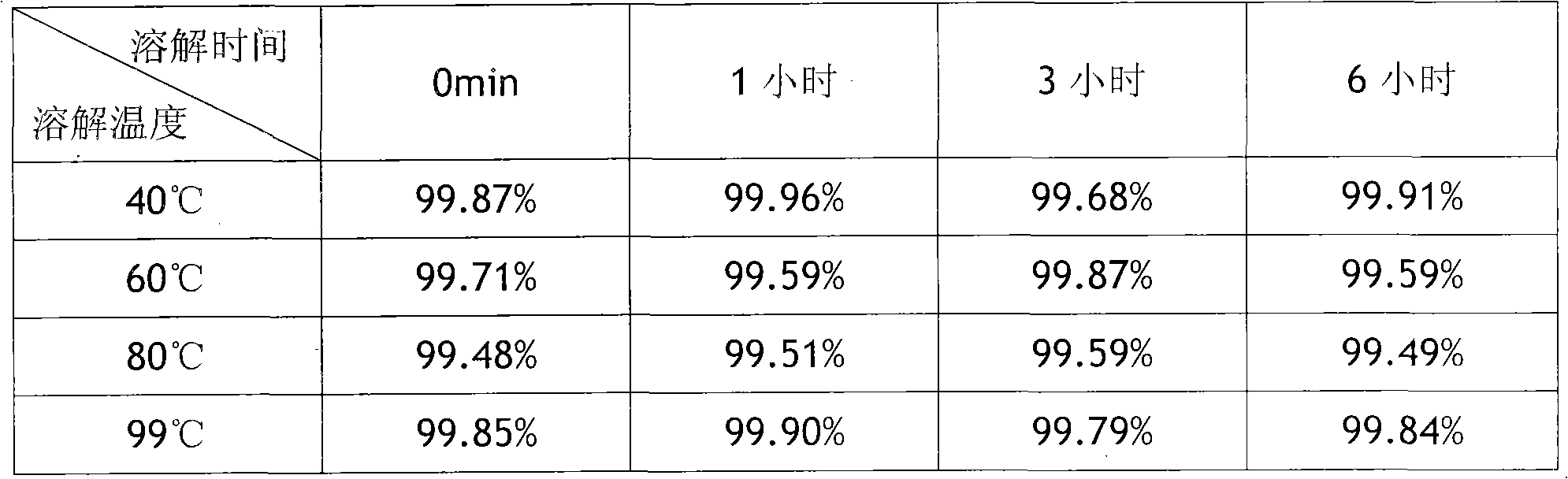
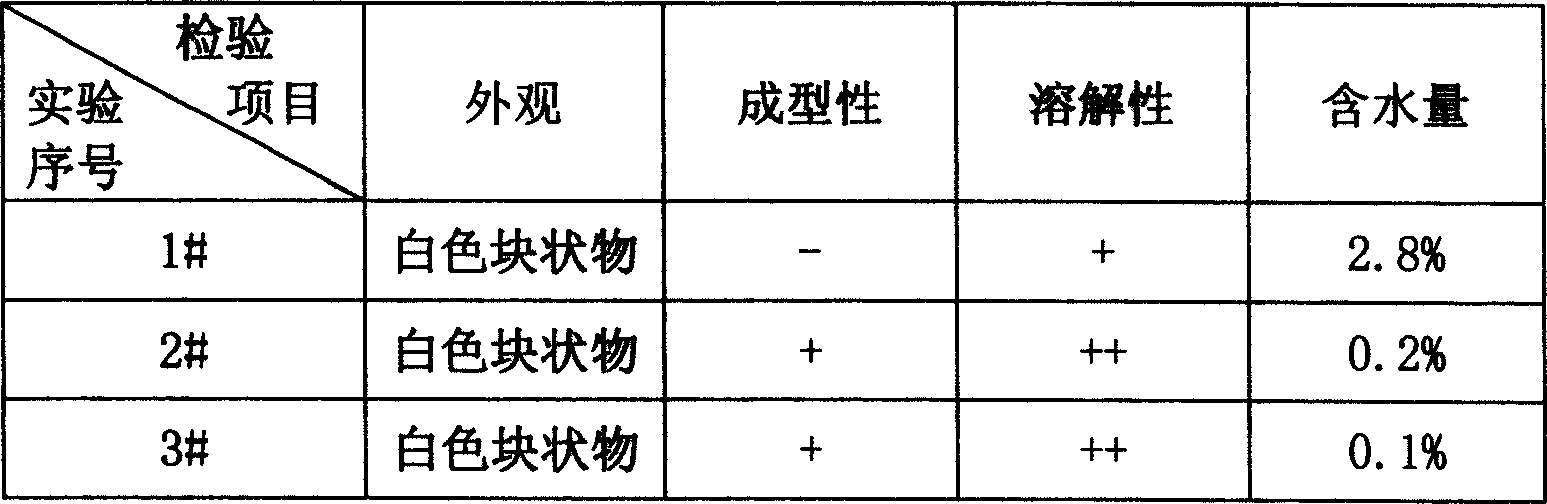
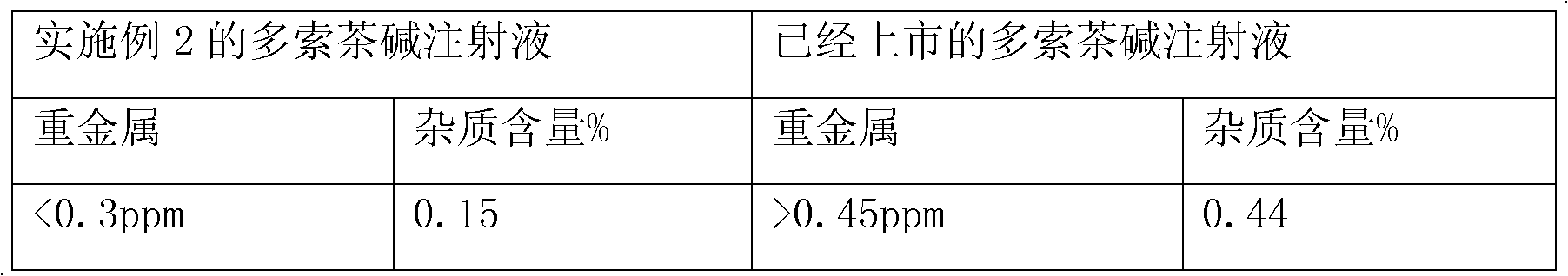
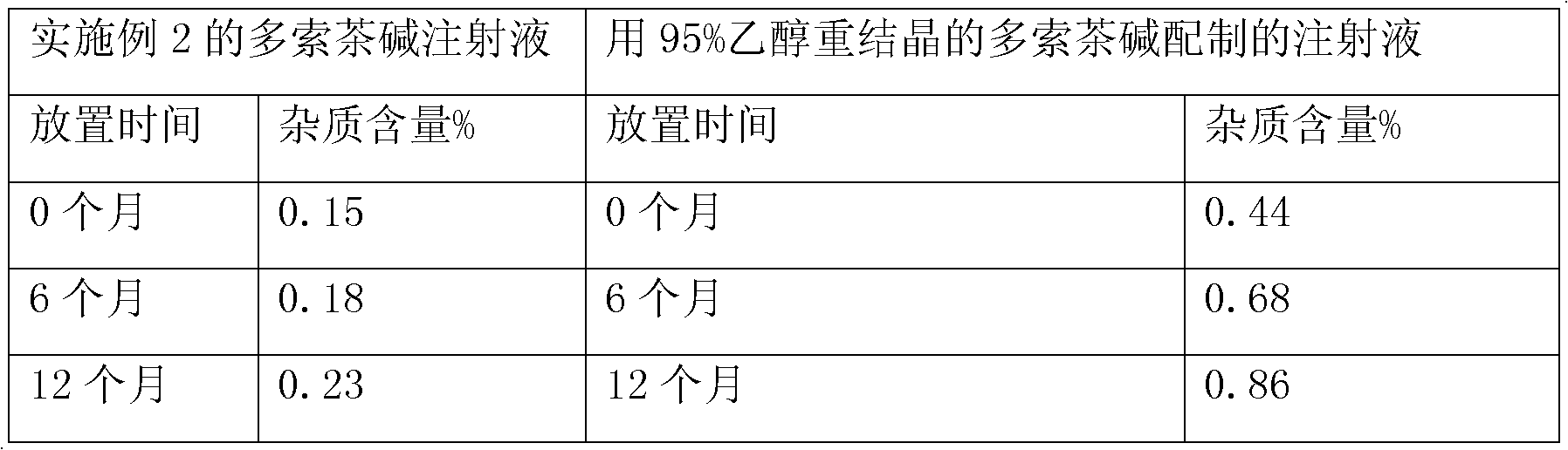
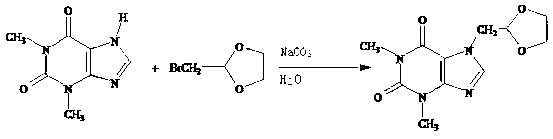
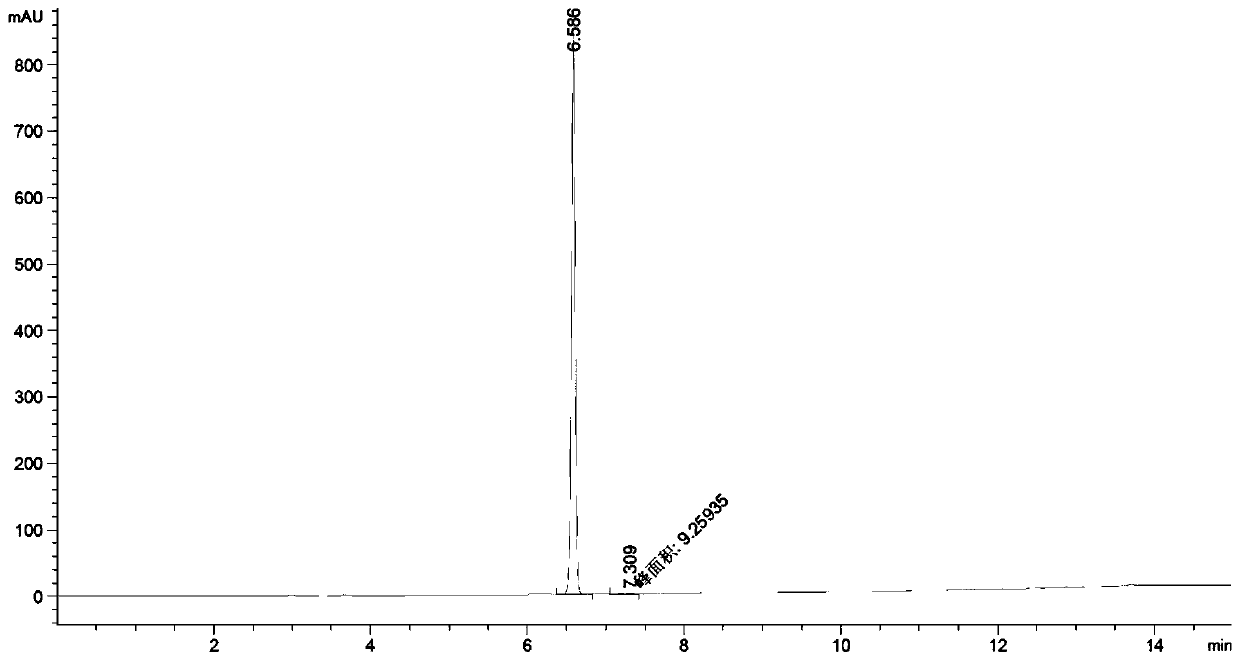
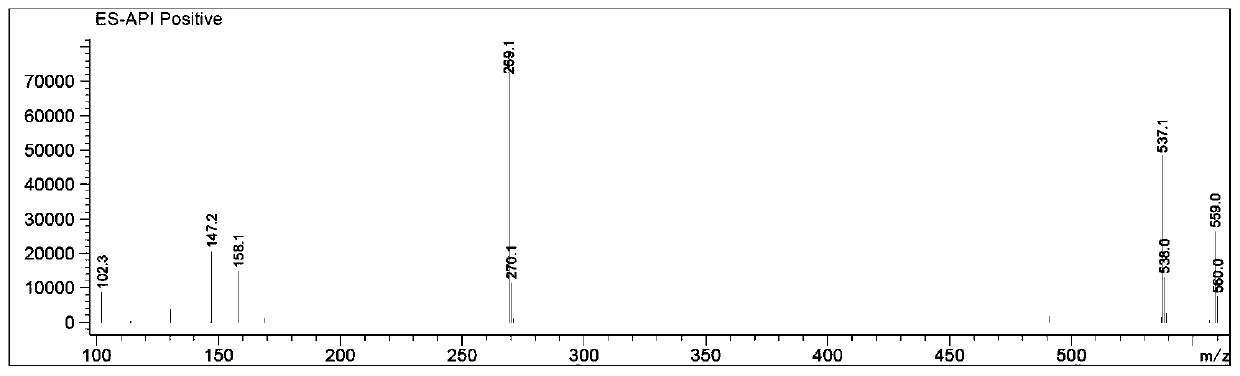
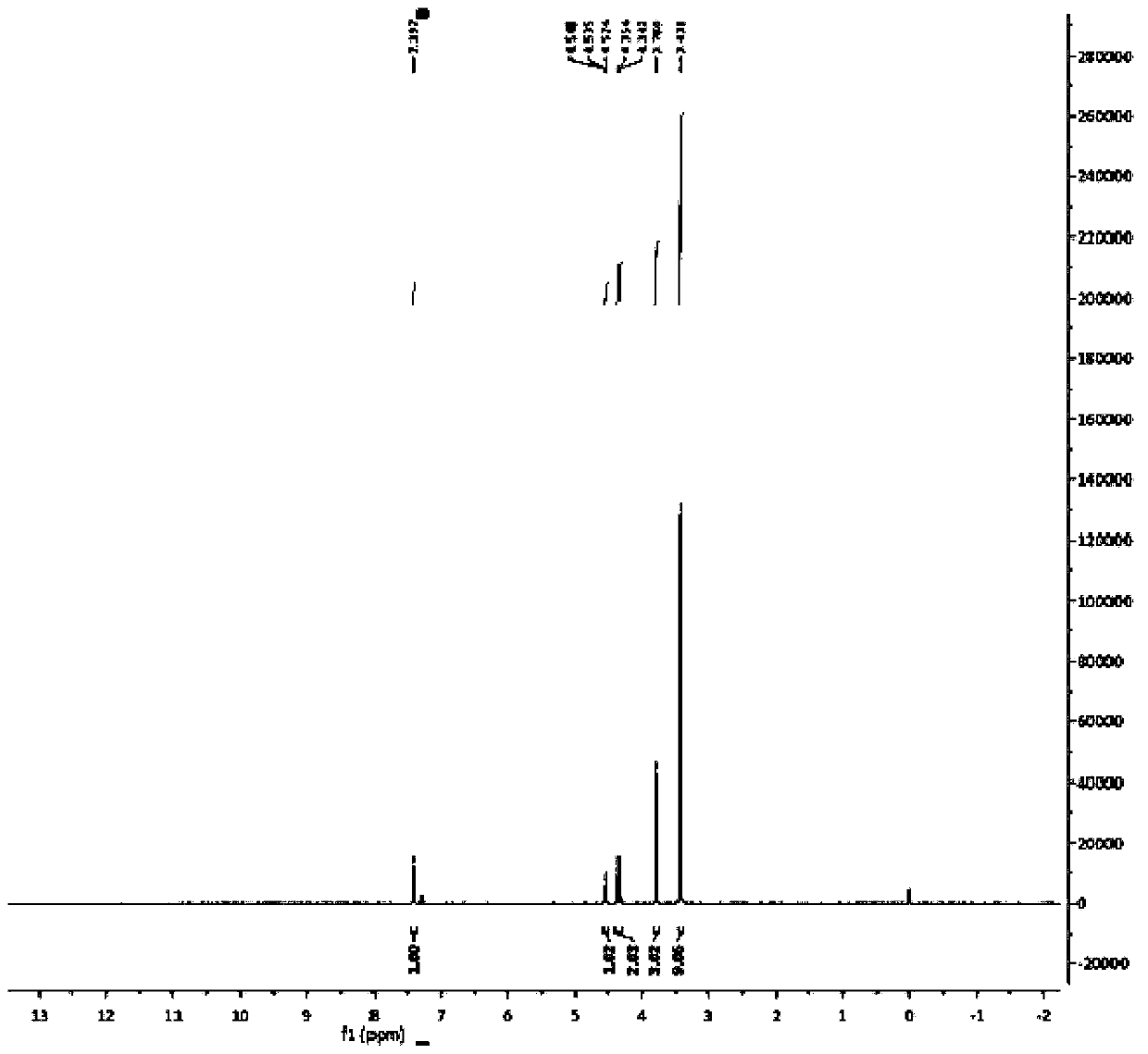
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Doxofylline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
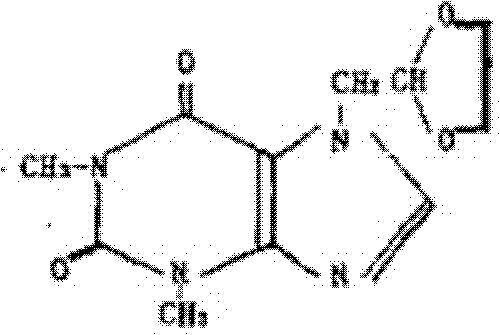
Doxofylline (also known as doxophylline) is a xanthine derivative drug used in the treatment of asthma.
Preparation method of small-volume doxofylline freeze-dried powder injection as well as product and device thereof
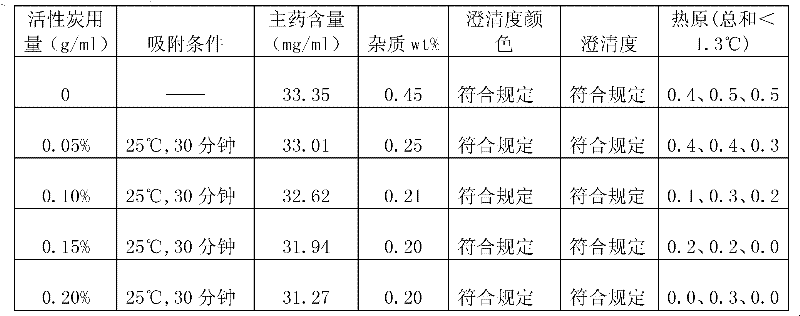
InactiveCN101953803AReduce volumeLow costPowder deliveryPharmaceutical product form changeActivated carbonDoxofylline
The invention discloses a preparation method of a small volume doxofylline freeze-dried powder injection. The preparation method is characterized by comprising the following steps: weighing doxofylline and auxiliary materials of the prescription amount, and adding the injection water of 80 to 90 DEG C to prepare a concentrated solution, and fully stirring so as to dissolve the materials; adding active carbon, stirring uniformly the mixture, heating to boil, and filtering; adding the dilute solution which is prepared by the residual injection water of 80 to 90 DEG C into the filtrate, keeping the temperature no lower than 80 DEG C, fully stirring, carrying out micro-filtering, and continuing to keep the temperature not lower than 80 DEG C; and filling into a 20ml ampoule, freeze-drying, pressing and sealing to obtain the product. The method of the invention has the beneficial effect of effectively reducing the packaging size so as to lower the packaging cost and the transport cost, and greatly reducing the energy consumption of the freeze-drying and evaporating so as to reduce the cost further due to that the preparation amount filled into the ampoule is only 7 to 8 ml before the freeze-drying.
