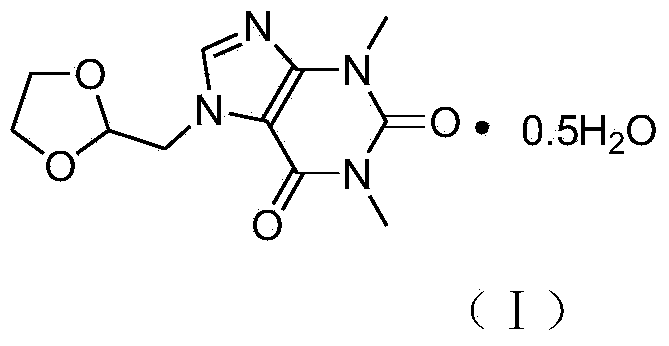
Doxofylline hemihydrate
A technology of doxofylline and hydrate, which is applied in the field of doxofylline hemihydrate and its preparation, can solve the problem that the amount of impurities cannot be effectively controlled, there are many types of impurities, and there is no refining method for doxofylline hemihydrate. Detailed research and disclosure of issues to achieve the effect of reducing market risk, high solubility, and simple methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In a 5000ml reaction bottle equipped with stirring, thermometer and condenser, add 100g of doxofylline, 0.5g of dimethylformamide (DMF) and 1000ml of water-ethanol=1:10 mixture, start stirring, Heat up to 55°C-60°C, heat up to reflux for 1 hour, cool down to room temperature naturally, then cool to 5°C, continue to stir and crystallize for 1 hour, filter, and dry at 50°C for 3 hours under nitrogen atmosphere to obtain doxofylline 90.8 grams of hemihydrate powder, the moisture measured by the Karl Fischer method is 3.33%.
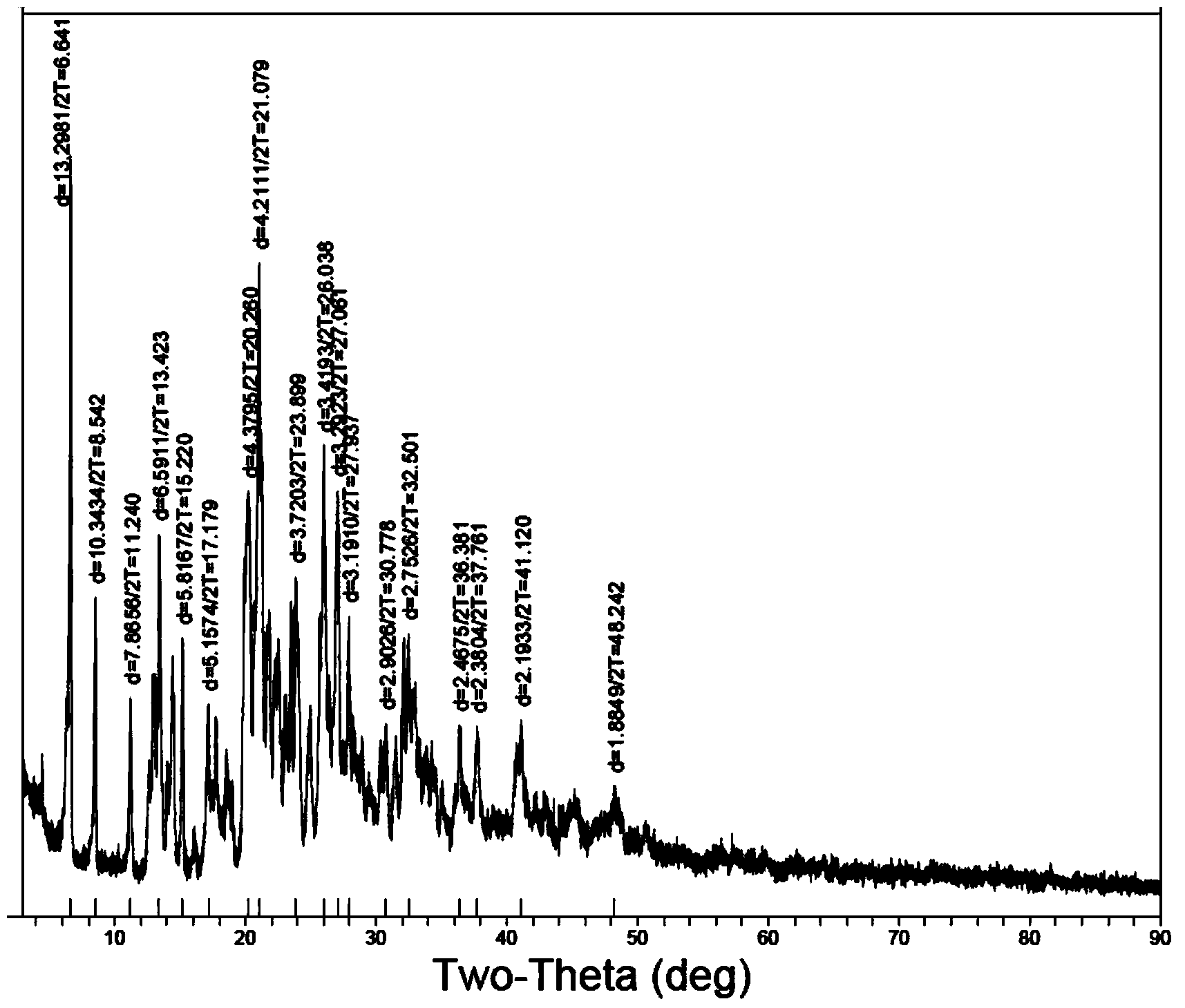
[0058] The X-ray diffraction pattern of the compound is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max2500 diffractometer; CuKa40Kv100mA; 2θ scanning range: 0-50°.
