation and root cause of disease and accelerate the canine recovery.
Owner:TIANJIN SHENGJI GRP CO LTD
Composition with skin-whitening, acne-removing and anti-allergic effects
InactiveCN103479803AOrganic active ingredientsCosmetic preparationsSide effectLincomycin Hydrochloride
The invention discloses a composition with skin-whitening, acne-removing and anti-allergic effects. The composition comprises Artemisia stelleriana, white poria, Chinese violets, Callicarpa macrophylla Vahl, Chenopodium ambrosioides, metronidazole, ketoconazole and clobetasol propionate cream and lincomycin hydrochloride and lidocaine hydrochloride gel. A preparation method of the composition comprises the steps as follows: the Artemisia stelleriana, the white poria, the Chinese violets, the Callicarpa macrophylla Vahl, the Chenopodium ambrosioides and the metronidazole tablets are ground into fine powder and mixed into the ketoconazole and clobetasol propionate cream and the lincomycin hydrochloride and lidocaine hydrochloride gel, the mixture is stirred uniformly and prepared into beautifying cream. The composition has the skin-whitening, acne-removing and anti-allergic effects and is remarkable in effect and free of side effects.
Owner:陈庆军
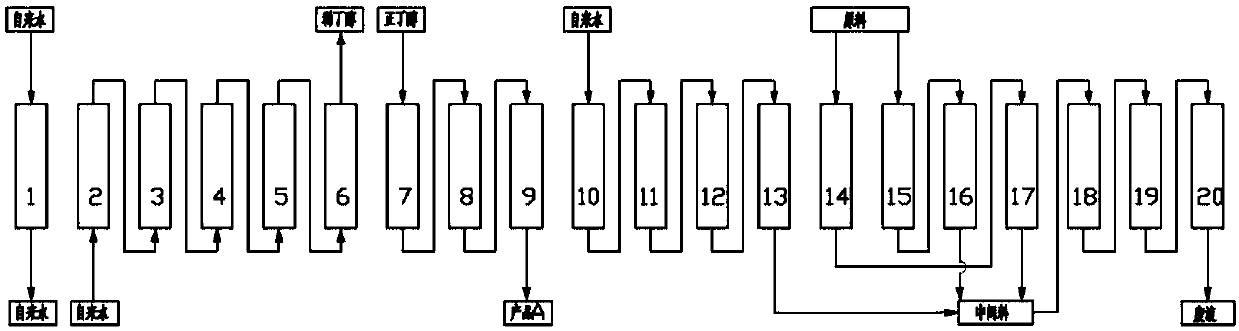
Continuous chromatography separation and purification method for lincomycin
ActiveCN107619421AComposition is stableStable concentrationSugar derivativesSugar derivatives preparationChromatographic separationState of art
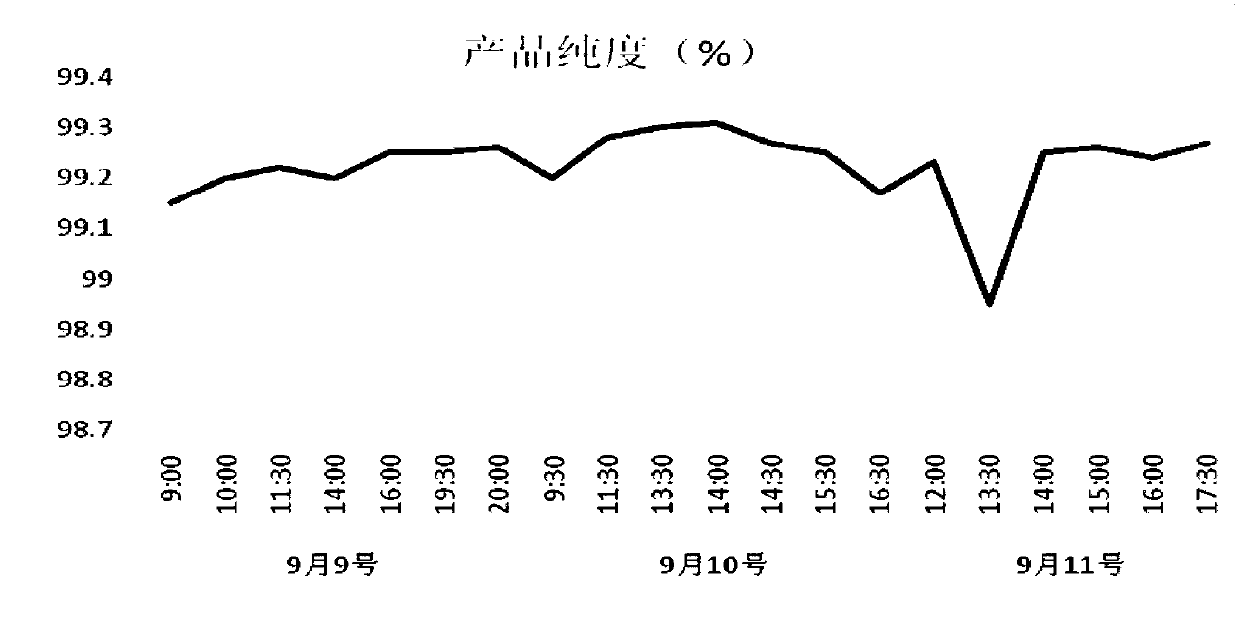
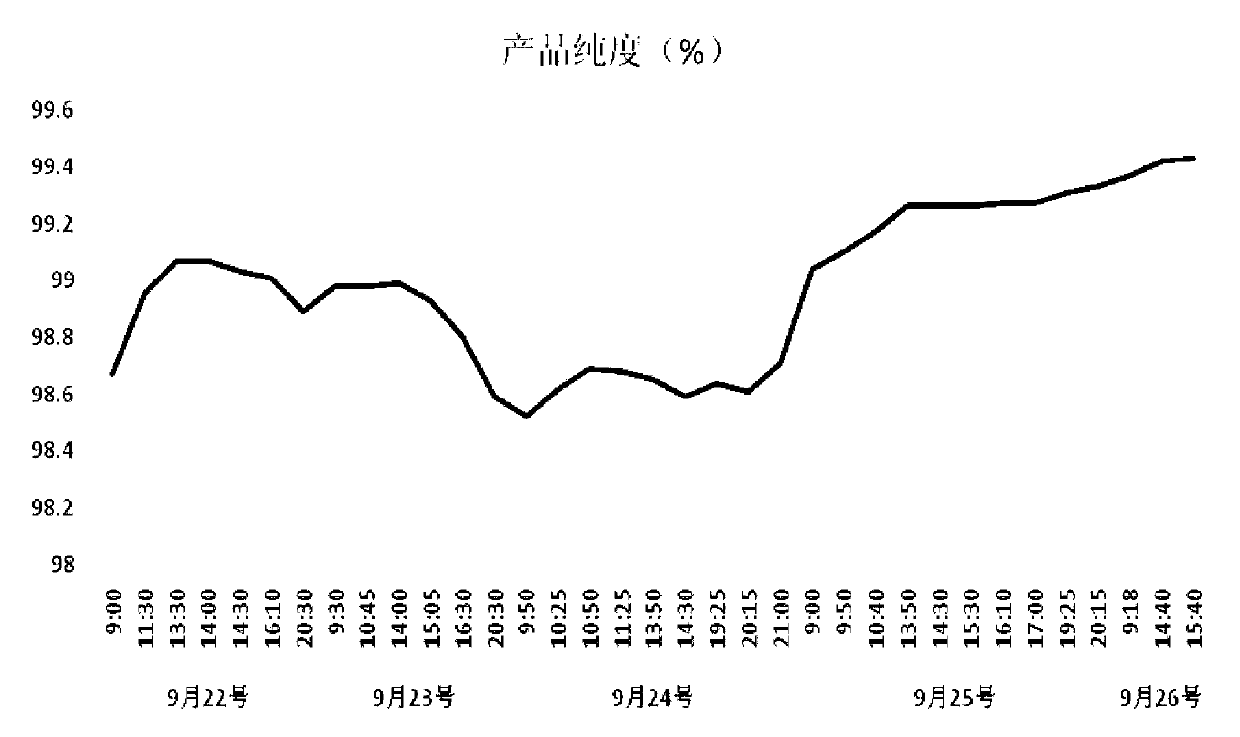