Owner:天津市铭泰医药科技有限公司
Doxufylline for injection and its preparing method
The present invention relates to a doxofylline for injection and its preparation method. It contains doxofylline and excipient, their mixing ratio is 1:1-1:10, the described excipient is mannitol, lactose, glucose and sucrose. Its preparation method adopts freeze-drying method, and said invention also provides the concrete steps of said method.
Owner:天津市铭泰医药科技有限公司
Doxofylline venous injection with small volume as well as preparation method and quality control method thereof
ActiveCN101647776AReduce health impactReduce the impactInorganic non-active ingredientsSurface/boundary effectDoxofyllineVein injection
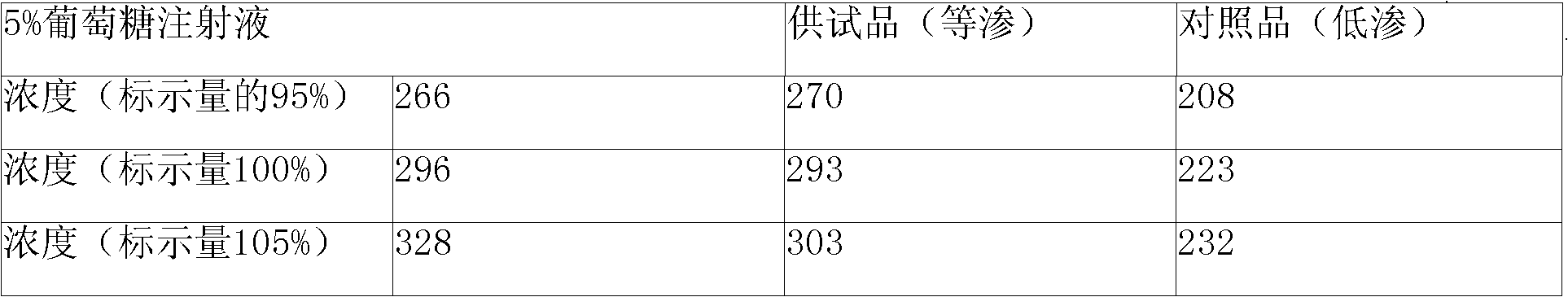
The invention discloses Doxofylline venous injection with a small volume and a preparation method thereof. The preparation unit of the isotonic small volume of doxofylline venous injection is 1-20ml,the weight percentage concentration of doxofylline is 1-4 percent, and the isotonic value of the venous injection is 257-340mosmol / kg. The invention enlarges the optional range of clinical applicationof doxofylline and provides the isotonic doxofylline venous injection with favorable security.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Freeze dried powder injecta of doxofylline and its preparation
The invention relates to a freeze dried powder injection of doxofylline and its preparation, wherein the injection comprises Doxofylline 0.05-0.5g, and at least one pharmaceutically acceptable excipient, pH modifier, anti-oxidant agent, and stabilizer, and is provided in the form of unit amount in the container. The advantages of the invention include easy medicament transport and preservation, prevention of freezing of the liquid injection in winter, increased medicament stability, thus can be applied for effectively treating bronchial asthma, lungs disease of bronchospasm, and chronic clogged pulmonary diseases.
