Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Bronchospasm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bronchospasm or a bronchial spasm is a sudden constriction of the muscles in the walls of the bronchioles. It is caused by the release (degranulation) of substances from mast cells or basophils under the influence of anaphylatoxins. It causes difficulty in breathing which ranges from mild to severe.
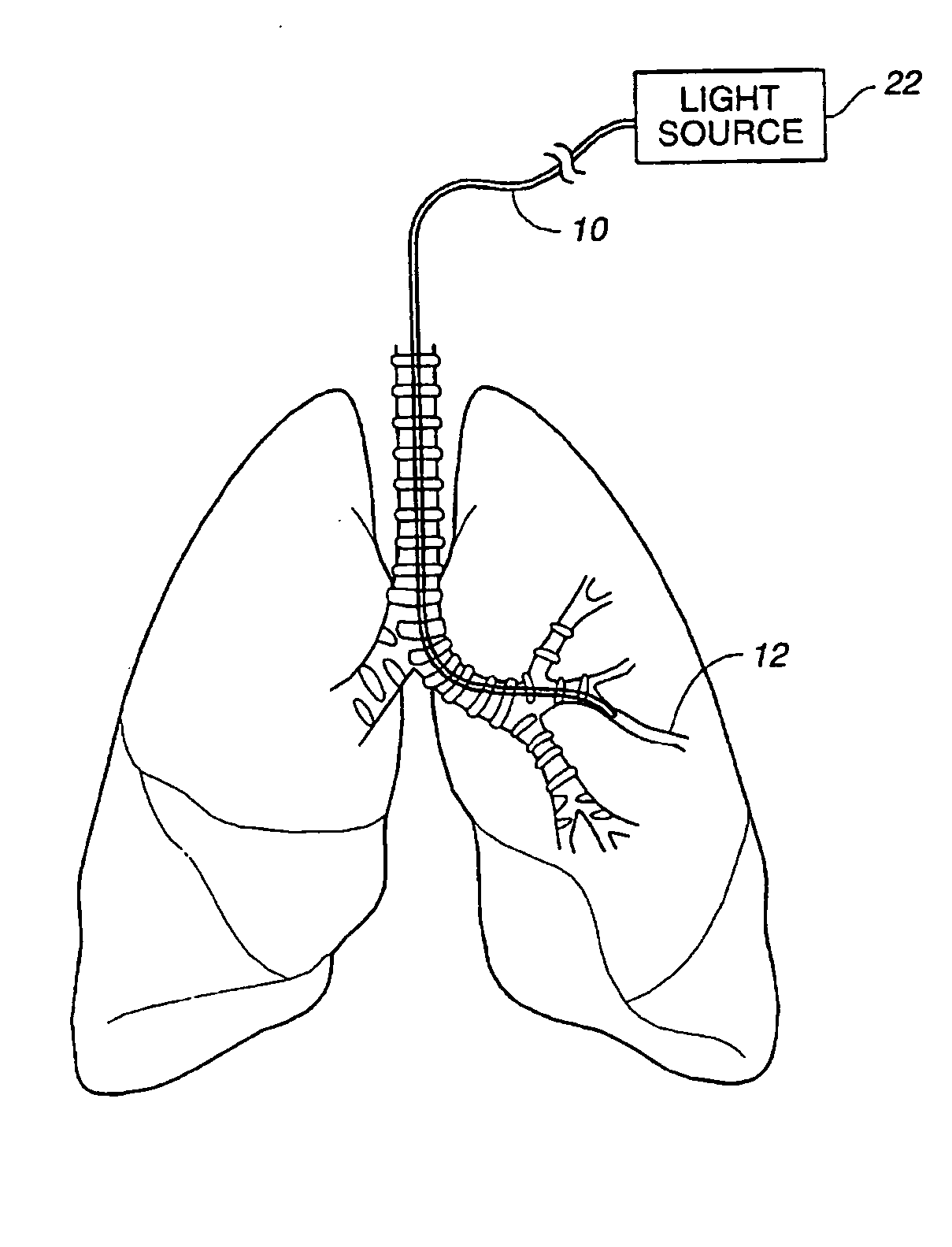
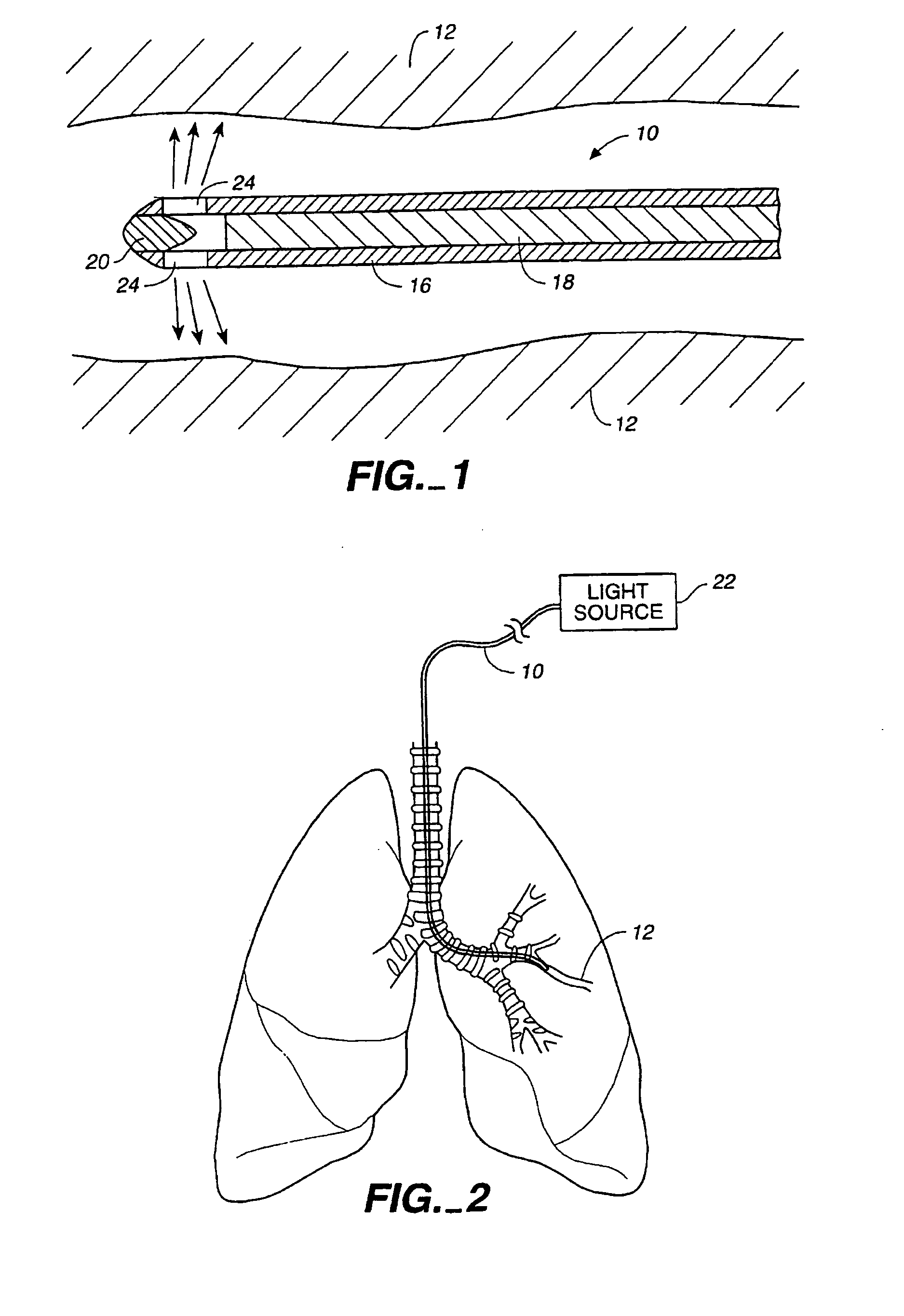
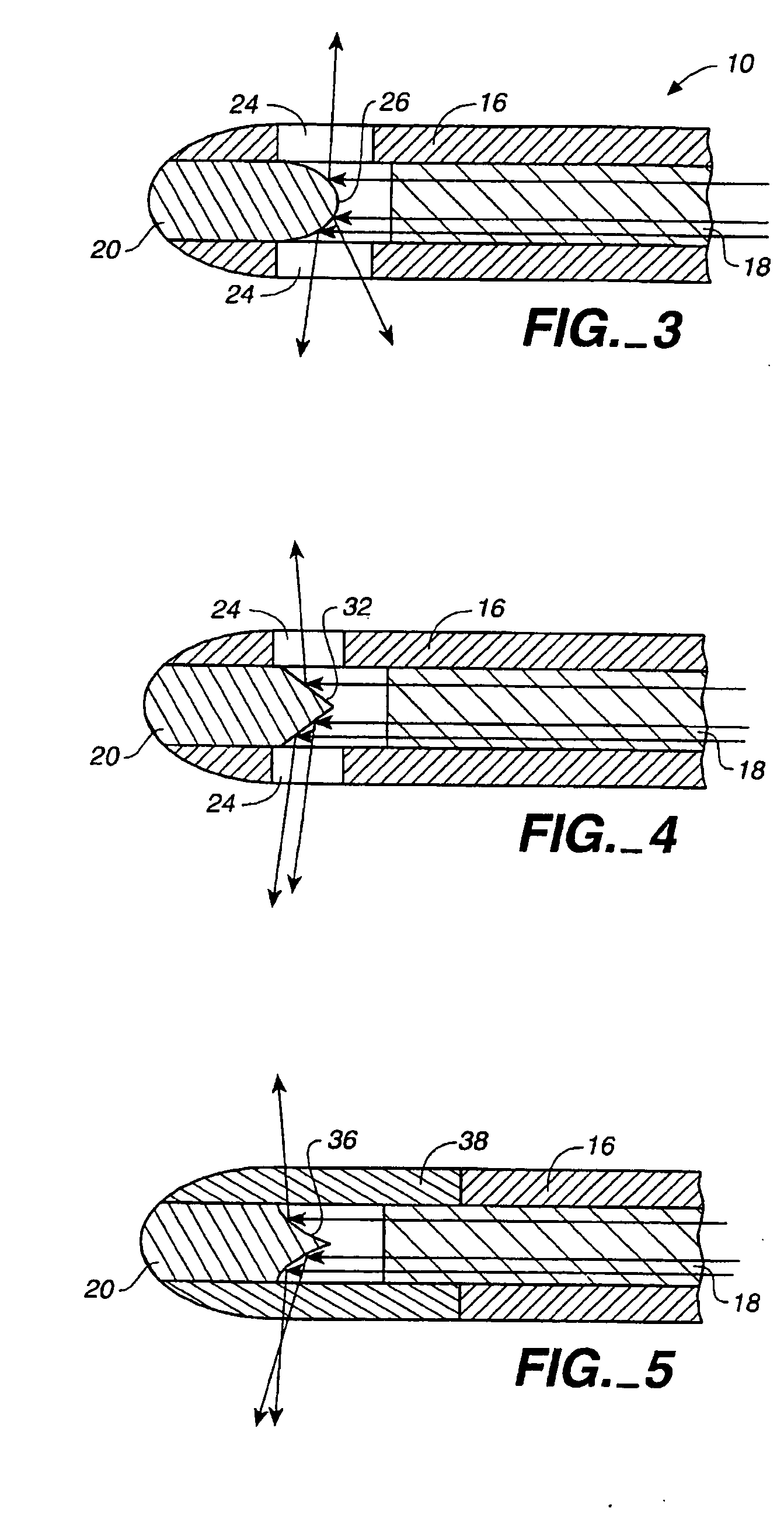
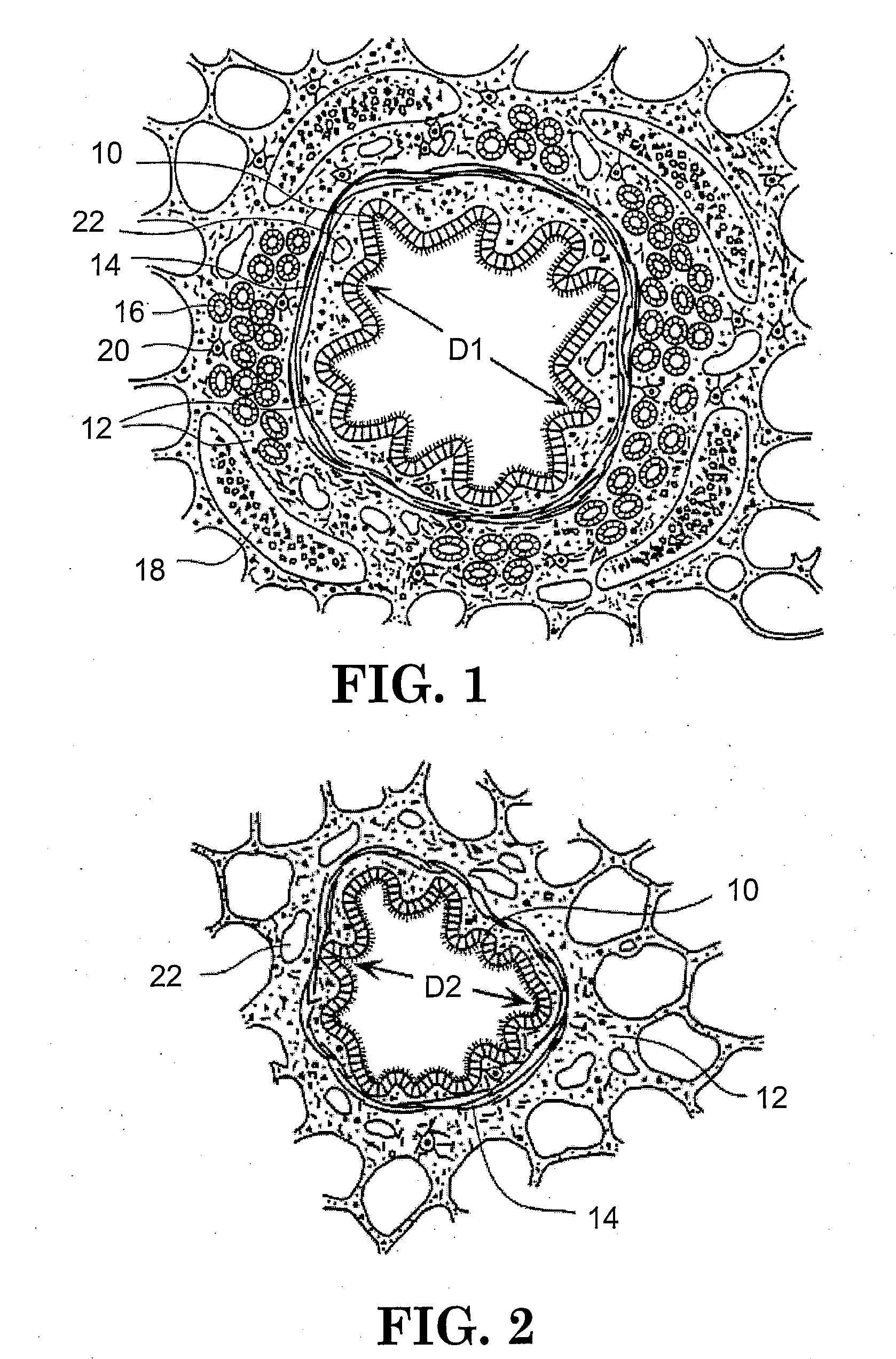
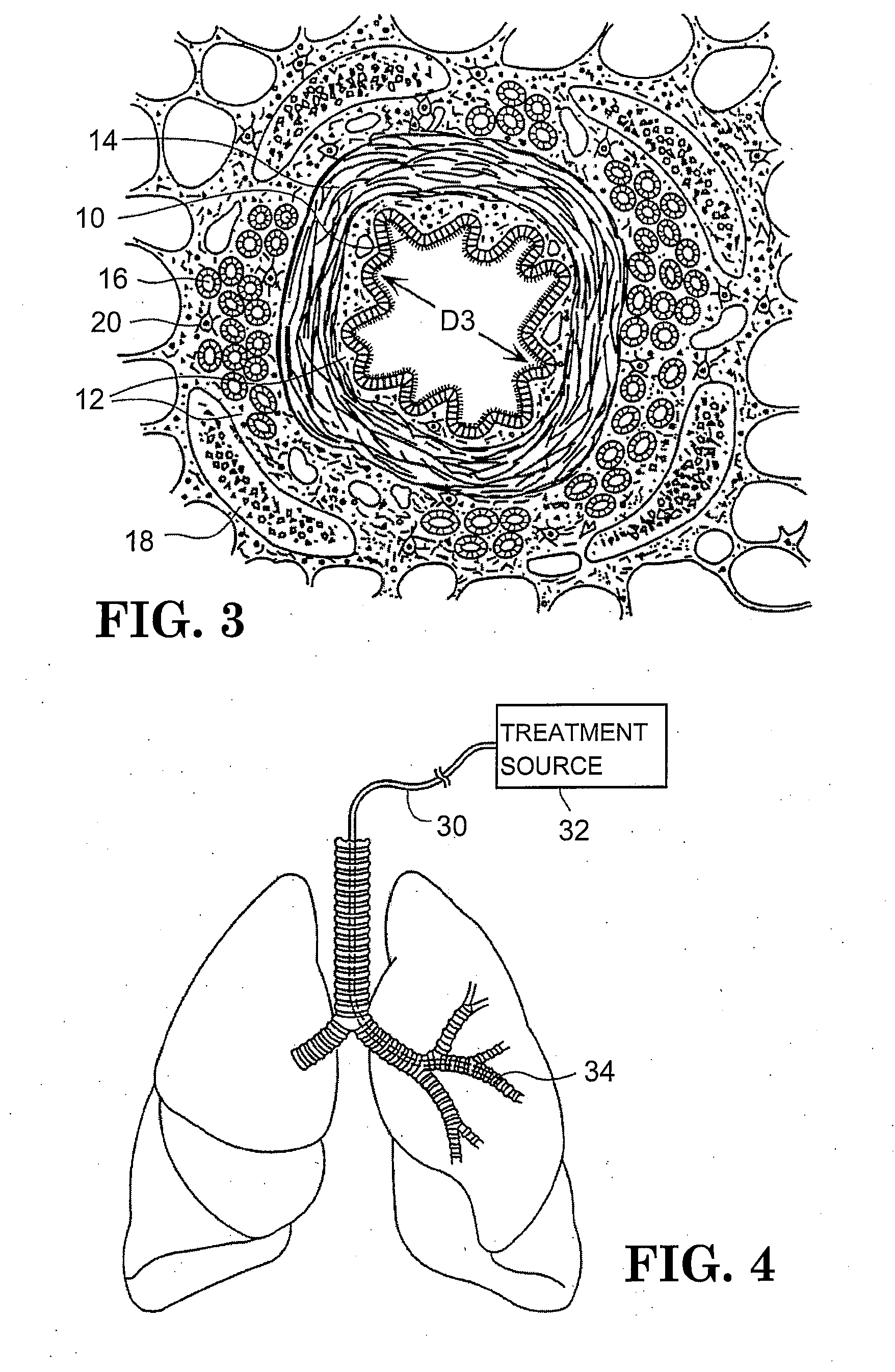
Method of treating airways in the lung
InactiveUS20050010270A1Reduce the amount requiredIncrease inner diameterSurgical instrument detailsLight therapyBronchospasmSmooth muscle spasm
A device and method for treating bodily conduits involves the application of energy to the smooth muscle tissue of the conduit walls to reduce the bulk of smooth muscle tissue and mucus glands. The irradiation treatment of the smooth muscle tissue causes a reduction in the amount of smooth muscle tissue over time which increases the inner diameter of the body conduit for improved fluid flow and prevents smooth muscle spasms. The treatment is particularly useful in the lungs for treatment of asthma to prevent bronchospasms, increase the airway diameter for improved air exchange, and reduce mucus secretions in the lungs.
Owner:BOSTON SCI SCIMED INC
Method for treating airways in the lung
InactiveUS20070106348A1Reduce the amount requiredIncrease inner diameterDiagnosticsMedical devicesBronchospasmSmooth muscle spasm
A device and method for treating bodily conduits involves the application of energy to the smooth muscle tissue of the conduit walls to reduce the bulk of smooth muscle tissue and mucus glands. The irradiation treatment of the smooth muscle tissue causes a reduction in the amount of smooth muscle tissue over time which increases the inner diameter of the body conduit for improved fluid flow and prevents smooth muscle spasms. The treatment is particularly useful in the lungs for treatment of asthma to prevent bronchospasms, increase the airway diameter for improved air exchange, and reduce mucus secretions in the lungs.