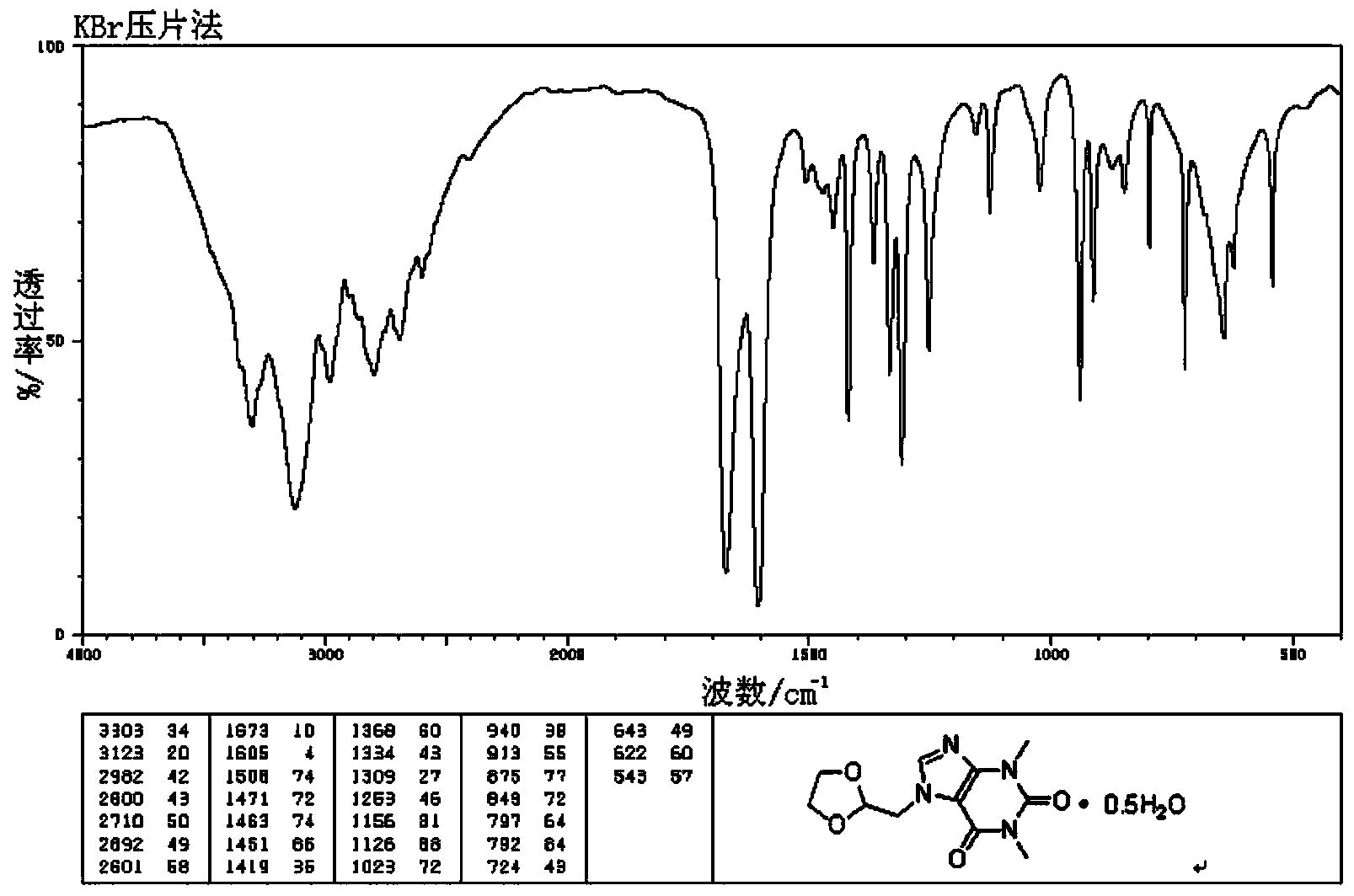
[0059] The infrared spectrum of the compound is shown in figure 2 , Determination with KBr tablet.
Embodiment 2
[0061] In a 5000ml reaction bottle equipped with stirring, thermometer and condenser, add 100g of doxofylline, 0.5g of dimethylformamide (DMF) and 800ml of water-ethanol=1:8 mixture, start stirring, Heat up to 55°C-60°C, heat up to reflux for 1 hour, cool down to room temperature naturally, then cool to 5°C, continue to stir and crystallize for 1 hour, filter, and dry at 50°C for 3 hours under nitrogen atmosphere to obtain doxofylline 91.8 grams of hemihydrate powders, the moisture measured by the Karl Fischer method is 3.18%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com