Owner:武汉安士医药科技有限公司
Doxofylline-contained liquid injection, preparation method and quality control method thereof
ActiveCN101569603AIncrease concentrationNot easy to precipitateComponent separationPharmaceutical delivery mechanismHigh concentrationDoxofylline
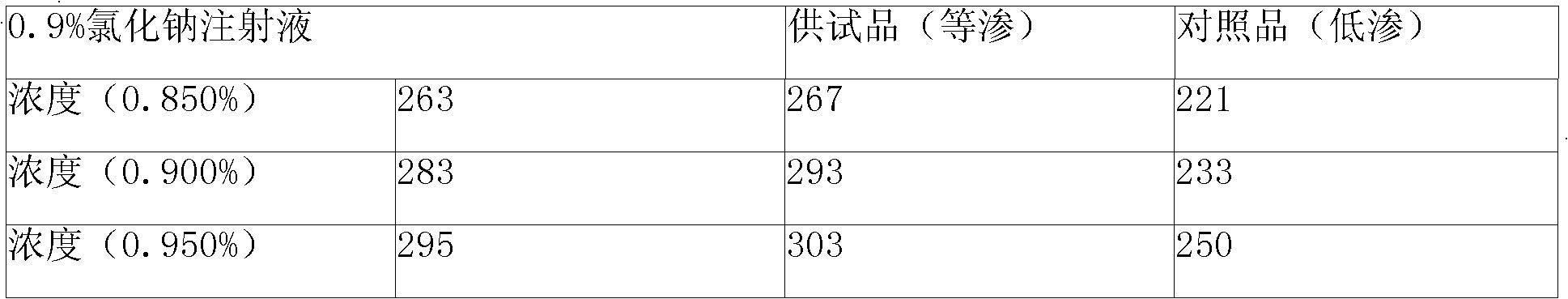
The invention relates to a doxofylline-containing liquid injection used for treating bronchiectasis, and provides the doxofylline with higher concentration. The injection comprises doxofylline, a water solvent, and a pH value regulating agent. The injection comprises the following components by weight percentage: 1 to 4 percent of the doxofylline, and 0.01 to 5 percent of the pH value regulating agent. The injection enriches specifications of the prior doxofylline injection, and brings convenience to the clinical application. The invention also provides a preparation method and a quality control method for the doxofylline liquid injection. Relevant materials in the doxofylline injection can be determined by an HPLC method to improve the product controllability.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Doxofylline compound and medicine composition thereof
ActiveCN103159769AThe prescription process is simpleImprove stabilityOrganic chemistryAntipyreticDoxofyllineFreeze-drying
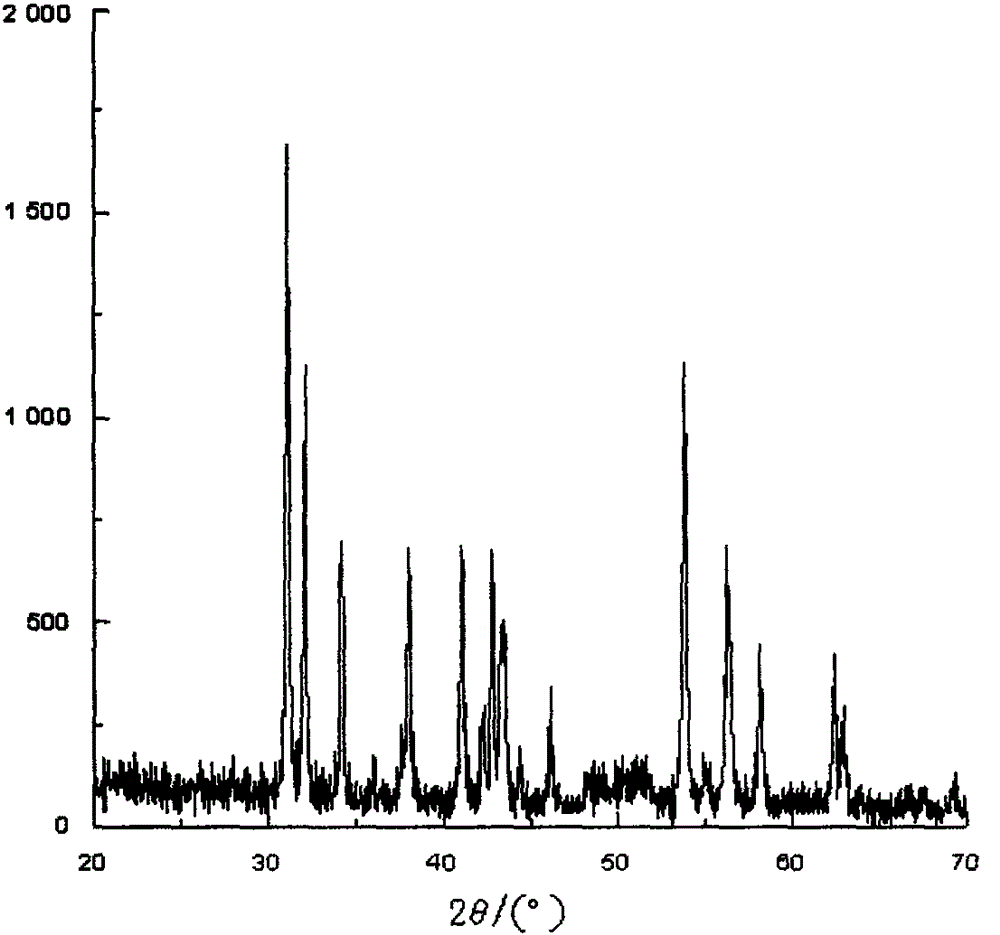
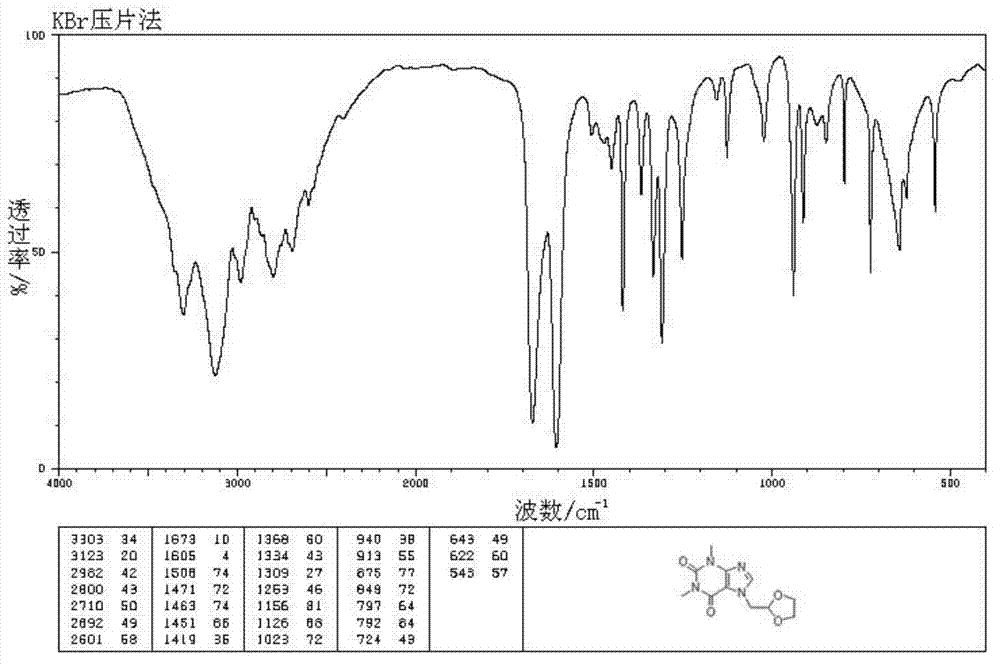
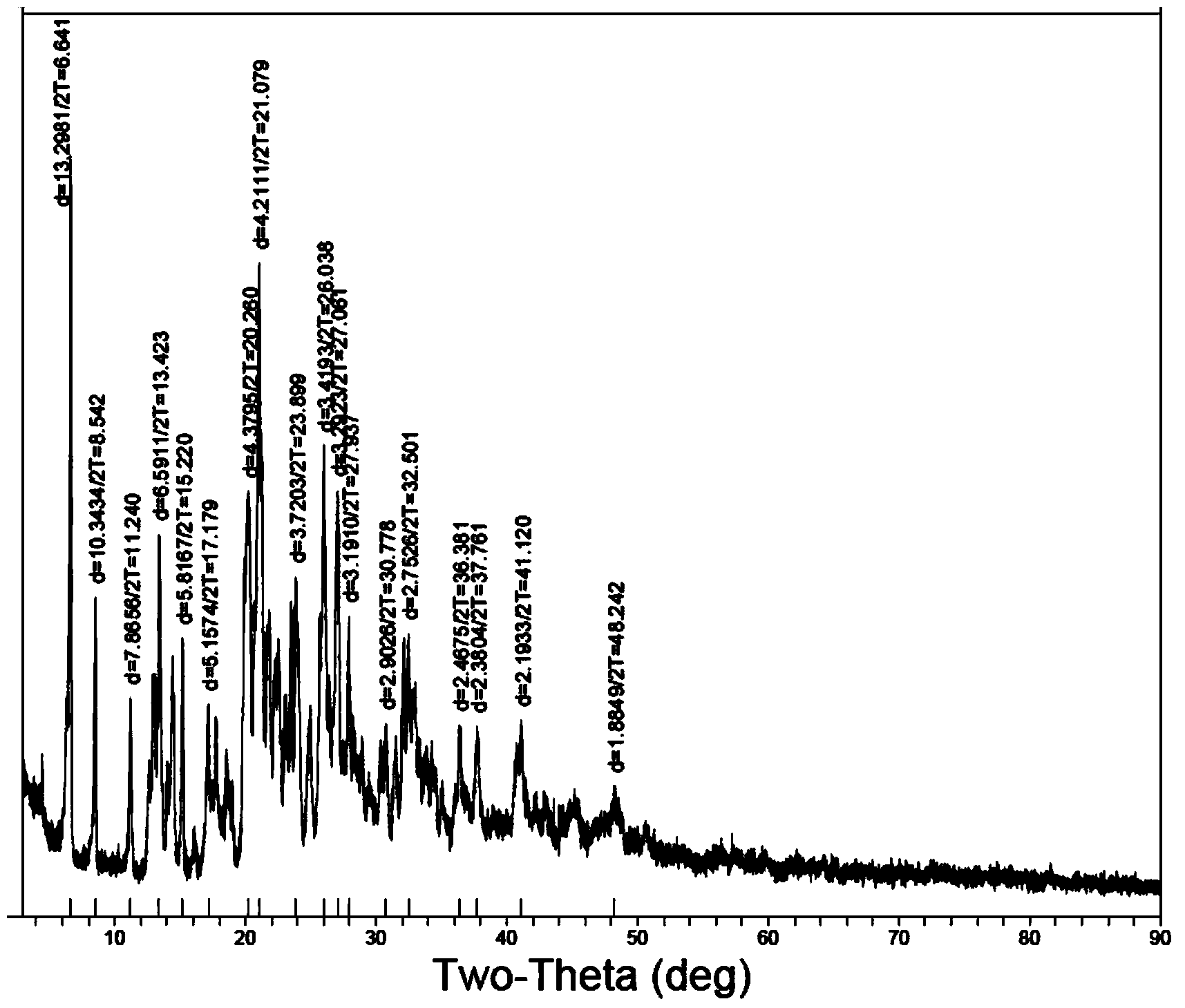
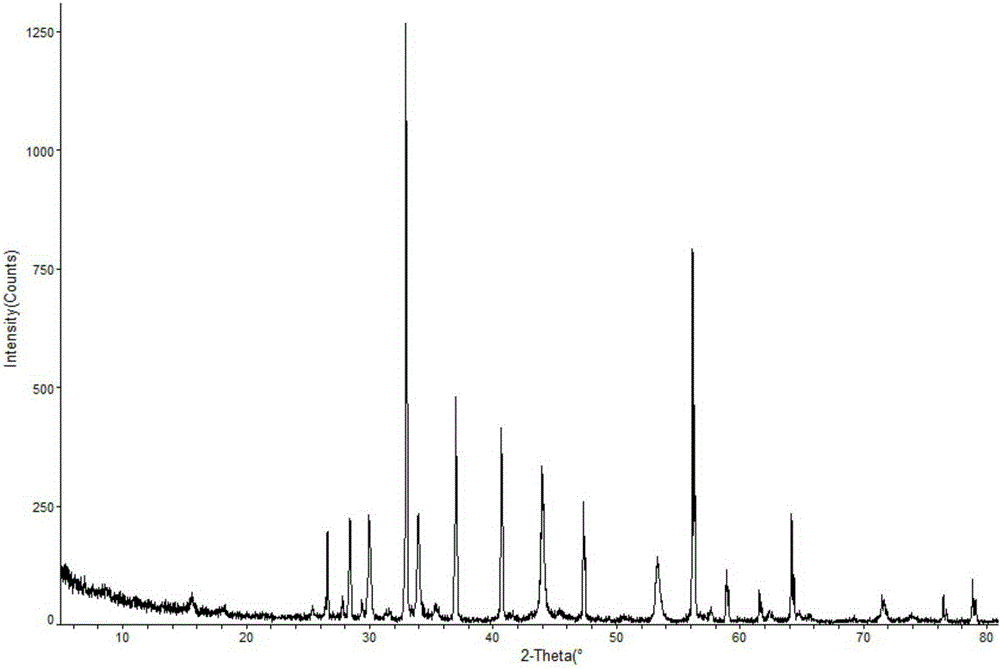
The invention relates to a doxofylline crystal compound. Determined by adopting X-ray powder diffraction, the doxofylline crystal compound has he characteristic peak at 2theta+ / -0.2 degrees of 11.8 degrees, 13.3 degrees, 16.8 degrees, 19.2 degrees, 20.8 degrees, 23.3 degrees, 26.3 degrees, 26.7 degrees, 28.2 degrees, 29.9 degrees, 31.3 degrees, 32.7 degrees, 36.8 degrees and 41.1 degrees. The invention also relates to a preparation and a medicine composition preparation containing the doxofylline compound, wherein the medicine composition preparation is freeze-dried powder, injection, tablets and capsules. The doxofylline freeze-dried powder, injection, tablets and capsules which are provided by the invention are simple in formulation and technology, have obviously improved stability and improve the safety and effectiveness of medicine using.