Owner:BOSTON SCI SCIMED INC
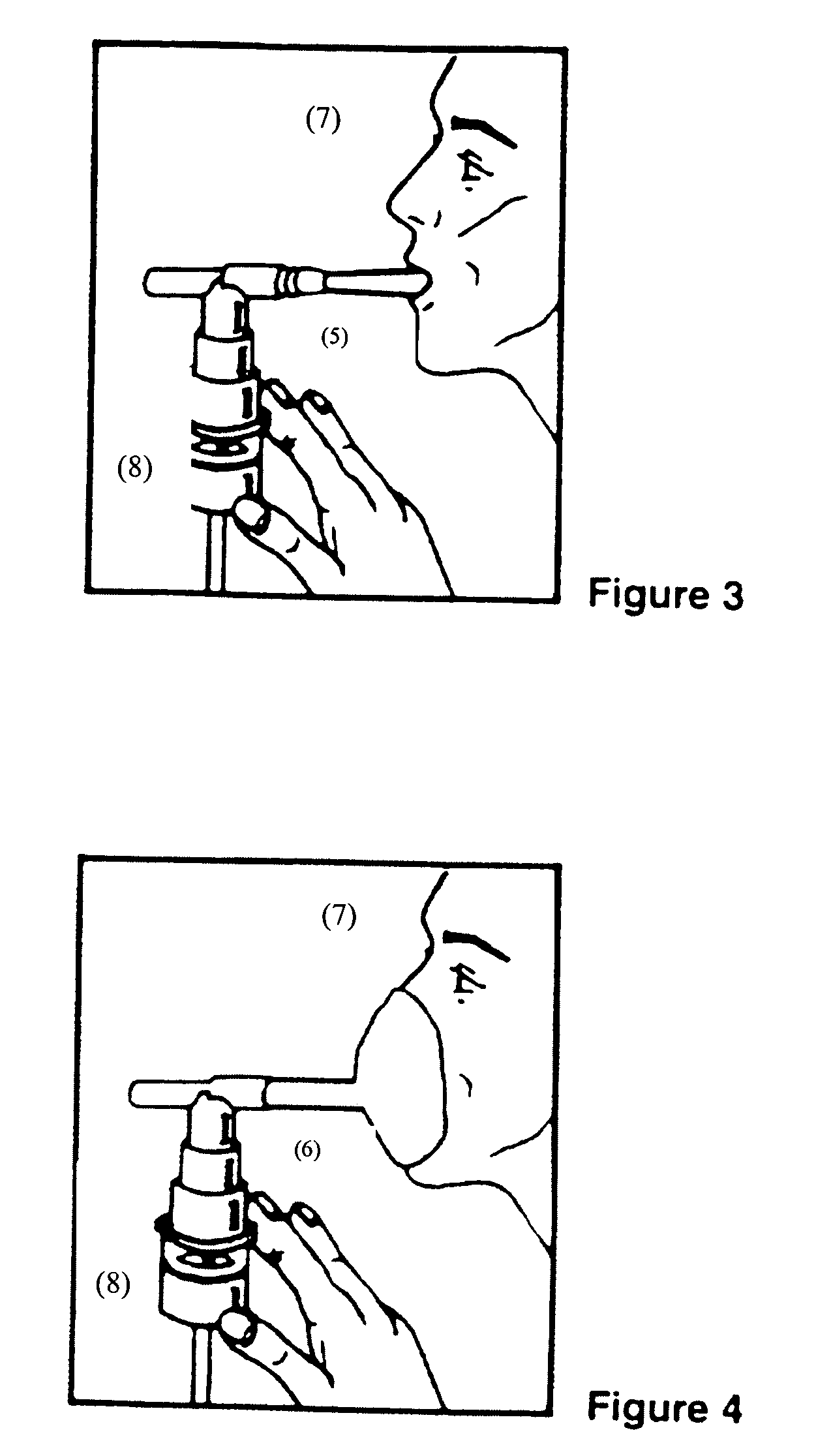
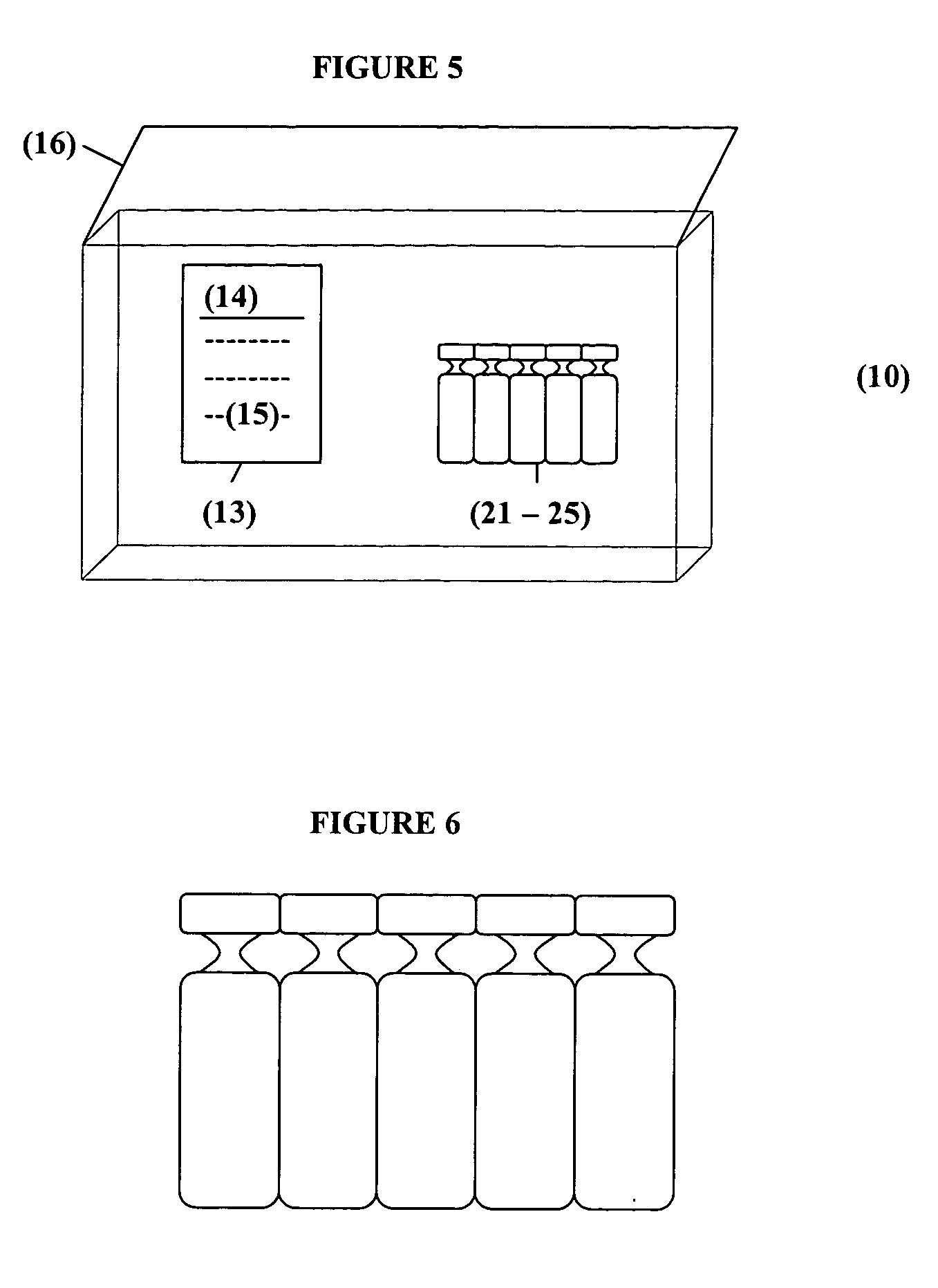
Intranasal Formulation of Epinephrine for the Treatment of Anaphylaxis
ActiveUS20150005356A1Fast onset timeSuitable for intranasal useBiocidePharmaceutical delivery mechanismBronchospasmInjection epinephrine
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
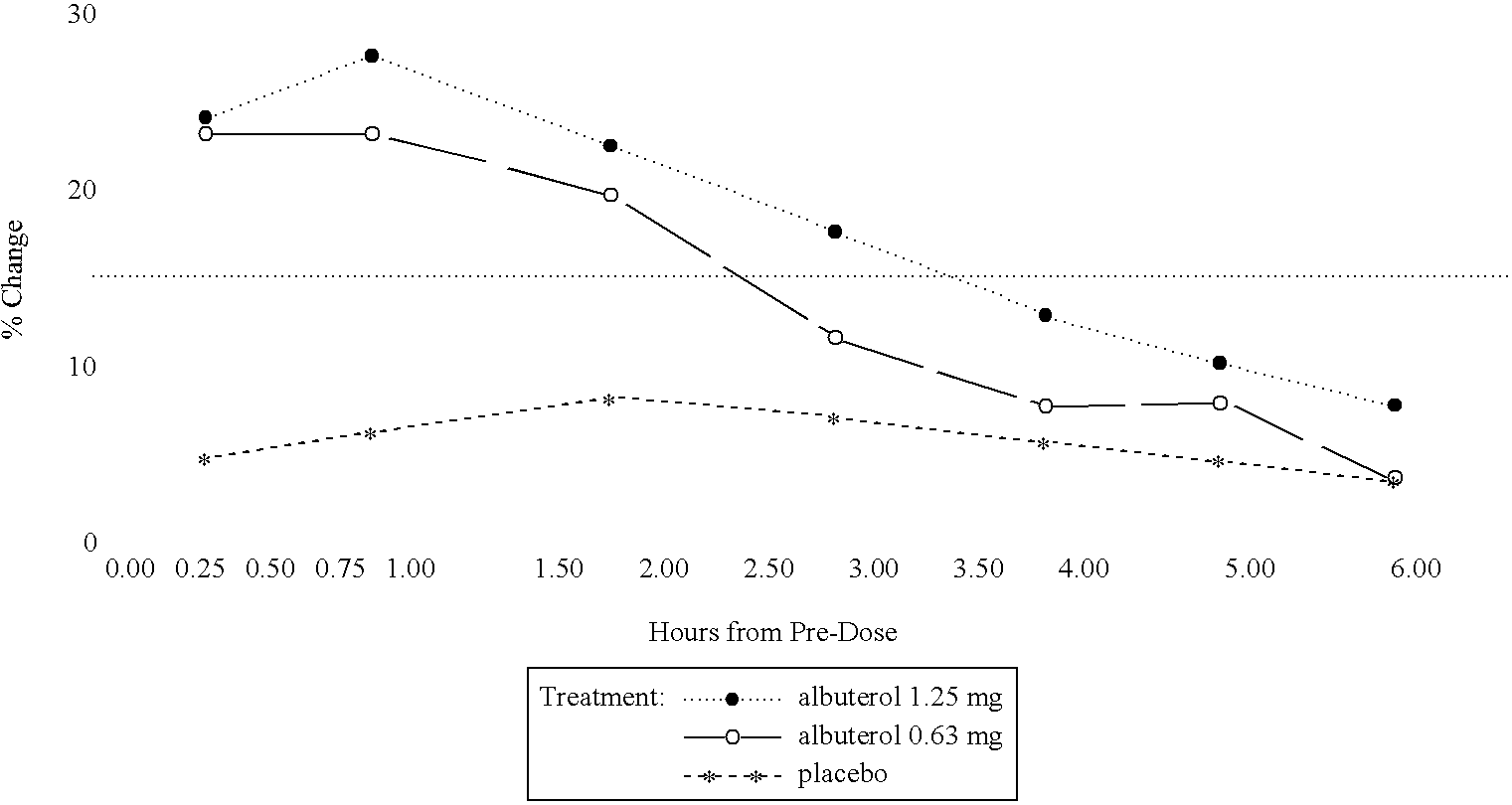
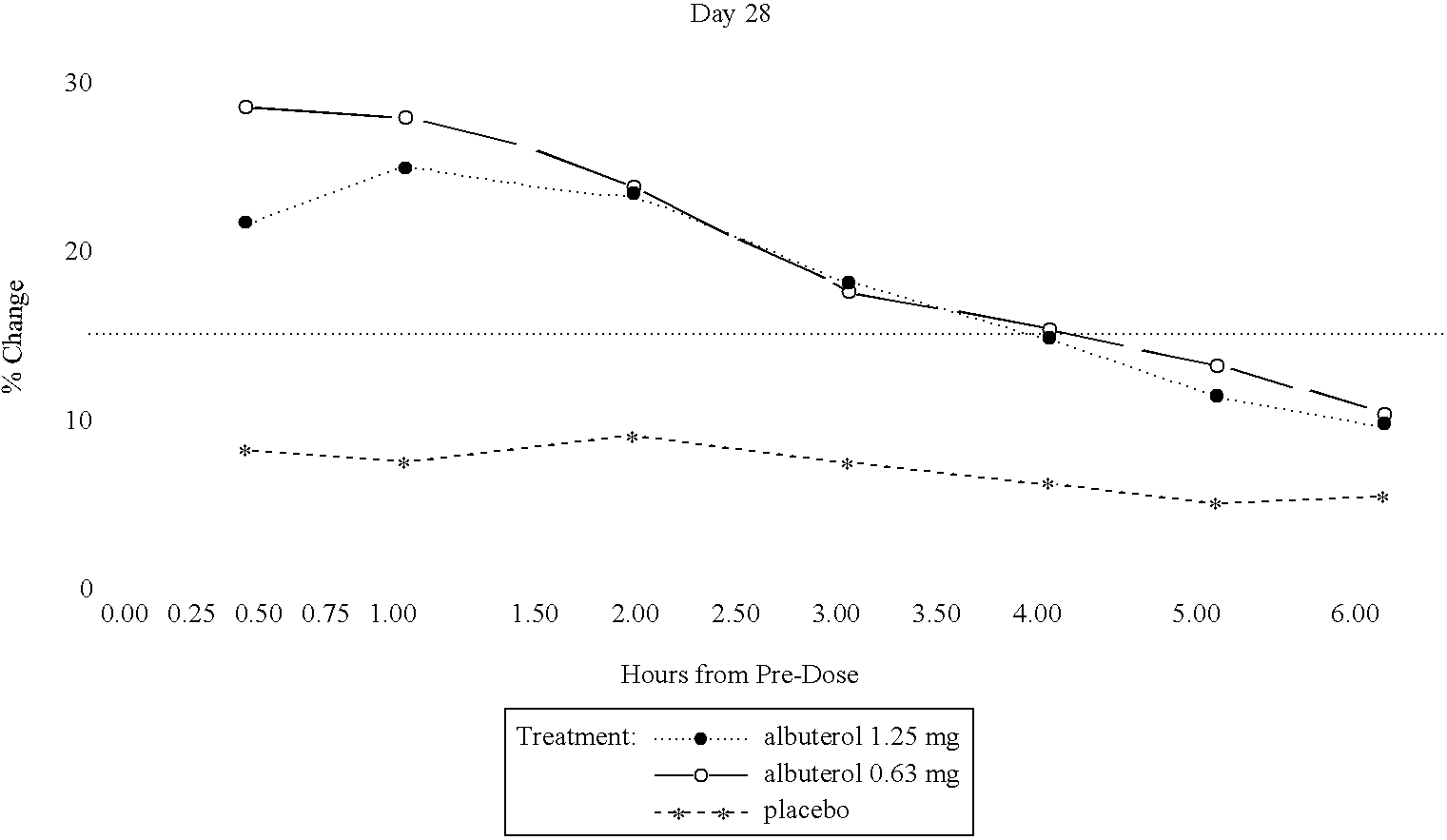
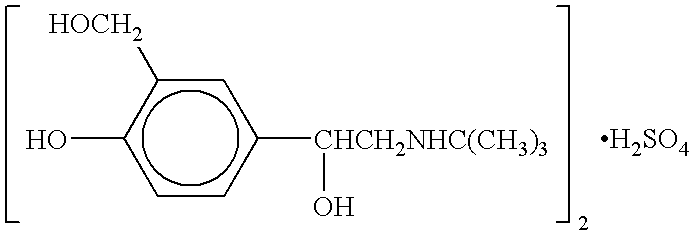
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
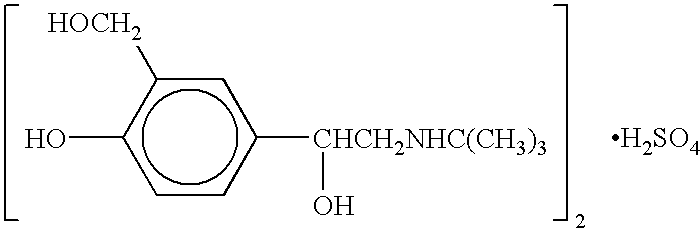
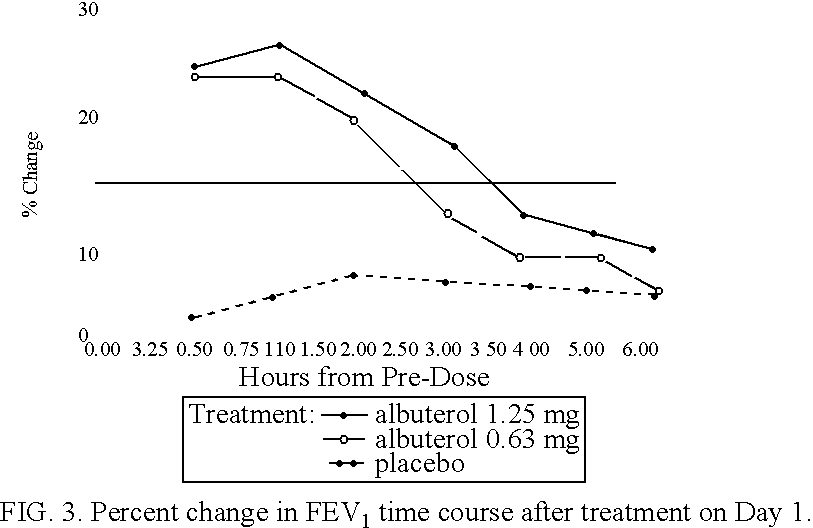
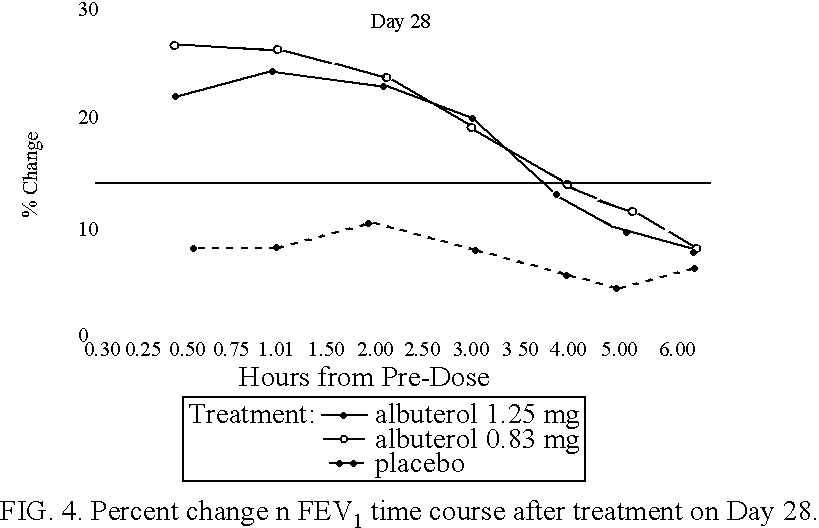
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
