Doxofylline venous injection with small volume as well as preparation method and quality control method thereof
A technology for intravenous injection and doxofylline, which is applied in medical formulas, measuring devices, medical preparations of non-active ingredients, etc., can solve the problems of large volume and achieve good safety and a wide range of options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
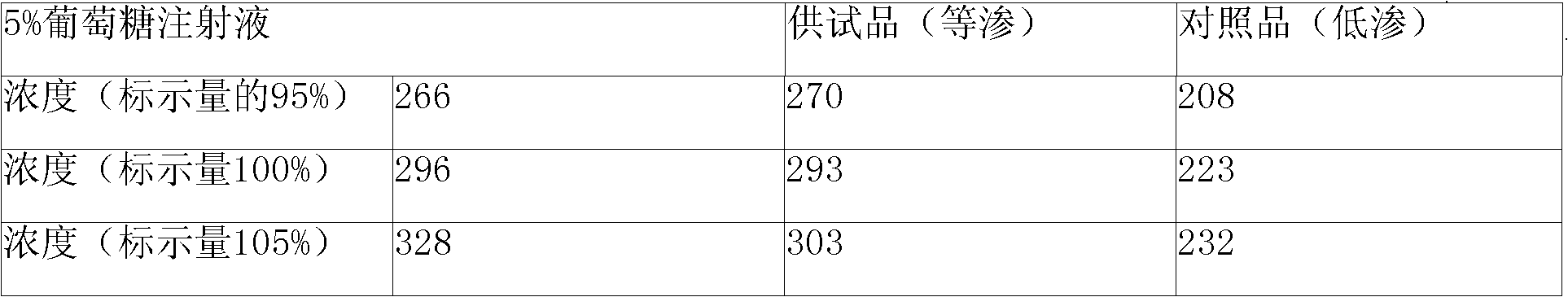
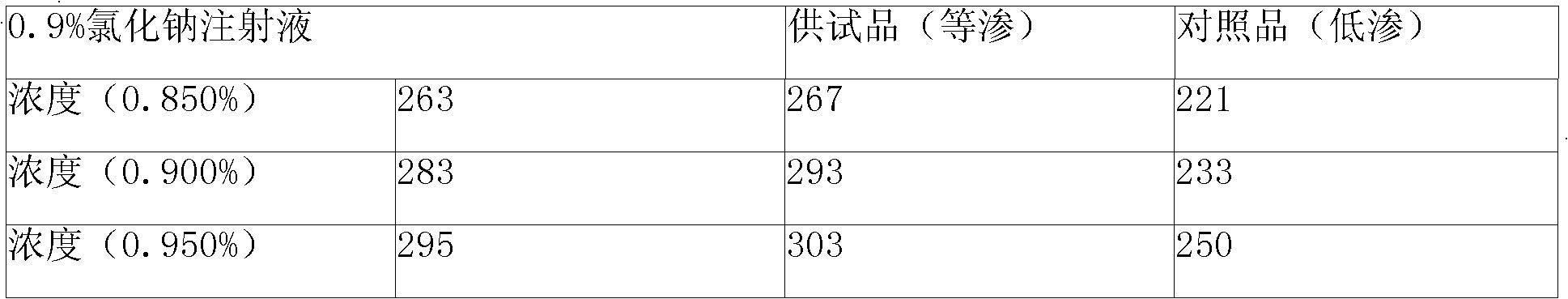
[0022] Example 1 Each 10ml: containing 0.1g doxofylline, adjust isotonicity with sodium chloride
[0023] Labeled prescription:
[0024] Doxofylline 100g
[0025] Sodium chloride 81g
[0026] Add water to 10000ml
[0027] - - - - - - - - - - - - - - - - - - -
[0028] Make 1000 sticks
[0029] Take 6000ml of water for injection, heat up, and add 115g of doxofylline under stirring (the amount of doxofylline absorbed by activated carbon is 15%, so add 15 grams more than the prescription amount, the following is for convenience of description, no longer note) and chlorination Sodium 81g, when the temperature of the liquid rises to about 80°C, add 5g of activated carbon after the powder is completely dissolved, continue stirring for 10 minutes, wait for the temperature to drop to 40°C, filter to remove carbon, and obtain a concentrated solution. Take the concentrated solution and add water for injection to make up to 10000ml, stir evenly, filter through a microporous membrane...
Embodiment 2
[0030] Example 2 Each 10ml: containing 0.1g doxofylline, adjust isotonicity with glucose
[0031] Labeled prescription:
[0032] Doxofylline 100g
[0033] Glucose 450g
[0034] Add water to 10000ml
[0035] - - - - - - - - - - - - - - - - - - - -
[0036] Make 1000 sticks
[0037] Take 6000ml of water for injection, heat up, add 115g of doxofylline and 450g of glucose under stirring, wait until the temperature of the liquid rises to about 80°C, add 5g of activated carbon after the medicine powder is completely dissolved, continue stirring for 10 minutes, wait until the temperature drops to 40°C, Filtrate to remove charcoal to obtain a concentrated solution. Take the concentrated solution and add water for injection to make up to 10000ml, stir evenly, and filter through a microporous membrane until the clarity is qualified, then take a sample to check the content and pH value. The content should be 95-105% of the labeled amount, and the pH value should be 4.5-5.5. Put it...
Embodiment 311
[0038] The preparation method of embodiment 311 is the same as that of embodiment 1 or 2, the difference is the consumption of doxofylline, the consumption and type of isotonic agent
[0039] Example 3
[0040] Example 7
[0041] The beneficial effects of the present invention will be further illustrated by test examples below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com