Owner:湖北美林药业有限公司
More stable doxofylline compound and pharmaceutical composite thereof
InactiveCN102367254ASlow degradationHigh purityPowder deliveryOrganic chemistryDoxofyllineActivated carbon
The invention relates to a more stable doxofylline compound and a pharmaceutical composite thereof. The preparation method of the doxofylline compound comprises the following steps: recrystallizing crude doxofylline with a mixed solvent of ethyl acetate and methanol for 1-3 times and decoloring with activated carbon to obtain off-white crystals, wherein the ratio of ethyl acetate to methanol is 1:2.
Owner:河北三禾实创生物科技有限公司
Sustaining agent of Duosuo theosine and its preparation
A slow-release doxofylline is composed of a fast-release component prepared from doxofylline (10-60%) and fast-release assistant and a slow-release component prepared from doxofylline (40-90%) and slow-release assistant. Its preparing process is also disclosed.
Owner:HEFEI HEYUAN PHARM TECH CO LTD
Method for preparing doxofylline
The invention discloses a method for preparing doxofylline. The preparation method comprises that anhydrous theophylline and bromoacetaldehyde ethylene acetal serve as basic materials, purified water serves as a solvent, and anhydrous sodium carbonate serves as a deacid reagent; the materials of the anhydrous theophylline, the anhydrous sodium carbonate and the solvent of the purified water are added in a reaction vessel to be stirred and heated, so that the anhydrous theophylline is dissolved completely; adding dropwise the bromoacetaldehyde ethylene acetal to perform reflux reaction, cooling crystallization and suction filtration are performed to obtain coarse product doxofylline; and the solvent of the purified water is added to dissolve the coarse product completely, activated carbon is added for decoloration, and cooling crystallization, suction filtration and drying are performed after decoloration to obtain the finished product doxofylline. According to the method for preparing doxofylline, the purified water is used as the solvent instead of organic solvents, so that the problem of residual organic solvent in a prior doxofylline preparation process is solved, and the doxofylline is conducive to being taken by patients.
Owner:KAIFENG MINGREN PHARMA
Compound sodium sulfadimidine injection liquid for pig and preparation method thereof
InactiveCN101606945ADelay drug resistanceIncreased sensitivityAntibacterial agentsOrganic active ingredientsDoxofyllineTrimethoprim
The invention discloses a compound sodium sulfadimidine injection liquid for a pig and a preparation method thereof, aiming at providing a compound kanamycin sulfate injection liquid which has fast effect for treating toxoplasmosis of the pig, addresses both the symptoms and root causes, reduces the time and dosage of used drug and has convenient drug usage, and a preparation method with simple technique and easy implementation. Each 100L injection liquid comprises: 5 to 20 sodium sulfadimidine, 1 to 10kg of lincomycin hydrochloride, 5 to 20kg of doxofylline, 1 to 4kg of trimethoprim, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 40kg of propylene glycol, 10kg of 95% alcohol and the balance of water for injection. The injection liquid has fast effect for toxoplasmosis of the pig, addresses both the symptoms and root causes, can effectively treat and prevent secondary infection of bacterium, reduces drug resistance of pathogenic microorganism and leads the pig to accelerate restoring. Simultaneously, the compound sodium sulfadimidine injection liquid is an injection liquid and has convenient use.
Owner:TIANJIN SHENGJI GRP CO LTD
Compound enrofloxacin injection for animals and preparation thereof
InactiveCN101301291AIncrease weightDelay drug resistanceOrganic active ingredientsAntiinfectivesDoxofyllineAntimicrobial drug
The invention discloses a compound enrofloxacin injection for animals and a preparation method thereof, which aims to provide the enrofloxacin injection for the animals and the preparation method thereof, wherein, the enrofloxacin injection for the animals can treat swine enzootic pneumonia, is quick to take effect, decreases drug resistance, and can treat symptoms and root causes; and the preparation method has a simple process and is easy to realize. The injection comprises the following compositions by weight portion: 0.5 to 5kg of enrofloxacin, 1 to 10kg of lincomycin hydrochloride, 0.01 to 0.05kg of sodium hydroxide, 0.1 to 2kg of doxofylline, 0.2kg of sodium sulfite, 0.01kg of EDTA-2Na, 30kg of propanediol, and the balance being water for injection. The injection adopts a compound preparation of an antimicrobial drug and antibiotics; due to the combined use of the enrofloxacin and the lincomycin hydrochloride as well as the doxofylline, the injection is quick to take effect to the swine enzootic pneumonia, is effective and highly efficient, reduces the probability of the drug resistance, can apparently relieve clinical symptoms caused by the swine enzootic pneumonia, can treat symptoms and root causes, and increase the weight of pigs.
Owner:TIANJIN SHENGJI GRP CO LTD
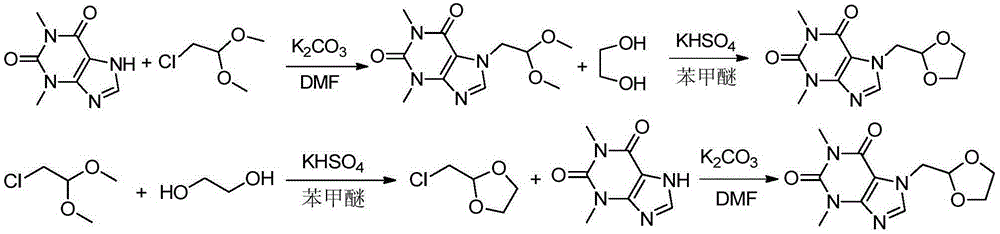
Preparation methods of doxofylline
The invention discloses preparation methods of doxofylline. The preparation methods include the steps: under the action of an acid-binding agent, carrying out a condensation reaction of theophylline and halogenated acetaldehyde dimethyl acetal in a polar solvent, to obtain an intermediate 7-(2,2-dimethoxy ethyl)theophylline; and then, with soluble hydrosulfate as a catalyst, carrying out a condensation cyclization reaction of the intermediate 7-(2,2-dimethoxy ethyl)theophylline with ethylene glycol in a solvent, to obtain doxofylline; or, firstly, carrying out a condensation cyclization reaction of halogenated acetaldehyde dimethyl acetal and ethylene glycol to obtain halogenated acetaldehyde ethylene acetal, and carrying out a condensation reaction with theophylline, to obtain doxofylline. The soluble hydrosulfate is used as the catalyst and replaces conventional p-toluenesulfonic acid, moreover, and anisole and other solvents are used for replacing methylbenzene used in a conventional reaction route, so that under a condition of ensuring high yield of the product, the toxicity of the solution in the synthesis reaction is reduced, and the difficult problem that residual toxicity easily exists in the finished product is solved.