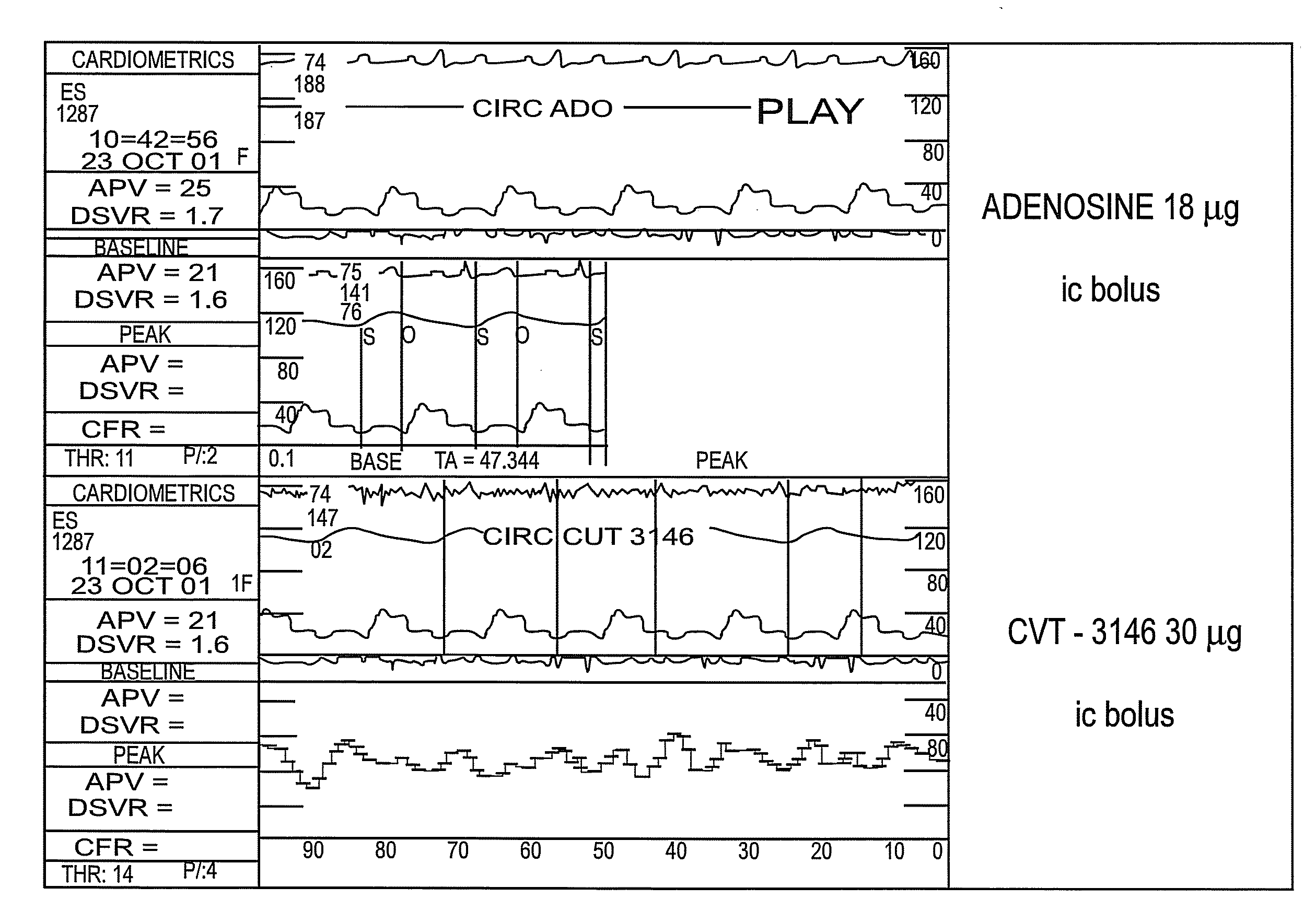
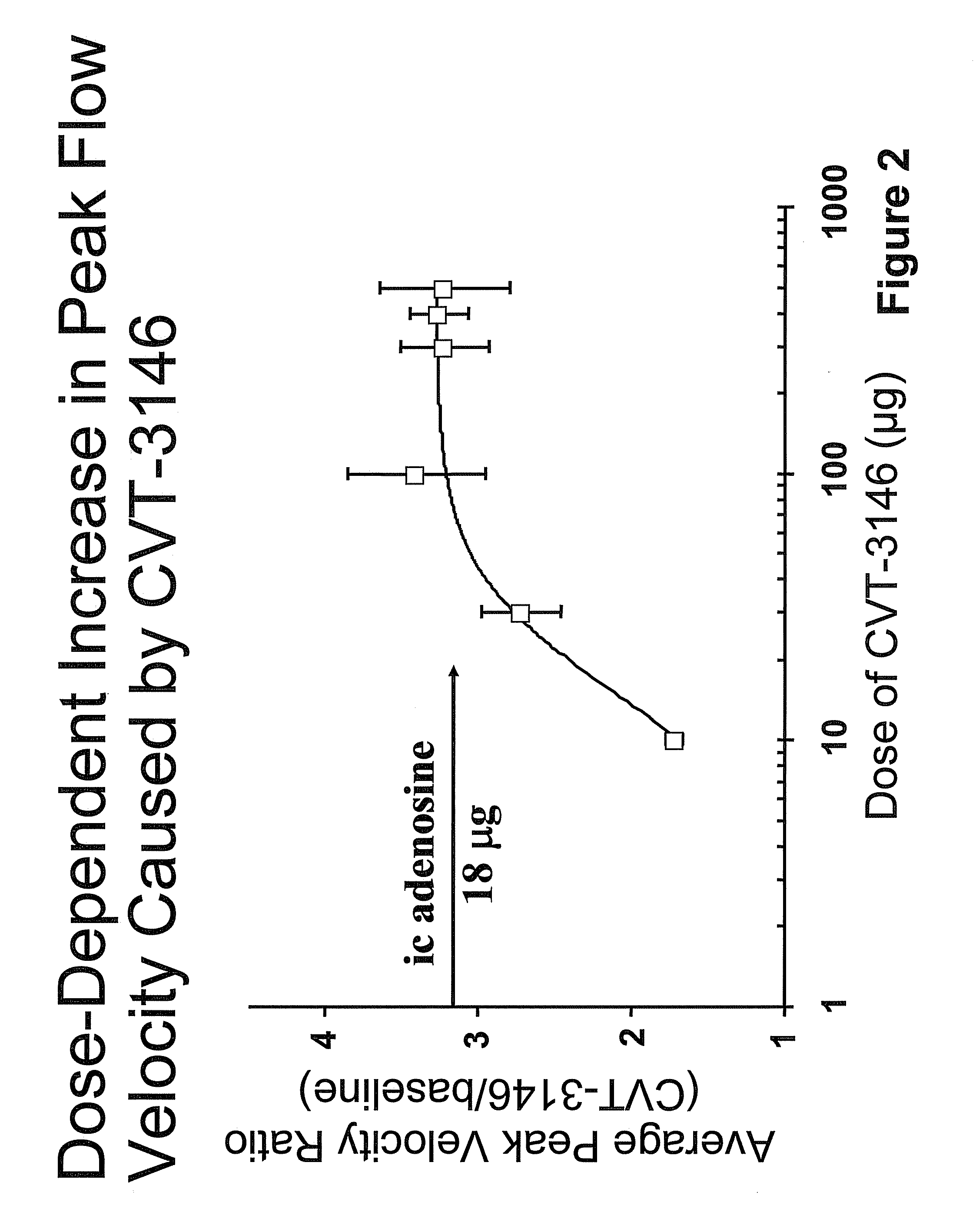
Methods for Myocardial Imaging in Patients Having a History of Pulmonary Disease
InactiveUS20080170990A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsBronchospasmCardiac muscle
The present application discloses methods for myocardial imaging in human patients having a history of pulmonary disease such as asthma, bronchospasm, chronic obstructive pulmonary disease, pulmonary fibrosis, pulmonary inflammation, or pulmonary hypertension, comprising administrating doses of one or more A2A adenosine receptor agonists to a mammal undergoing myocardial imaging and detecting and / or diagnosing myocardial dysfunction.