The invention relates to a continuous chromatography separation and purification method for lincomycin. Efficient separation of component A of lincomycin is realized by using a continuous chromatography technology. The method comprises the following steps: enabling a lincomycin fermented filter liquor obtained through biological fermentation to enter into a continuous chromatography system, carrying out adsorption, impurity washing, lincomycin elution, eluant collection and chromatographic column regeneration, concentrating a collected eluant and then carrying out salt decoloration to obtain lincomycin hydrochloride. The lincomycin obtained by adopting the method is high in yield, high in purity, low in cost and environmentally-friendly, and is applicable for industrial production. The method has the advantages of short production process time and high product yield, the content of lincomycin hydrochloride (dry basis) in the prepared final product reaches more than 99%, and the disadvantages that the lincomycin hydrochloride produced in the prior art is not high in content and long in production process time are overcome.
Owner:JIANGXI GUOYAO PHARMA LLC +1
Whitening acne-removing cream
InactiveCN1762376ANo side effectsHigh antibacterial activityOrganic active ingredientsAntimycoticsMedicineLincomycin Hydrochloride
The invention provides a whitening and moisturizing acne-removing cream comprising the following raw materials (by weight percentage): Ketoconazole and Clobetasol propionate cream 20-40%, metronidazole 5-20%, Lincomycin hydrochloride and Lidocaine hydrochloride gel 35-55%, and Malingua hydrochloride 5-20%.
Owner:白长义
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com