Owner:浙江北生药业汉生制药有限公司
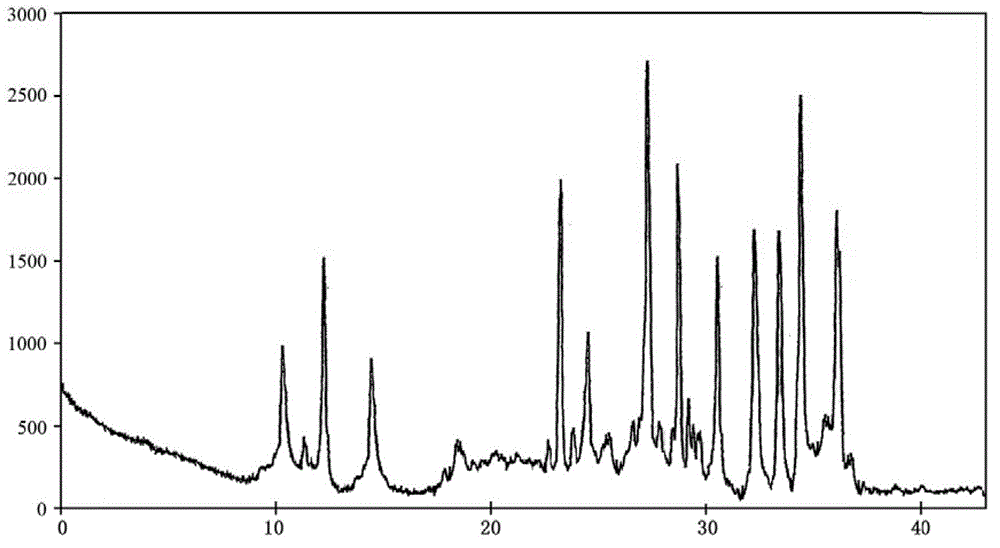
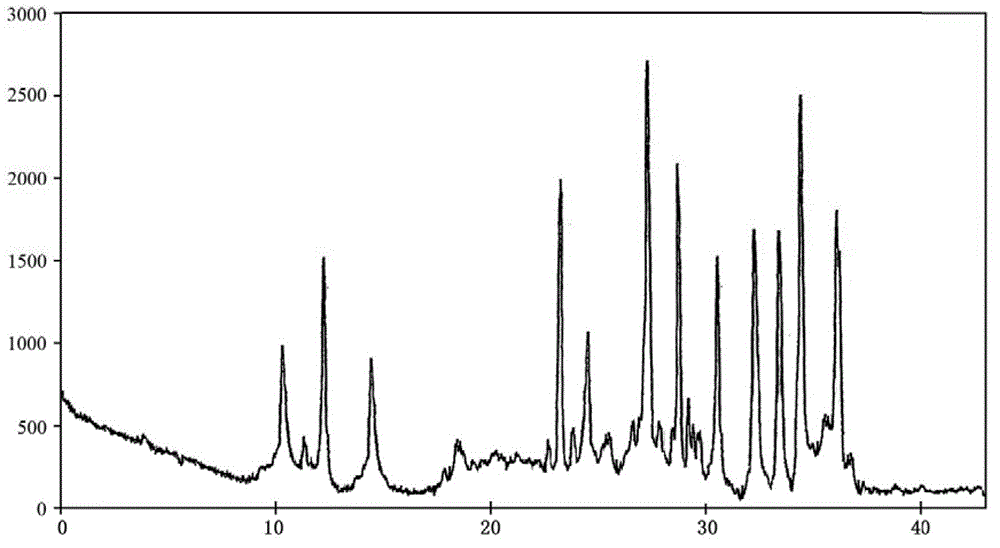
Doxofylline crystalline compound and lyophilized powder thereof
InactiveCN103145713AImprove solubilityNot easy to precipitatePowder deliveryOrganic chemistryDoxofyllineX-ray
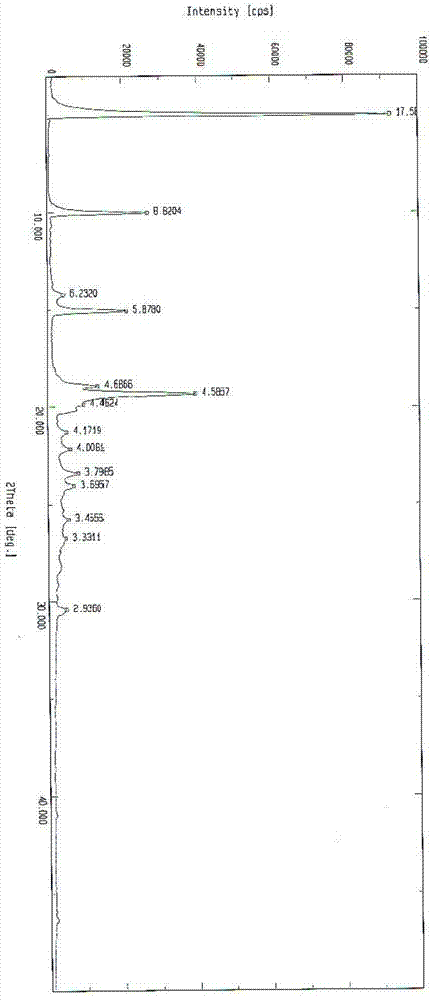
The invention relates to a doxofylline crystalline compound and lyophilized powder thereof. The doxofylline crystalline compound is measured by a powder X-ray diffraction measurement method; and an X-ray powder diffraction pattern represented by a diffraction angle of 2(theta)+ / -0.2 degrees shows characteristic diffraction peaks at the positions of 10.21 degrees, 12.12 degrees, 14.39 degrees, 23.32 degrees, 24.43 degrees, 27.23 degrees, 28.60 degrees, 30.54 degrees, 32.28 degrees, 33.32 degrees, 34.38 degrees and 36.12 degrees. The re-dissolubility of the doxofylline crystalline compound provided by the invention is obviously improved, solid is not easily separated out after long-time placement, and the medication safety of a patient is greatly improved.
Owner:SHANXI PUDE PHARMA CO LTD
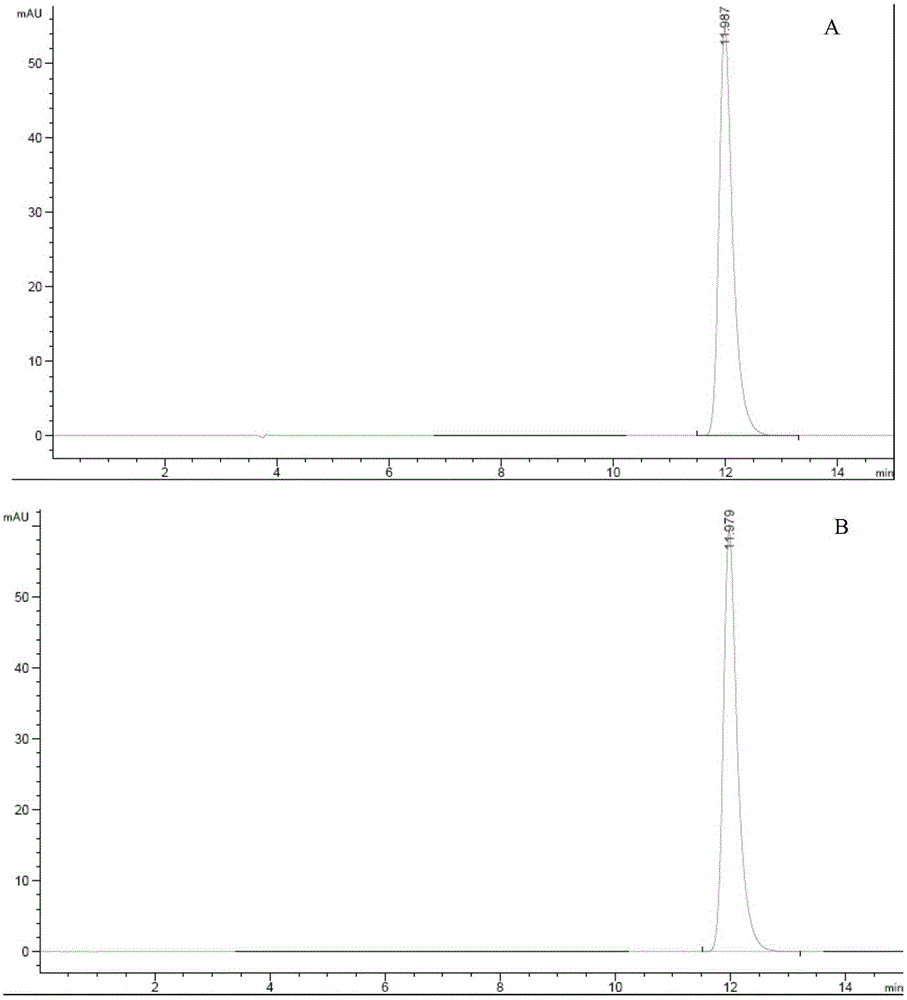
Doxofylline injection-related substance determination method
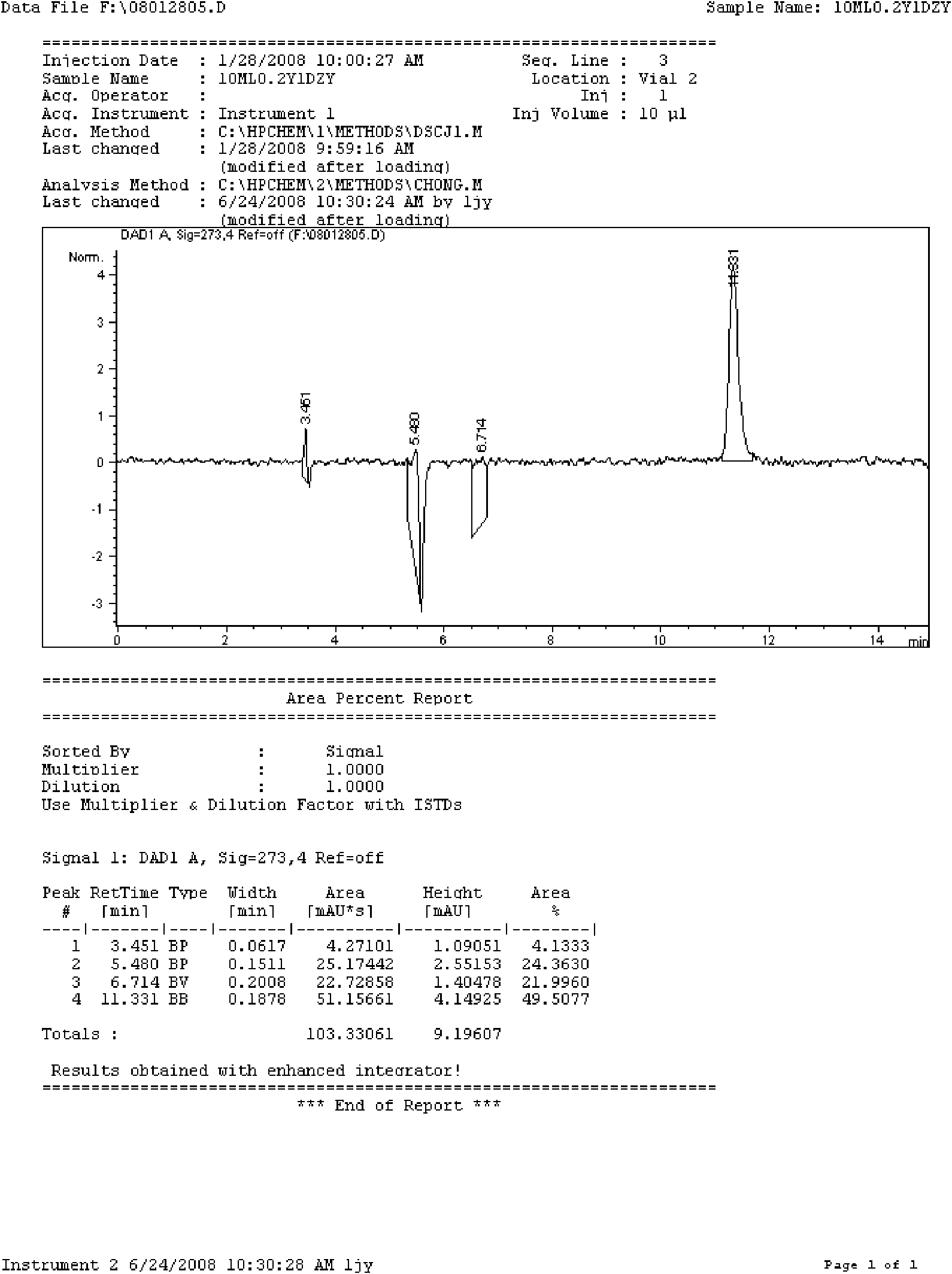
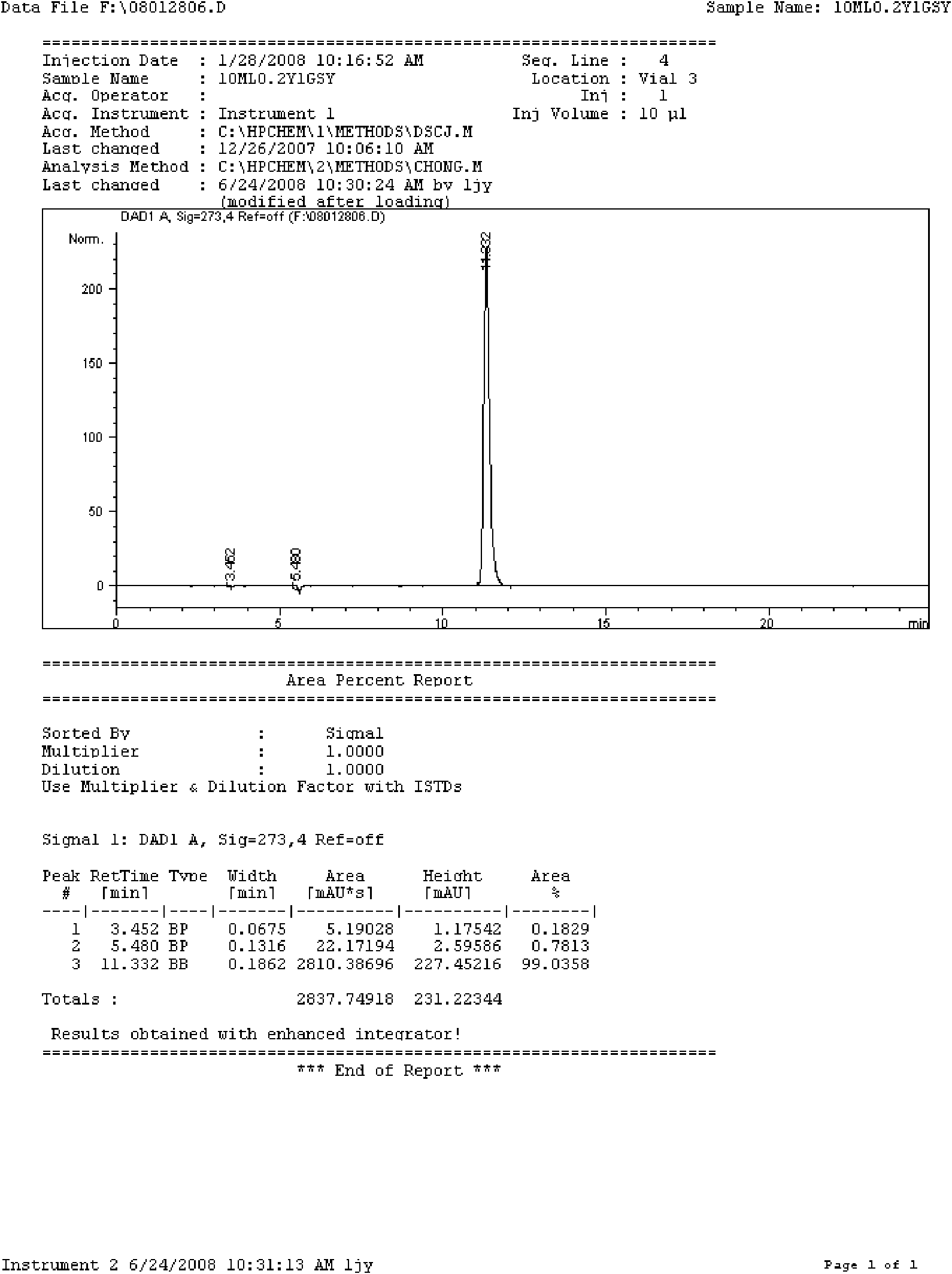
The invention relates to a doxofylline injection-related substance determination method. According to the method, a high-efficiency liquid chromatography is used for determination. The invention provides a method capable of effectively controlling the doxofylline injection-related substances by using the high-efficiency liquid chromatography, which has the beneficial effects being good in durability, simple and rapid in operation, high in system suitability. According to the method, at least three reference substances containing theophylline, theophylline ethanol and theophylline acetaldehyde are used for the determination of the related substances. At the same time, the invention provides impurity reference substances such as impurity A, impurity B, impurity C, impurity D, impurity E, impurity G, impurity caffeine, methylxanthine, impurity C', impurity D', impurity E', impurity 2, theophylline acetic acid and the like for the determination of the doxofylline injection-related substances, so that the related substances caused by different synthetic routes, and preparation processes and storage processes of preparations. The invention also provides a synthesis method for separating and synthesizing the reference substances containing impurity A, impurity A'', impurity B, impurity C, impurity D, impurity E, impurity G and the like for the determination of the doxofylline injection-related substances.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Doxofylline freeze-dried powder injection
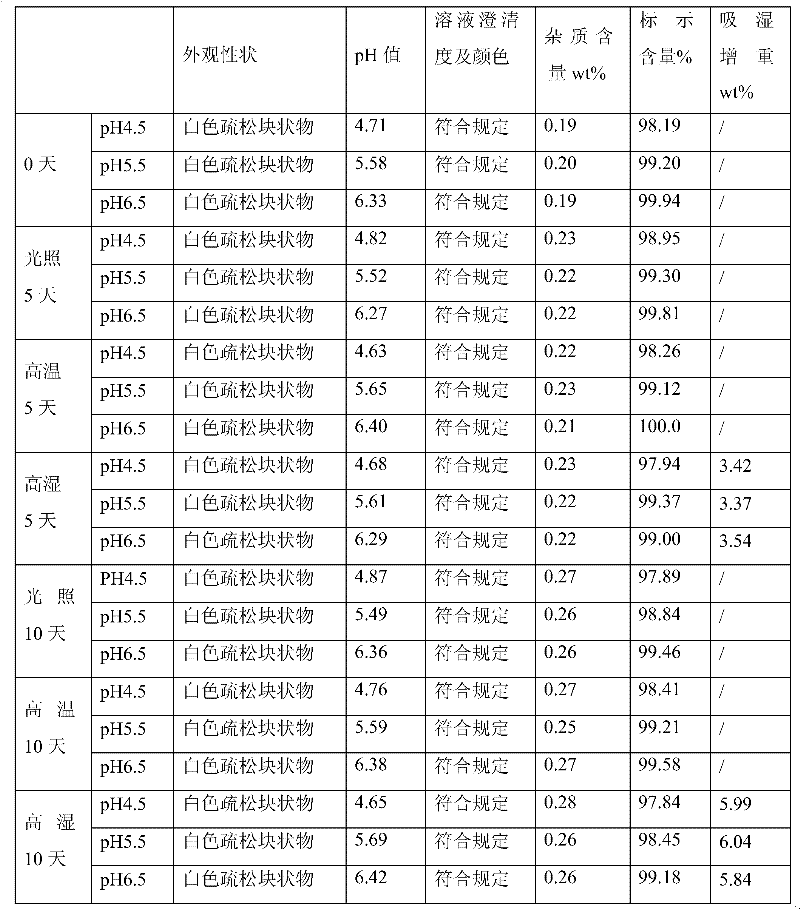
InactiveCN102512385AReduce energy consumption for freeze-drying and evaporationReduce manufacturing costPowder deliveryPharmaceutical non-active ingredientsDoxofyllinePenicillin
The invention relates to a freeze-dried powder injection, in particular to a doxofylline freeze-dried powder injection. The injection is characterized by being prepared according to the following preparation method: adding 200 g of doxofylline and 150 g of mannitol into a container with scales; adding injection water to 4,800 ml; stirring while adding; adding 4.8 g of active carbon according to a standard that 1 mg of active carbon is added per 1 ml of solution; stirring and adsorbing for 30 minutes; decarburizing; adjusting pH value to be 4.5-6.5 by using 0.01 mol / L hydrochloric