Owner:TPG AXON LEX SUB TRUST +1
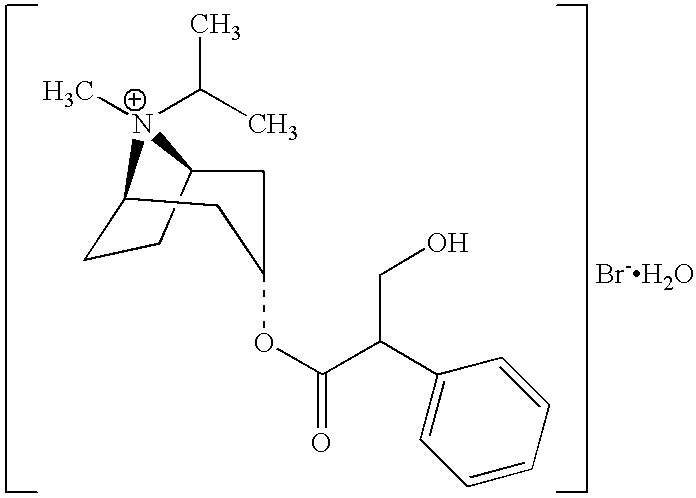
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS6632842B2Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030149007A1Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
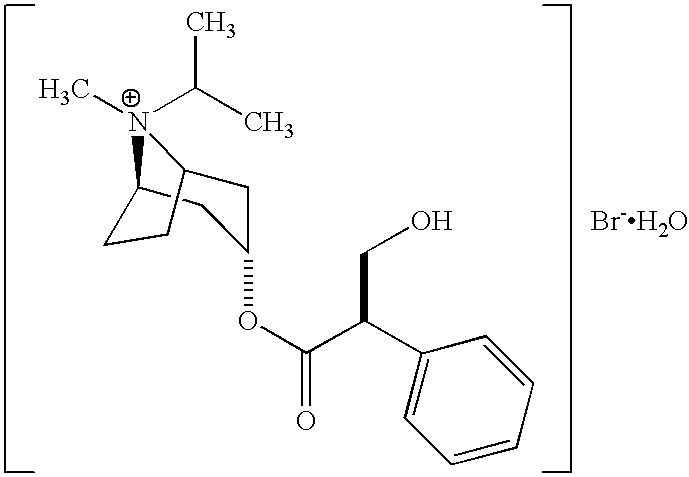
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:MYLAN SPECIALTY
Methods of use of compounds which inhibit the stem cell signaling pathway
InactiveUS6989248B2Avoid reactionAntipyreticMicrobiological testing/measurementHypopigmentationBronchospasm
This invention provides a method of preventing or treating in a subject contact dermatitis which comprises administering to the subject an amount of a compound capable of inhibiting the stem cell factor signaling pathway effective to prevent or treat contact dermatitis so as to thereby prevent or treat contact dermatitis in the subject. This invention also provides a methods of preventing or treating in a subject hyperpigmentation, asthma, cutaneous inflammation, anaphylaxis and bronchospasm, mastocytosis, tumors which express activated kit, and conception.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Treatment of Respiratory Disease
InactiveUS20080020048A1High and immediate onsetSuitable for treatmentBiocidePowder deliveryBronchospasmDisease
Glycopyrrate or an analogue thereof is useful for the treatment of bronchospasm or as a rescue medication.
Owner:SOSEI R&D LIMITED
Intranasal formulation of epinephrine for the treatment of anaphylaxis
ActiveUS9789071B2Increases absolute nasal residency timeMaximal stability of unstable APIsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsBronchospasmNose
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
Methods for inhibiting cutaneous inflammation and hyperpigmentation
InactiveUS6977159B1Avoid reactionInhibit inflammationAntipyreticAnalgesicsHypopigmentationBronchospasm
This invention provides a method of preventing or treating in a subject contact dermatitis which comprises administering to the subject an amount of a compound capable of inhibiting the stem cell factor signaling pathway effective to prevent or treat contact dermatitis so as to thereby prevent or treat contact dermatitis in the subject. This invention also provides a method of preventing or treating in a subject hyperpigmentation, asthma, cutaneous inflammation, anaphylaxis and bronchospasm, mastocytosis, tumors which express activated kit, and conception.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
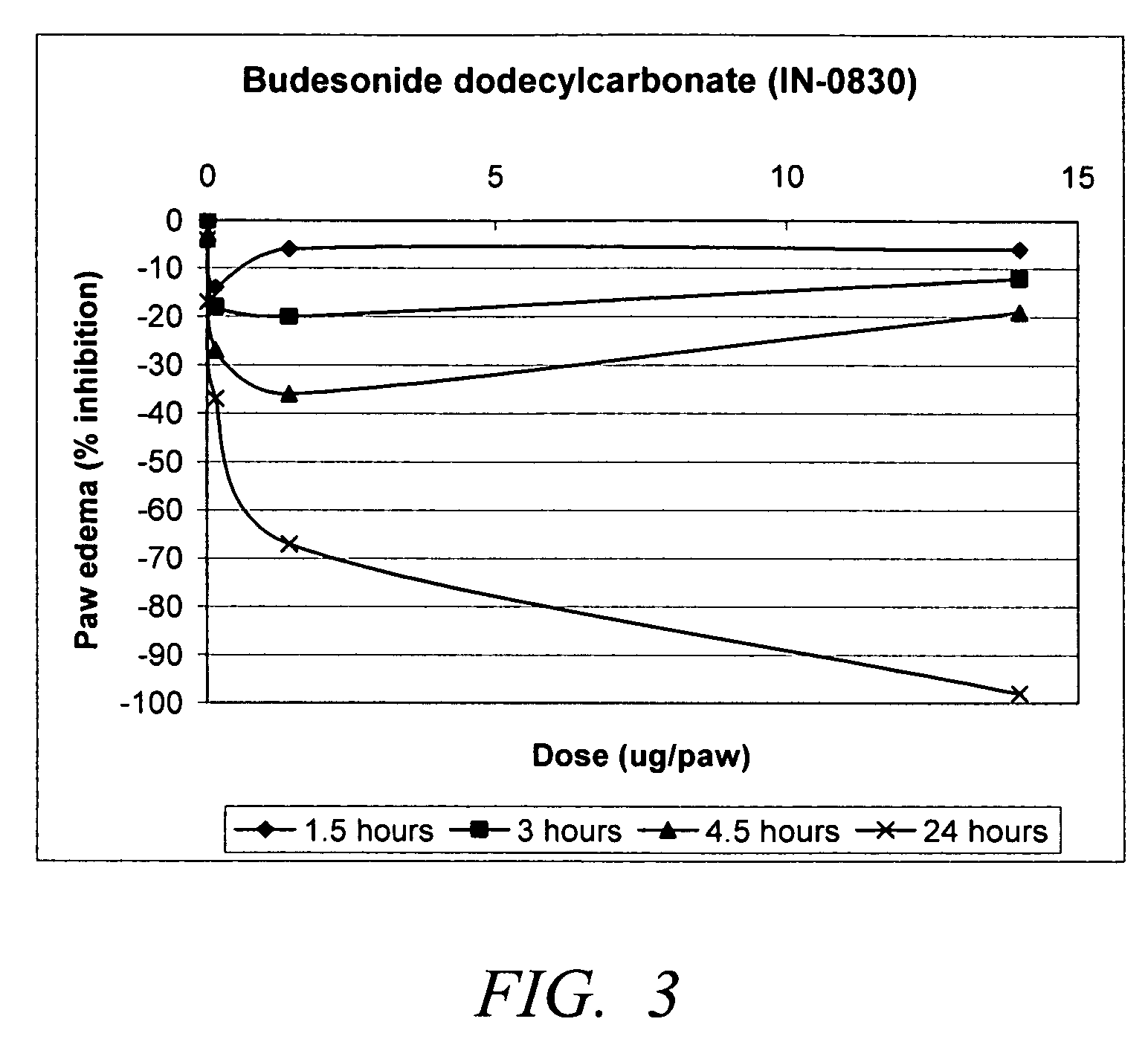
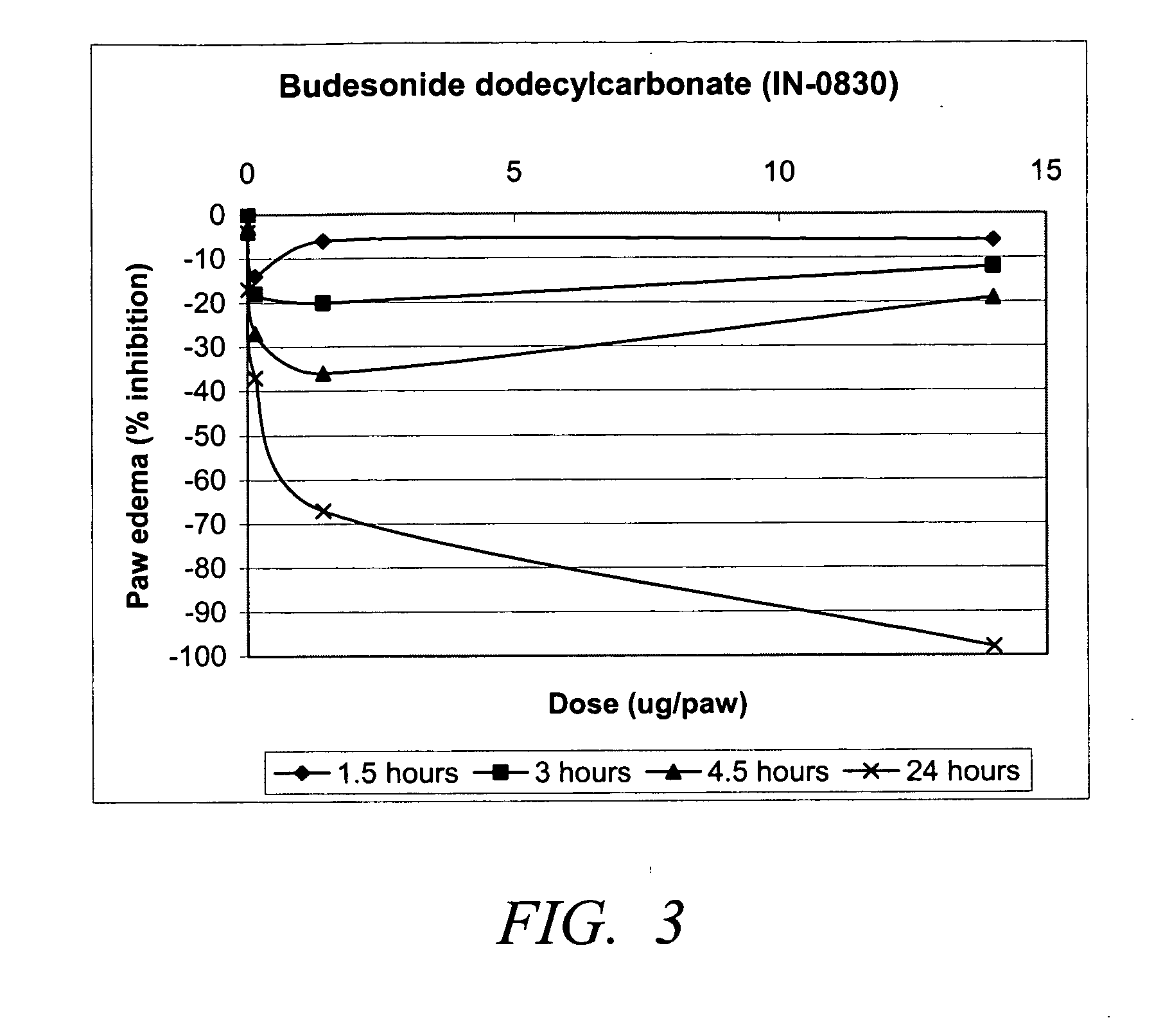
Carbonate and carbamate modified forms of glucocorticoids in combination with B2 adrenergic agonists
Compositions containing β2 adrenergic agonists in combination with carbonates and carbamates of the formulaand in combination with related steroid carbonates and carbamates are disclosed. The compositions are useful for treating bronchospasm, for inducing bronchodilation and for treating rhinitis, asthma, and chronic obstructive pulmonary disease (COPD) and inflammatory diseases, particularly by inhalation.