acid solution; adding injection water to 6,000 ml; removing bacteria and filtering; then filling in the amount of 5.8-6.3 ml per bottle; and finally, placing the filled injection into a freeze-dryer to freeze-dry, pressing a plug after the freeze-drying is finished, and capping to obtain the freeze-dried powder injection. The freeze-dried powder injection has the advantages that: the process is adjusted scientifically and rationally, so that the allocation quantity filled in a penicillin bottle before freeze-drying is about 6 ml; and therefore, the freeze-dry evaporation energy consumption is reduced, and the production cost is reduced.
Owner:REYOUNG PHARMA
Doxofyline osmotic pump controlled releasing preparation and its production
InactiveCN1552325AStable blood concentrationLittle side effectsPharmaceutical delivery mechanismRespiratory disorderDoxofyllineCellulose acetate
An osmotic pump type release controlled doxofylline tablet is composed of the core consisting of the medicine layer prepared from doxofylline, osmosis-promoting polymer, osmosis promoter, diluent and lubricant and the push-pull layer prepared from osmosis promoter, coloring agent and lubricant, and the coated layer prepared from cellulose acetate, polyethanediol and acetone or chloroform. Its preparing process is also disclosed. Its advantages are long release controlling time (12-24 hr), and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Synthetic method of doxofylline
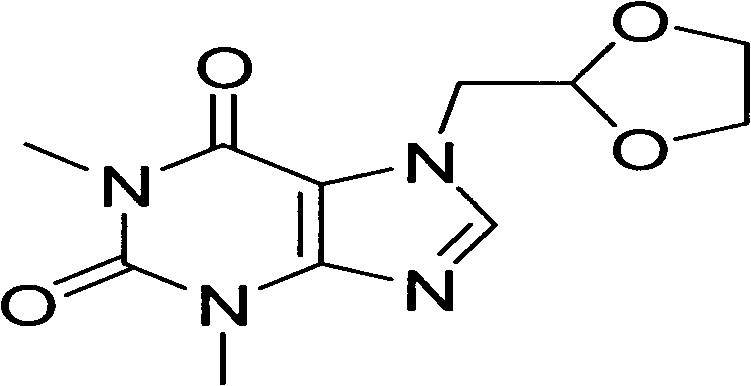
InactiveCN108840872AEase of industrial productionShort reaction timeOrganic chemistryDoxofyllineOrganic synthesis
The invention discloses a synthetic method of doxofylline and belongs to the technical field of organic synthesis. The method comprises the following step: theophylline and 2-chloromethyl-1,3-dioxolane are subjected to a reaction at 60-110 DEG C in an aprotic solvent under the action of alkali and a phase transfer catalyst, and doxofylline is synthesized, wherein the mole ratio of theophylline, 2-chloromethyl-1,3-dioxolane and the alkali is 1:1-2:1-3, the phase transfer catalyst accounts for 3%-5% of the molar weight of theophylline, and the phase transfer catalyst is selected from one or moreof tetrabutylammonium chloride, tetrabutylammonium bromide and tetrabutylammonium fluoride. The provided new synthetic method of doxofylline is short in reaction time and only needs 3-6 h, is low inreaction temperature which is lower by 20-30 DG C than that of the conventional method, is high in yield which is 10% or higher than that of the conventional method, and is beneficial to industrial production of the drug.
Owner:HUBEI HUNTIDE BIOTECH
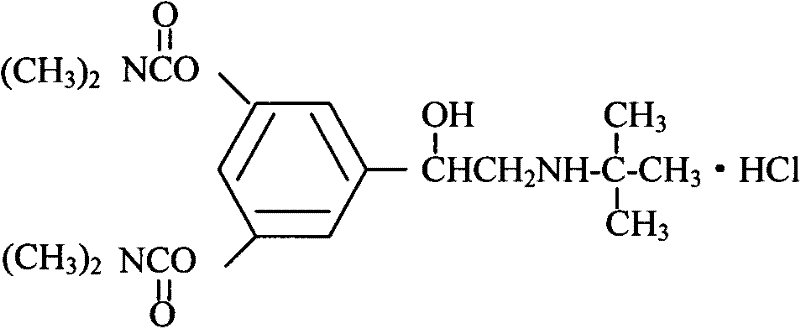
Bambutero hydrochloride and doxofylline-contained compound preparation and preparation method thereof
InactiveCN102440992AGive full play to the mechanism of joint actionCan produce synergistic effects in the treatment of asthmaRespiratory disorderEster active ingredientsLiquid glucoseDoxofylline
The invention discloses a bambutero hydrochloride and doxofylline-contained compound preparation, which comprises tablet and syrup, wherein main medicines are bambutero hydrochloride and doxofylline, auxiliary materials comprise corn starch, lactose, gelatin, talcum powder, magnesium stearate, liquid glucose, cane sugar, vitamin C, sodium metabisulfite, edetate disodium, orange juice flavouring agent, edible toner and purified water. The compound preparation has good co-therapy function to bronchial asthma, chronic bronchitis, emphysema and other lung diseases which are relevant to concurrent bronchospasm.
Owner:岳阳新华达制药有限公司
Preparation method of doxofylline injection
ActiveCN109833293AExcellent low temperature dispersion stabilityReduce contentPharmaceutical delivery mechanismPharmaceutical non-active ingredientsDoxofyllineFiltration
The invention discloses a preparation method of doxofylline injection. The method comprises the following steps of enabling hydroxypropyl-beta-cyclodextrin to dissolve in water for injection, and regulating the pH value with acid; adding amino acids, and performing stirring until a dissolving state is obtained so as to obtain an hydroxypropyl-beta-cyclodextrin aqueous solution containing amino acids; adding doxofylline, performing stirring, and regulating the pH value with alkali; and performing filtration with a 0.45[mu]m millipore filter, performing subpacking in vials, and performing hot pressing for sterilization. The concentration of the doxofylline injection is greater than that of injection for sale, and the doxofylline injection has excellent low temperature decentralized stabilityand chemical stability.
Owner:浙江长典药物技术开发有限公司
Doxofylline compound and medicine composition thereof
InactiveCN105037361AHigh clinical safetyGood water solubilityOrganic chemistry methodsPill deliveryDoxofyllineX-ray
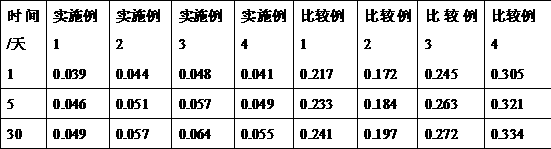
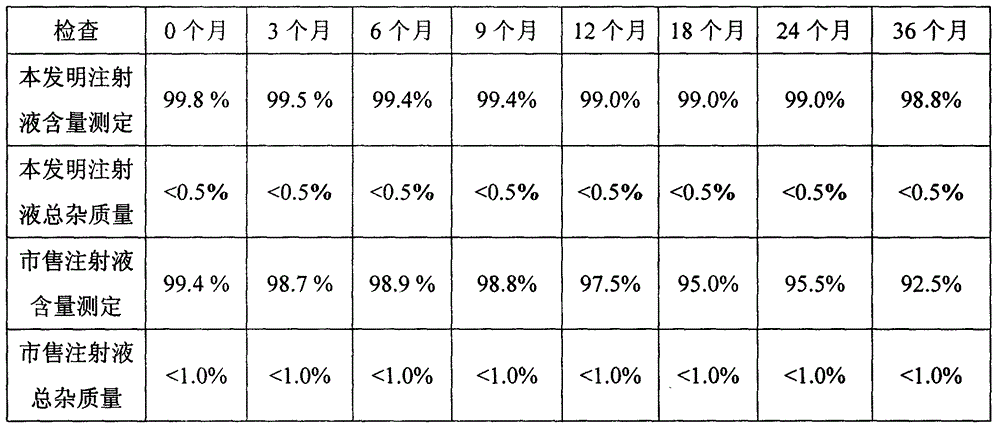
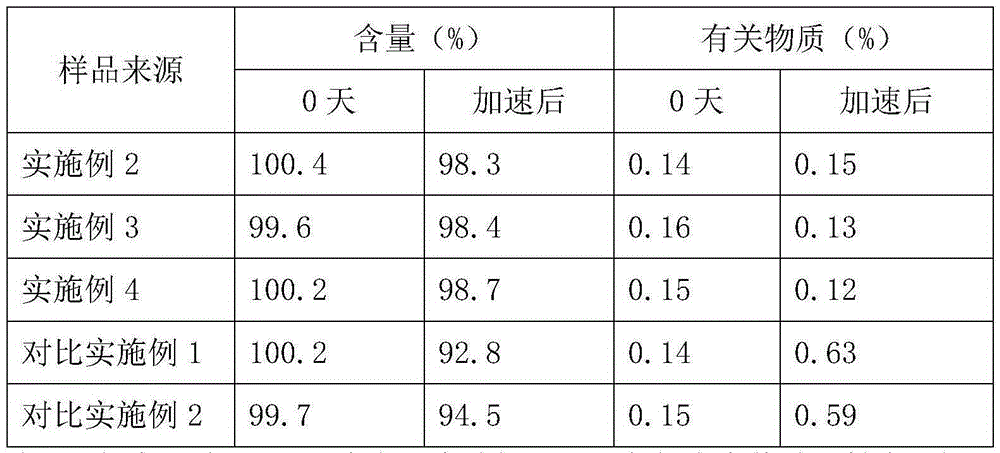
The invention belongs to the technical field of medicines and in particular provides a doxofylline compound and a medicine composition thereof. Characteristic peaks of X-ray powder diffraction, measured by using a Gu-Kalpha ray, of the doxofylline compound are shown at 31.1 degrees, 32 degrees, 34.3 degrees, 37.5 degrees, 41.3 degrees, 43 degrees, 53.8 degrees, 56.5 degrees, 58 degrees and 62.5 degrees. The melting point of the doxofylline compound is 136-137 DEG C. The doxofylline compound has crystal purity higher than 99.9%. Doxofylline has good stability. Tablets and injections prepared by using the doxofylline medicine composition containing doxofylline have good stability and have total impurity rates lower than 0.5% after being placed for 36 months.
Owner:杭州科源医药技术有限公司
High-solubility doxofylline compound
The invention belongs to the technical field of medicines, and particularly relates to a doxofylline compound and a preparation method thereof. The space group of the doxofylline compound is Cc; and cell parameters are as follows: alpha is 90.00 degrees, gamma is 90.00 degrees, and z is 4. The doxofylline compound provided by the invention has high purity and good stability; even if in a high-humidity condition, the weight gain caused by moisture absorption is not obvious, and related substances do not grow; and compared with doxofylline in other crystalline states, the doxofylline compound is higher in solubility.
Owner:天津梅花生物医药科技有限公司
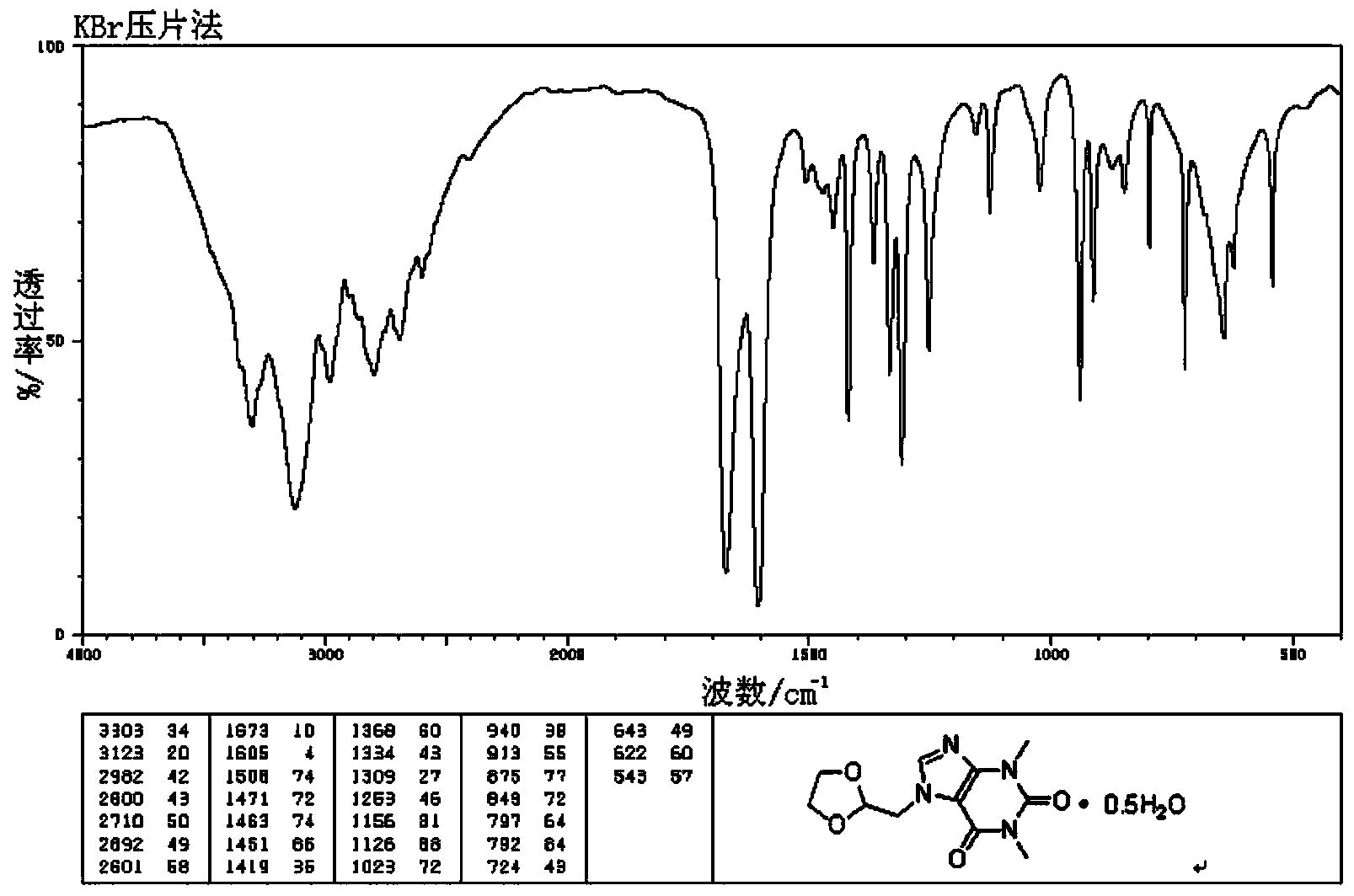
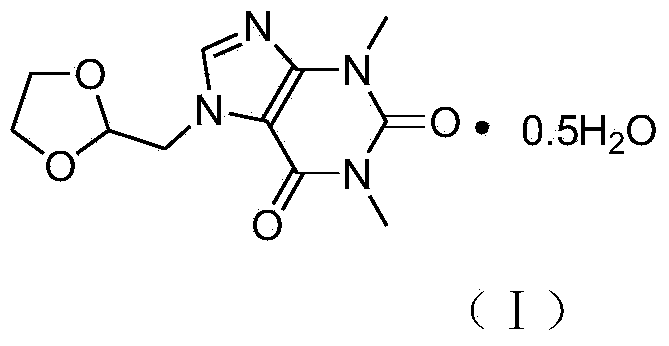
Doxofylline hemihydrate
The invention specifically relates to a doxofylline hemihydrate, which belongs to the field of medical technology. The doxofylline hemihydrate provided by the invention has good stability and has no obvious moisture absorption and weight gain under a high humidity condition, and related substances do not increase; compared with doxofylline in other crystalline states, the doxofylline hemihydrate has high solubility. A preparation method for the doxofylline hemihydrate comprises the following steps: weighing doxofylline, putting weighed doxofylline in a reaction vessel, adding a proper volume of an ethanol aqueous solution, successively carrying out stirring, heating reflux, natural cooling to room temperature and cooling to 5 DEG C, continuing stirring for crystallization for 1 h, carrying out filtering and then carrying out drying at a temperature of 50 DEG C in a nitrogen atmosphere so as to obtain the doxofylline hemihydrate. Chemical purity of the doxofylline hemihydrate reaches 99.9%, and total impurities are less than 0.08%.