Owner:SUNOVION PHARMA INC
Freeze dried powder injecta of doxofylline and its preparation
The invention relates to a freeze dried powder injection of doxofylline and its preparation, wherein the injection comprises Doxofylline 0.05-0.5g, and at least one pharmaceutically acceptable excipient, pH modifier, anti-oxidant agent, and stabilizer, and is provided in the form of unit amount in the container. The advantages of the invention include easy medicament transport and preservation, prevention of freezing of the liquid injection in winter, increased medicament stability, thus can be applied for effectively treating bronchial asthma, lungs disease of bronchospasm, and chronic clogged pulmonary diseases.
Owner:武汉安士医药科技有限公司
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030203930A1Relieve bronchospasmBiocideDispersion deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Novel ultrasonic atomization sputum aspirator
InactiveCN106422006AAvoid damageResolve complicationsMedical devicesCatheterBronchospasmTracheal mucosa
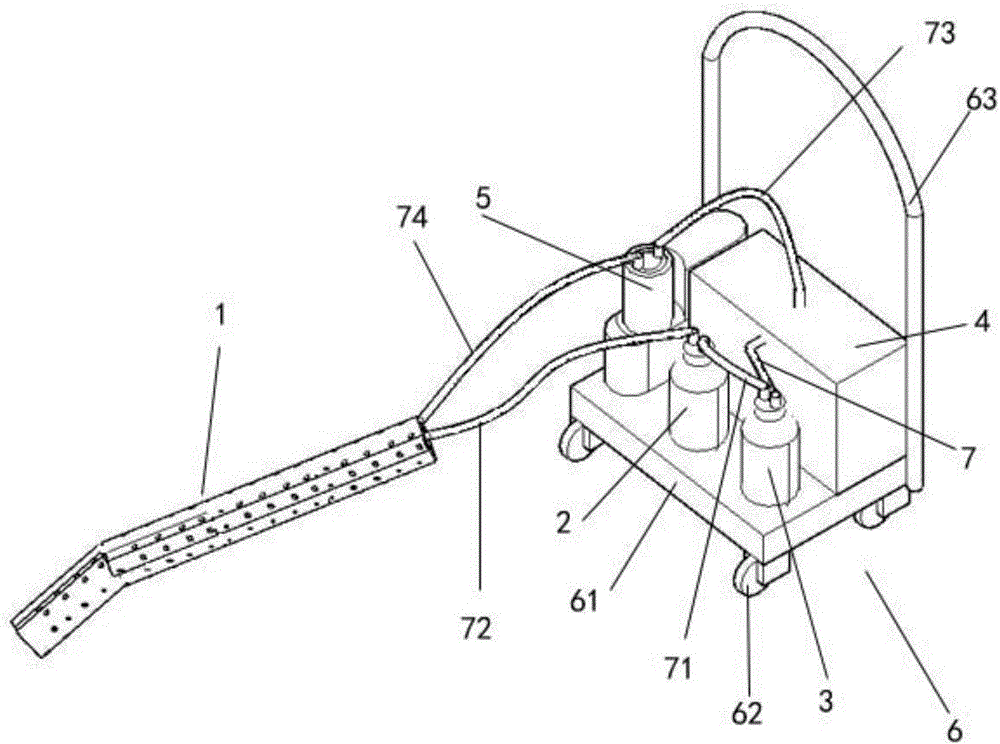
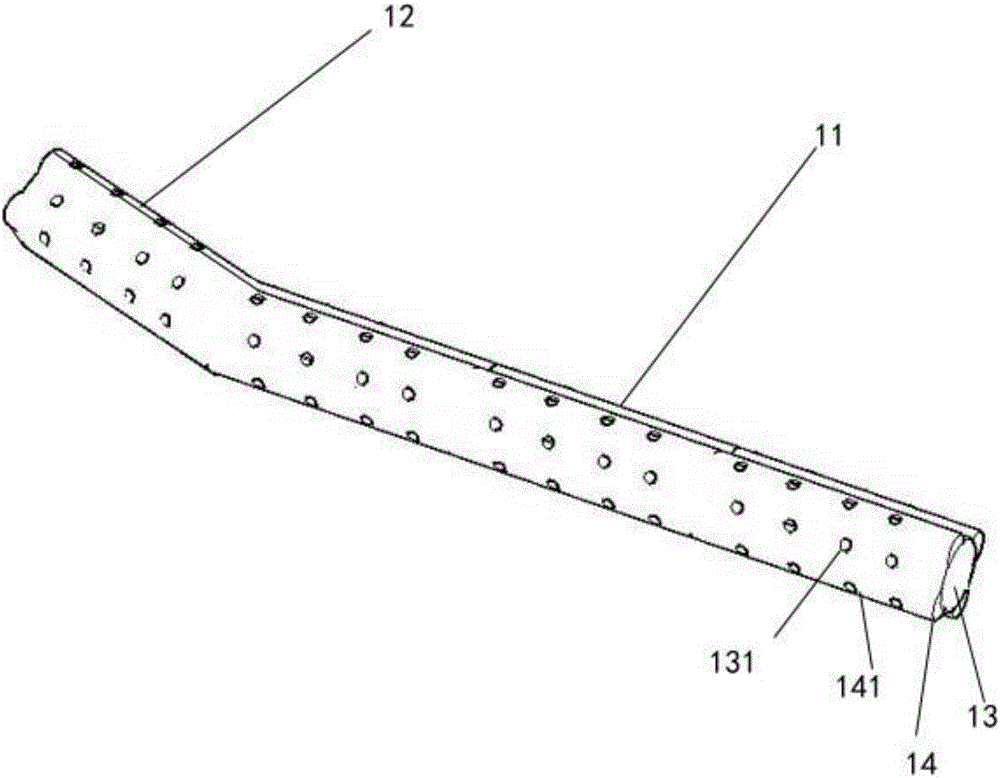
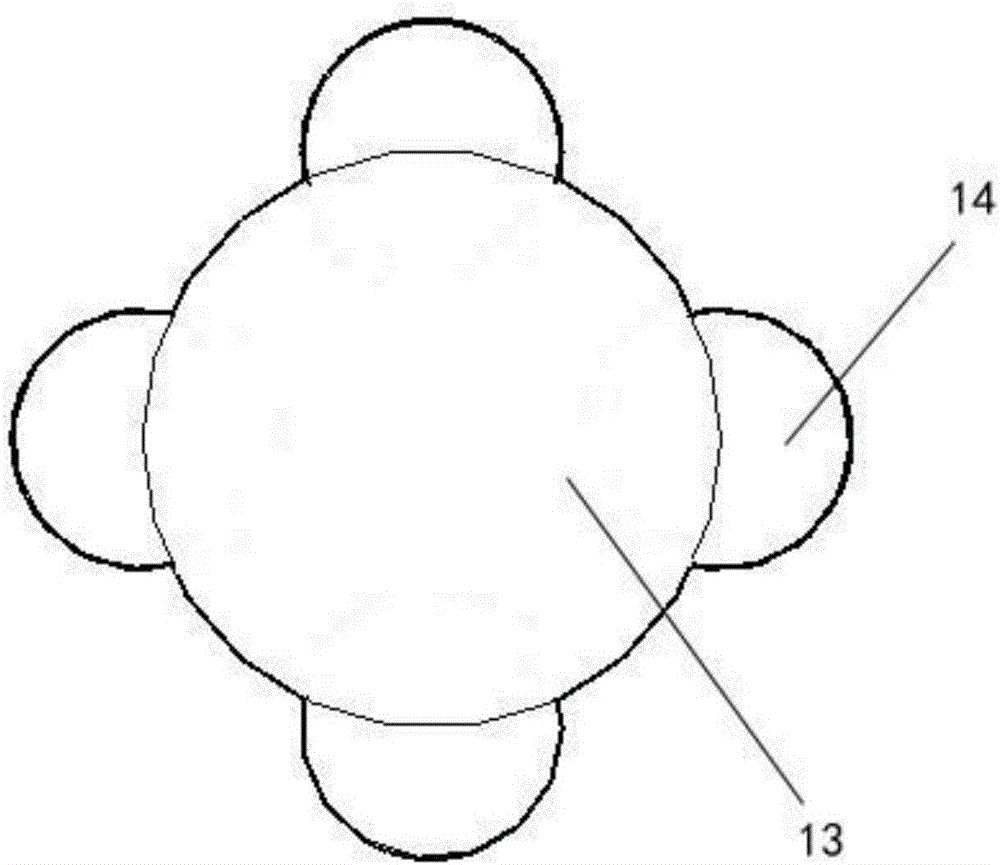
The invention relates to a novel ultrasonic atomization sputum aspirator. The ultrasonic atomization sputum aspirator comprises a sputum aspiration pipe, a sputum liquid bottle, a negative pressure bottle, a negative pressure control device, an ultrasonic atomizer, a thermostat and a base; the far end of sputum aspiration pipe is of an arc structure, the sputum aspiration pipe comprises an inner pipe and an outer pipe, first side holes are formed in the inner pipe, second side holes are formed in the outer pipe, the first side holes and the second side holes are staggered at the axial position, and connecting lines formed by connecting the first side holes and the second side holes in a staggered mode are spirally distributed. The novel ultrasonic atomization sputum aspirator can reduce injury of tracheal mucosa, carry out shaping, aspirate sputum in different airflow directions, carry out atomization and humidifying treatment through ultrasound and completely solve complication problems of existing equipment such as tracheal mucosa injury, pulmonary atelectasis, bronchospasm, hyoxemia, infection, artificial airway blocking and sputum aspiration pipe blocking.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
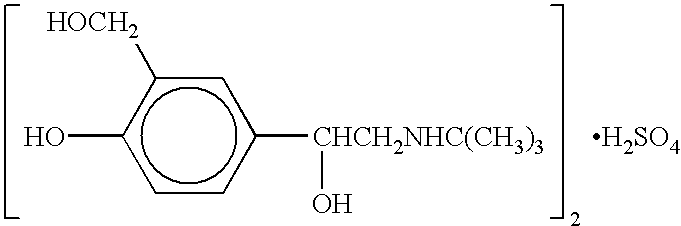
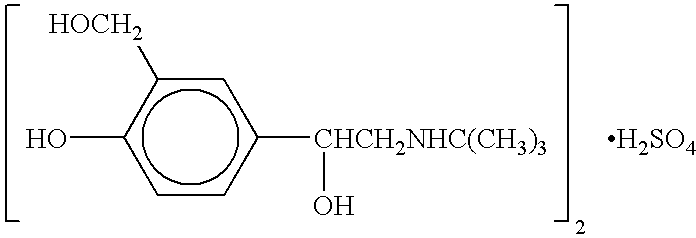
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.75 mg or about 1.5 mg albuterol sulfate.