Owner:天津梅花生物医药科技有限公司
Compound kanamycin sulfate injection liquid for pig and preparation method thereof
InactiveCN101606946ADelay drug resistanceIncreased sensitivityAntibacterial agentsAntipyreticDoxofyllineTreatment effect
The invention discloses a compound kanamycin sulfate injection liquid for a pig and a preparation method thereof, aiming at providing a compound kanamycin sulfate injection liquid which has fast effect for treating pant disease of the pig, addresses both the symptoms and root causes, reduces the time and dosage of taking drug and has convenient drug usage, and a preparation method with simple technique and easy implementation. Each 100L injection liquid comprises: 5 to 20 billion units of kanamycin monosulfate, 1 to 10kg of lincomycin hydrochloride, 0.1 to 2kg of doxofylline, 0.05kg of dexamethasone sodium phosphate, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na, 10kg of dimethyl acetamide and the balance of water for injection. The injection liquid reasonably mixes usage of all the components by a plurality of types of drugs, has fast effect for treating pant disease of the pig, addresses both the symptoms and root causes, reduces drug resistance of pathogenic microorganism and leads the pig to accelerate restoring. Simultaneously, the compound kanamycin sulfate injection liquid has convenient use, and can achieve the treatment effect of a plurality of types of drugs.
Owner:TIANJIN SHENGJI GRP CO LTD
Compound Ofloxacin solution and preparation method thereof
InactiveCN101879179AEffective cough and asthma reliefAvoid drug resistanceAntibacterial agentsDigestive systemBacteroidesDisease
The invention discloses a compound Ofloxacin solution and a preparation method thereof, aiming at providing a solution with the advantages of treating digestive tract infections and respiratory tract infections of birds with rapid effect, treating both principal and secondary aspects of diseases and reducing drug-applied times and a preparation method thereof. The solution can be injected or orally taken and proper treating methods and administration approaches can be adopted according to disease states and the specific conditions of culturists. Every 100L of solutions comprises 1-10 kg of Ofloxacin, 3-15 kg of lincomycin hydrochloride, 0.05-0.5 kg of atropine sulfates, 0.2-2 kg of doxofylline, 0.01 kg of EDTA (Ethylene Diamine Tetraacetic Acid)-2Na, 10 kg of dimethylacetylamide and 0.5 L of hydrochloric acid. Because the Ofloxacin and the lincomycin hydrochloride are adopted as antibacterial components, the compound Ofloxacin solution can effectively solve the problems of bacterial drug resistance and takes effect on common bacteria causing the digestive tract infections and the respiratory tract infections; and meanwhile, the doxofylline can effectively relieve cough and asthma and inhibit respiratory symptoms, and the atropine sulfates have the rapid effects of inhibiting glandular secretion and reducing the liquid contents of digestive tracts and the respiratory tracts.
Owner:天津市天合力药物研发有限公司
Doxofylline composition injection and preparation method thereof
The invention relates to a doxofylline composition injection and a preparation method thereof, in particular to a small-dose doxofylline composition injection and a preparation process thereof. According to the preparation method, citric acid and an acidic buffer solution of sodium citrate are compatible with doxofylline, the mass ratio scheme of the optimal composition of three substances under different contents (0.1g / 10ml, 0.2g / 10ml and 0.3g / 20ml) of the doxofylline is optimized, screened and established, and the preparation process for optimizing the scheme is formulated correspondingly. Therefore, the technical problem of low physical and chemical stability of the small-dose doxofylline composition injection in the processes of transportation and storage is solved, and the quality of medicines is improved.
Owner:UNKNOWN
Aerosol inhalation preparation for treating bronchial asthma
InactiveCN106344544AImprove stabilityImprove atomization effectDispersion deliverySolution deliveryDoxofyllineSolvent
The invention relates to an aerosol inhalation preparation for treating bronchial asthma. The aerosol inhalation preparation contains doxofylline taken as an active substance, a solvent, a stabilizer and / or a pharmacologically acceptable carrier. According to the doxofylline aerosol inhalation preparation made from the components at specific ratio provided by the invention, the character, PH, insoluble particle, content, related substance and osmotic pressure are not obviously changed after 0-month acceleration, so that the doxofylline aerosol inhalation preparation by the invention is higher in stability. Meanwhile, the doxofylline aerosol inhalation solution has excellent atomization effect and high absorptivity and the effective dose below 5mu m reaches up to 50% or above.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Anti-bronchial asthma doxofylline injection
InactiveCN105287373AImprove stabilityReduce typesOrganic chemistryPharmaceutical delivery mechanismDoxofyllineCombinatorial chemistry
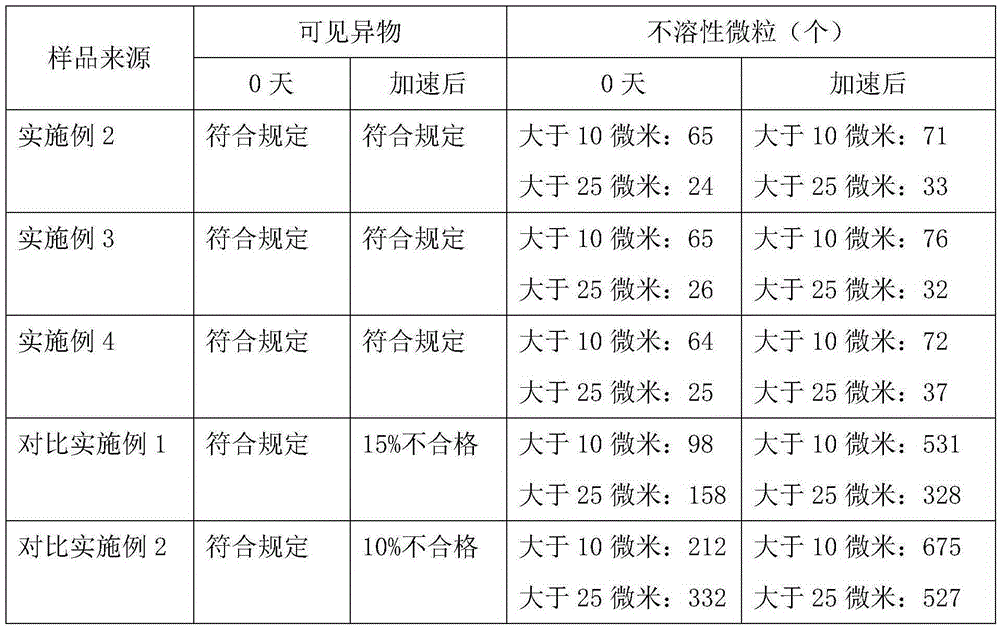
The invention discloses anti-bronchial asthma doxofylline injection, and belongs to the technical field of medicine. Doxofylline is a crystal, and the novel crystal form of the doxofylline provided by the invention is different from a crystal form structure of the prior art; experiments find that a compound with the novel crystal form is high in purity, good in fluidity, good in stability, low in purity content, not easy for humidity absorption and safe and reliable in clinic, and powder-injection prepared by utilizing the compound with novel crystal form is good in stability, stable after compatibility with a solvent, extremely low in insoluble particle content, and very suitable for clinical application.
Owner:李正梅
3D printing doxofylline orally disintegrating tablets and preparation method thereof
ActiveCN107669648AFast releaseSmall toxicityPharmaceutical non-active ingredientsPill deliveryDoxofyllineOlder people
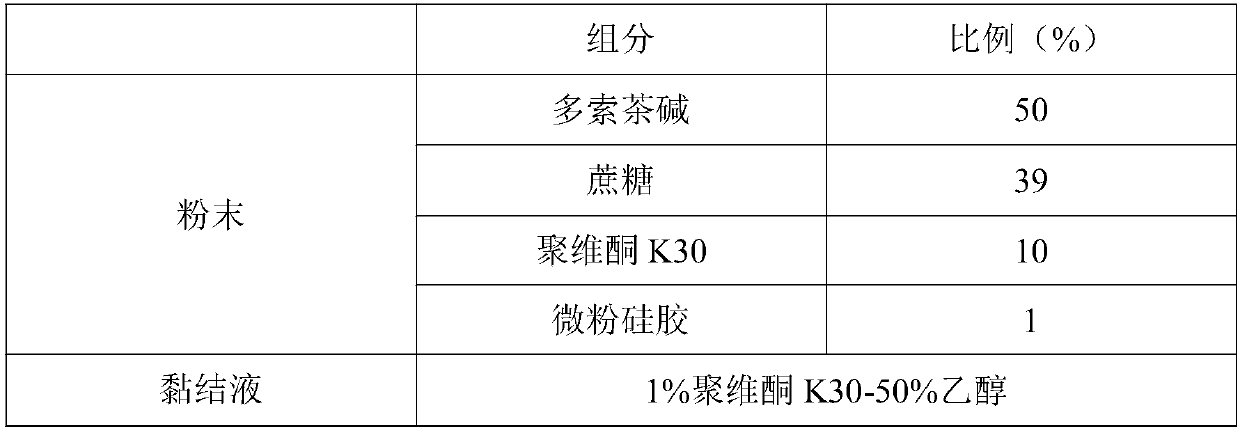
The invention discloses 3D printing doxofylline orally disintegrating tablets which are mainly prepared from, by mass, 40-60% of doxofylline, 20-40% of filling agent, 0-10% of disintegrating agent, 2-10% of binder, 0.1-1.0% of micropowder silica gel and 0-2% of corrigent by adopting 3D printing technology. The invention further discloses a preparation method of the doxofylline orally disintegrating tablets. The doxofylline orally disintegrating tablets are high in drug release speed, can improve drug taking compliance of patients and is especially convenient for old people, children and peoplehaving trouble in swallowing to take.
Owner:江苏互竑生物医学有限公司
Doxofylline injection
ActiveCN104958300AIncreased durabilityEasy to operateOrganic chemistryPharmaceutical delivery mechanismAcetic acidDoxofylline
The invention relates to a doxofylline injection-related substance determination method. According to the method, a high-efficiency liquid chromatography is used for determination. The invention provides a method capable of effectively controlling the doxofylline injection-related substances by using the high-efficiency liquid chromatography, which has the beneficial effects being good in durability, simple and rapid in operation, high in system suitability. According to the method, at least three reference substances containing theophylline, theophylline ethanol and theophylline acetaldehyde are used for the determination of the related substances. At the same time, the invention provides impurity reference substances such as impurity A, impurity B, impurity C, impurity D, impurity E, impurity G, impurity caffeine, methylxanthine, impurity C', impurity D', impurity E', impurity 2, theophylline acetic acid and the like for the determination of the doxofylline injection-related substances, so that the related substances caused by different synthetic routes, and preparation processes and storage processes of preparations. The invention also provides a synthesis method for separating and synthesizing the reference substances containing impurity A, impurity A'', impurity B, impurity C, impurity D, impurity E, impurity G and the like for the determination of the doxofylline injection-related substances.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Doxofylline impurity and preparation method thereof
The invention provides a doxofylline impurity and a preparation method thereof. The impurity is 9-(2,2-dimethoxyethyl)-1,3-dimethyl-1H-purine-2,6(3H,9H)-diketone. A compound provided by the inventioncan be used as a standard substance for detecting ingredients of a doxofylline finished product, thereby improving the accurate positioning and characterization of the detection and analysis of doxofylline finished product for the impurities, strengthening the control of the impurities, and further improving the quality of the doxofylline finished products.
Owner:四川蓝励医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com