Owner:DEY
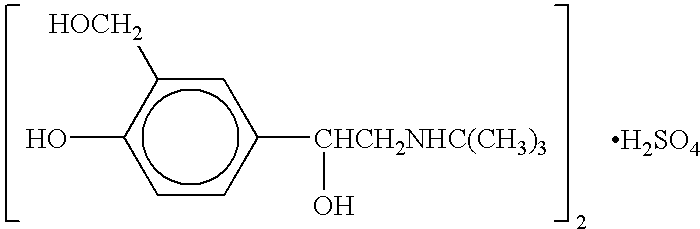
Levalbuterol hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN101103963ALittle side effectsGood curative effectOrganic active ingredientsPill deliveryBronchospasmAsthmatic bronchitis
The invention relates to a levalbuterol hydrochloride orally disintegrating tablet and the preparation method; wherein the levalbuterol hydrochloride orally disintegrating tablet comprises an active pharmaceutical ingredient, the levalbuterol hydrochloride, and pharmaceutical necessities. The pharmaceutical necessities include a disintegrant, a filling agent, a masking agent and a corrective. The raw material ingredients calculated by weight ratio are 0.79-2.5 per cent levalbuterol hydrochloride, 50-60 percent disintegrant, 20-40 per cent filling agent, 1.58-10 per cent masking agent and 10-12 per cent corrective, which are made into the orally disintegrating tablets by direct powder compression process. The tablets of the invention is taken easily without water, disintegrated quickly and takes effect quickly, high in bioavailability, less stimulatory function to gastrointestinal tract, and applicable to prevention and cure of bronchial asthma, asthmatic bronchitis, and bronchospasm of an emphysema patient.
Owner:HARBIN UNIV OF COMMERCE
Albuterol inhalation soultion, system, kit and method for relieving symptoms of pediatric asthma
InactiveUS20030140920A1Relieve bronchospasmRespiratorsOrganic active ingredientsBronchospasmSalbutamol
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:DEY
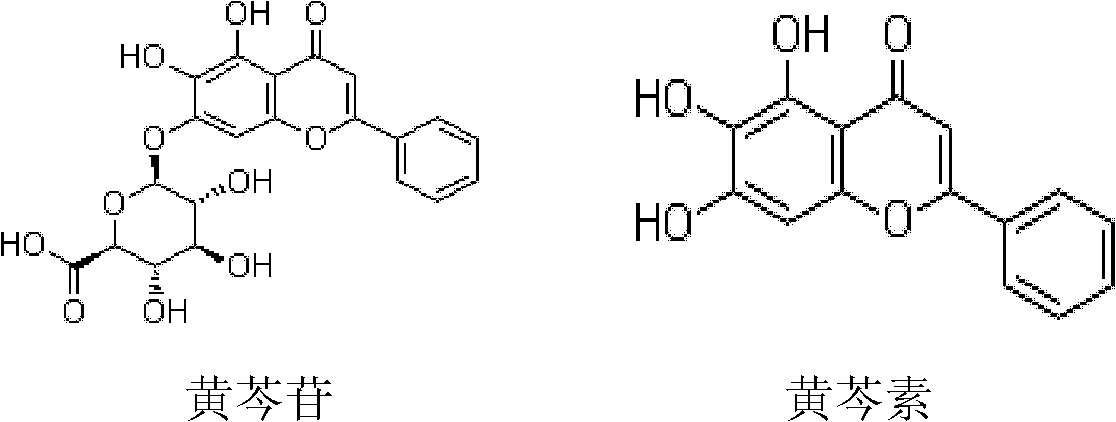
Application of high-purity baicalin or baicalein to preparation of inhaled asthma relieving medicament
InactiveCN102302504AAcute seizure controlRelieve spasmsOrganic active ingredientsPharmaceutical delivery mechanismBronchospasmSide effect
The invention discloses application of high-purity baicalin or baicalein to the preparation of inhaled asthma relieving medicaments. The inhaled asthma relieving medicaments comprise the formulations of inhaled solution or inhaled powder sprays, wherein the inhaled solution is prepared from the baicalin or the baicalein and pharmaceutical adjuvants A, and the pharmaceutical adjuvants may be one or more of solvents, latent solvents, surfactants or antioxidants; the inhaled powder sprays are prepared from the baicalin or the baicalein or pharmaceutical adjuvants B, and the pharmaceutical adjuvants B may be one or more of the solvents, the antioxidants or carriers; and the purity of the baicalin or the baicalein is more than 90 percent. Experiments proves that the inhaled asthma relieving medicaments prepared from the high-purity baicalin or baicalein can control the acute paroxysm of asthma effectively, relieve bronchospasm and reduce the resistance of air passages, is high in medicinal effect, small in side effect, nontoxic and low in cost.
Owner:LOGISTICS UNIV OF CAPF
Carbonate and carbamate modified forms of glucocorticoids in combination with B2 adrenergic agonists
InactiveUS20050009798A1Good potencyEffective treatmentOrganic active ingredientsAntipyreticBronchospasmCarbamate
Compositions containing β2 adrenergic agonists in combination with carbonates and carbamates of the formula and in combination with related steroid carbonates and carbamates are disclosed. The compositions are useful for treating bronchospasm, for inducing bronchodilation and for treating rhinitis, asthma, and chronic obstructive pulmonary disease (COPD) and inflammatory diseases, particularly by inhalation.
Owner:SEPACOR INC
The treatment of respiratory disease
Glycopyrrate or an analogue thereof is useful for the treatment of bronchospasm or as a rescue medication.
Owner:SOSEI R&D LTD
Bambutero hydrochloride and doxofylline-contained compound preparation and preparation method thereof
InactiveCN102440992AGive full play to the mechanism of joint actionCan produce synergistic effects in the treatment of asthmaRespiratory disorderEster active ingredientsLiquid glucoseDoxofylline
The invention discloses a bambutero hydrochloride and doxofylline-contained compound preparation, which comprises tablet and syrup, wherein main medicines are bambutero hydrochloride and doxofylline, auxiliary materials comprise corn starch, lactose, gelatin, talcum powder, magnesium stearate, liquid glucose, cane sugar, vitamin C, sodium metabisulfite, edetate disodium, orange juice flavouring agent, edible toner and purified water. The compound preparation has good co-therapy function to bronchial asthma, chronic bronchitis, emphysema and other lung diseases which are relevant to concurrent bronchospasm.
Owner:岳阳新华达制药有限公司
Chinese medicinal atomizing preparation for treating chronic bronchitis
The invention discloses a Chinese medicinal atomizing preparation for treating chronic bronchitis, which is prepared from the following pure Chinese medicinal herbs with functions of clearing away heat and toxic material, regulating qi and strengthening the spleen, eliminating dampness and eliminating phlegm, and removing heat from the lung to relieve cough: liquoric root, heartleaf houttuynia herb, tangerine peel, ephedra herb, ajuga decumbens, almond and wild chrysanthemum. When being used for atomization inspiration of the patient, the Chinese medicinal atomizing preparation is beneficial for the mucous membrane of the respiratory tract to discharge secretions, sanies and pathogenic bacteria and has effect of provoking self-cleaning of the respiratory tract. The Chinese medicinal atomizing preparation can relieve bronchospasm, reduce mucous edema and liquefying bronchial secretions, promotes the control of the bronchial inflammatory process and the improvement of the ventilation function, and has good treatment effect and no side effect.
Owner:卢子民
2-(2,2-diarylethyl)-cyclamine derivative or salt, and synthesis and application and composition thereof
ActiveCN110372571AM receptor antagonist activity is goodHigh antagonistic activityNervous disorderOrganic chemistryBronchospasmDisease
The invention provides 2-(2,2-diarylethyl)-cyclamine derivative or salt, and synthesis and application and a composition thereof. Biological activity testing shows that the 2-(2,2-diarylethyl)-cyclamine derivative has great M receptor antagonistic activity, and can serve as pharmaceutical active ingredients to be used for treating muscarinic receptor mediated or regulated diseases comprising asthma, chronic obstructive pulmonary disease (COPD), overactive bladder (OAB), bronchospasm accompanied with COPD, visceral spasm, irritable bowel syndrome, Parkinson's disease, depression or anxiety, schizophrenia and related mental illness, and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Lung benefiting pill with 25 ingredients having the function of being homology of medicine and food
ActiveCN104958539AImprove cardiopulmonary functionUnswapUnknown materialsPill deliveryBronchospasmAngelica Sinensis Root
The invention discloses a lung benefiting pill with 25 ingredients having the function of being homology of medicine and food. The composition of the lung benefiting pill comprises sea buckthorn, radix pseudostellariae, polyporus umbellatus, platycodon grandiflorum, stir-baked rhizoma dioscoreae, milk steamed poria cocos, longan aril, north astragalus, baked perilla seed, baked semen raphani, baked semen brassicae, radix glehniae, radix salviae miltiorrhizae, spina date seed, gecko tails, cordyceps sinensis, almonds, fructus schisandrae, linden honey, salted alisma orientalis, atractylodes macrocephala koidz baked with bran, angelica sinensis, milk steamed polygonatum kingianum, radix ophiopogonis, raw lily and the like. The lung benefiting pill is advantageous to guarantee normal ventilation of alveolus pulmonis and relieves organism anaerobic condition. Due to the fact that the permeability of lung blood capillary is improved, gas exchange is facilitated, and the symptoms of dyspnea and coughing with expectoration are relieved. Weak people with dyspnea and chronic cough generally has the abnormal phenomenon of hemorheology, synchronic treating of phlegm and blood stasis can solve the root cause of sputum and the root cause of blood stasis, bronchospasm can be relieved, discharging of sputum is promoted, cohesive force of red blood cells can be relieved, the blood viscosity is lowered, the hyperviscosity of the weak patients with dyspnea and chronic cough can be relieved, the cardio-pulmonary function of the patients can be improved, and therefore, the lung benefiting pill has good effect on treatment of the patients of this kind.
Owner:刘水平
Tourmaline negative ion therapeutic apparatus
InactiveCN107744621ADoes not generate ozoneNo secondary pollutionElectrotherapyElectrical apparatusBronchospasmMedicine
The invention discloses a tourmaline negative ion therapeutic instrument, which relates to the technical field of medical equipment and solves the problems of energy consumption and corona of a high-pressure negative ion therapeutic instrument. It includes a breathing mask, a connecting hose, an anion generator, an air intake pipe, and Said breathing mask links to each other with negative ion generator through connecting flexible pipe, and negative ion generator is connected with air intake pipe from one end of breathing mask; Described negative ion generator interior arranges honeycomb body, and described honeycomb body ceramic material and tourmaline powder and carbon dioxide The cerium powder is compounded and fired. The invention has the advantages of simple structure, convenient operation, easy portability, health and energy saving. It is mainly used in the treatment of bronchitis, bronchospasm, asthma, rhinitis and other fields.
Owner:弘毅天承知识产权股份有限公司
Method of mediating Airway Smooth Muscle Construction Due to Airway Irritation
InactiveUS20100048732A1Mitigating bronchospasm or airway smooth muscle constrictionDecrease of constrictionRespiratorsBiocideBronchospasmIrritation
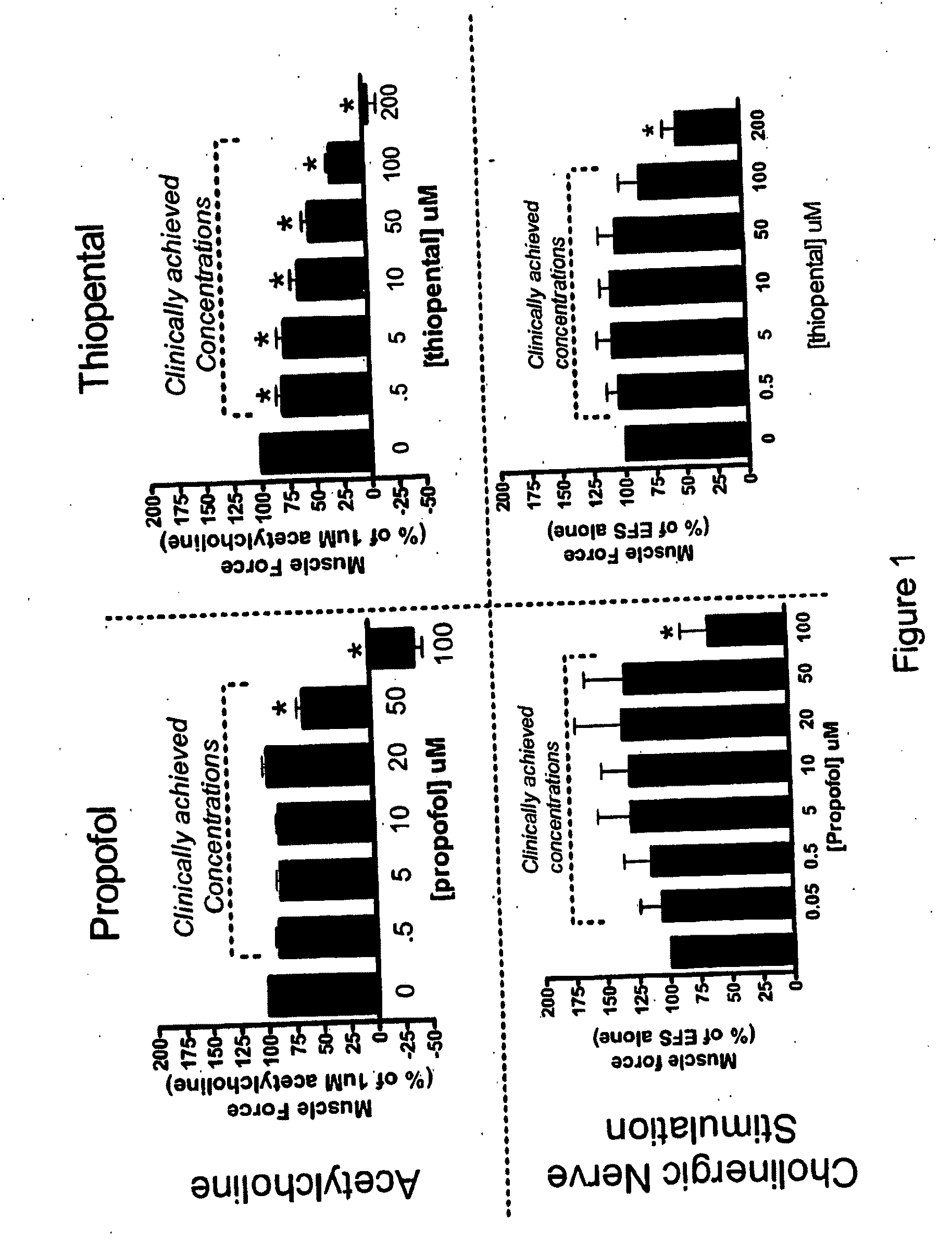
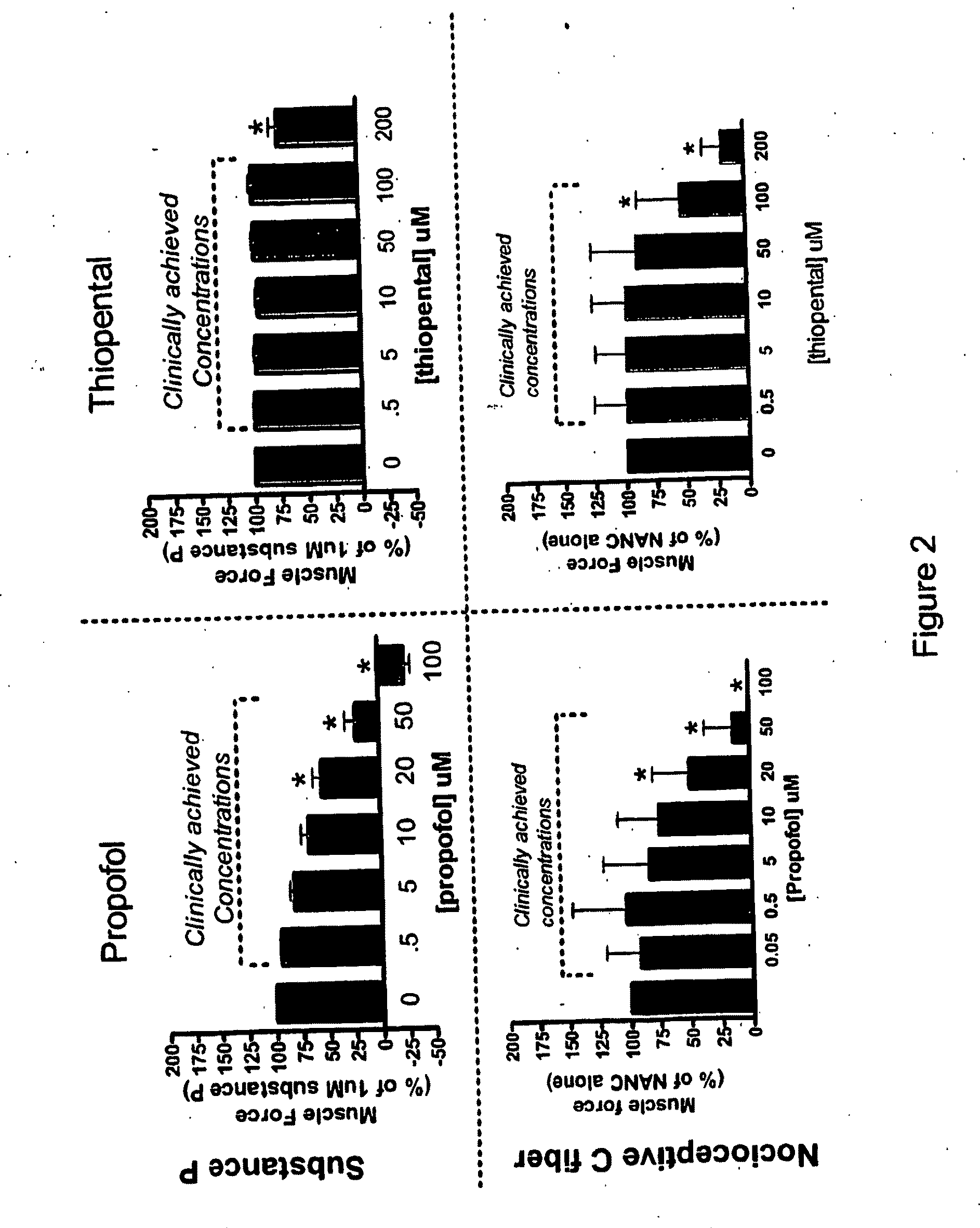
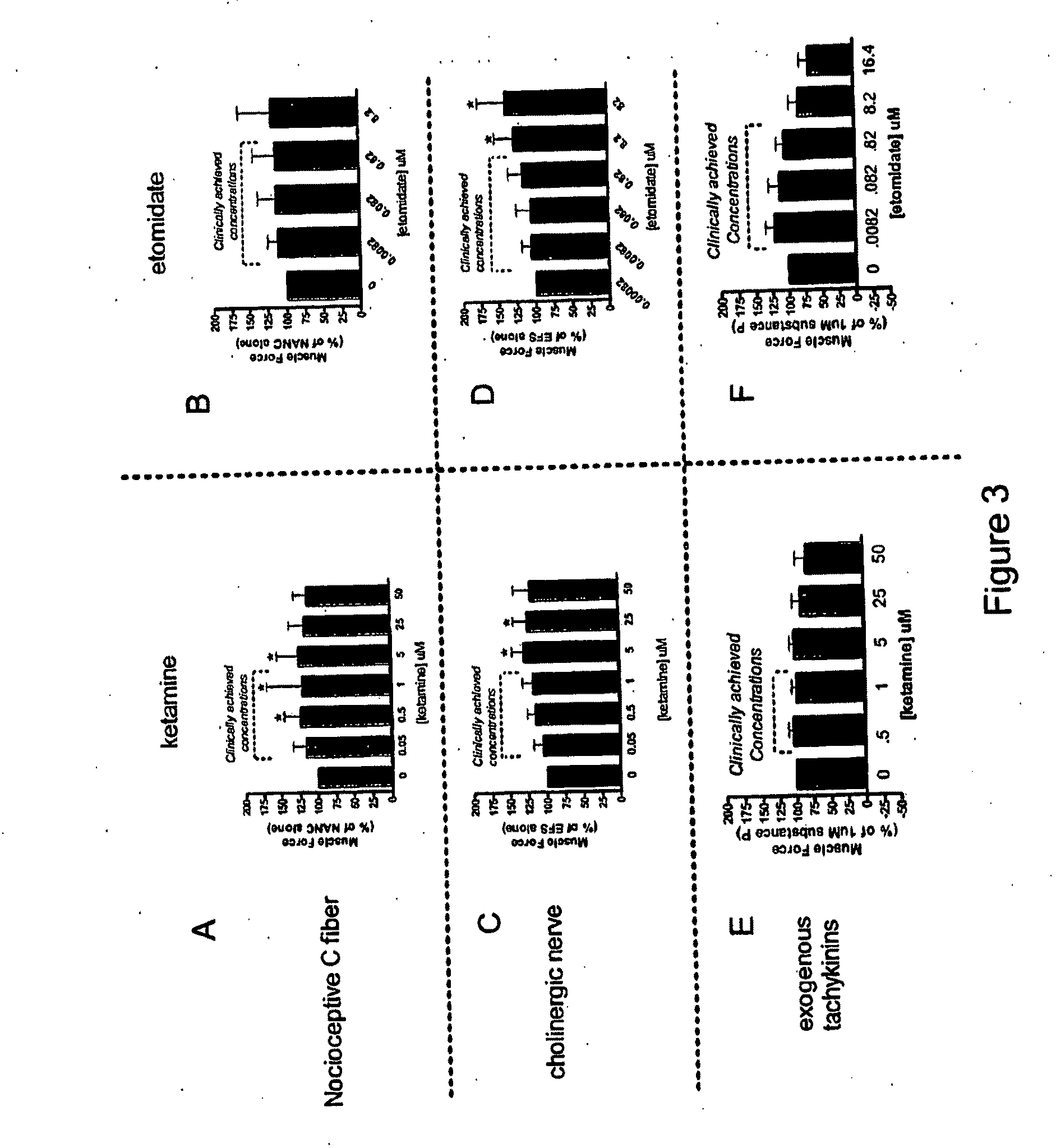
Methods of minimizing bronchospasm and contraction of airway smooth muscle due to irritation of the airway are provided. More particularly, a method is provided of mitigating bronchospasm or airway smooth muscle constriction due to irritation. This method includes administering to a subject in need of such treatment an amount of propofol or a derivative thereof effective to decrease the severity and / or duration of bronchospasm or airway smooth muscle constriction. Also provided are methods of up-regulating GABA mediated relaxation of airway smooth muscle at GABAA receptors expressed on airway smooth muscle and methods of anesthetizing a subject and minimizing bronchospasm or airway smooth muscle constriction due to irritation using propofol or a propofol derivative.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide in a 0.5 ml volume.
Owner:MYLAN SPECIALTY
Treatment of acute exacerbation of asthma and reduction of likelihood of hospitalization of patients suffering therefrom
InactiveUS20110117213A1Promote respirationReduce hospitalization ratesBiocideAnimal repellantsBronchospasmIntensive care medicine
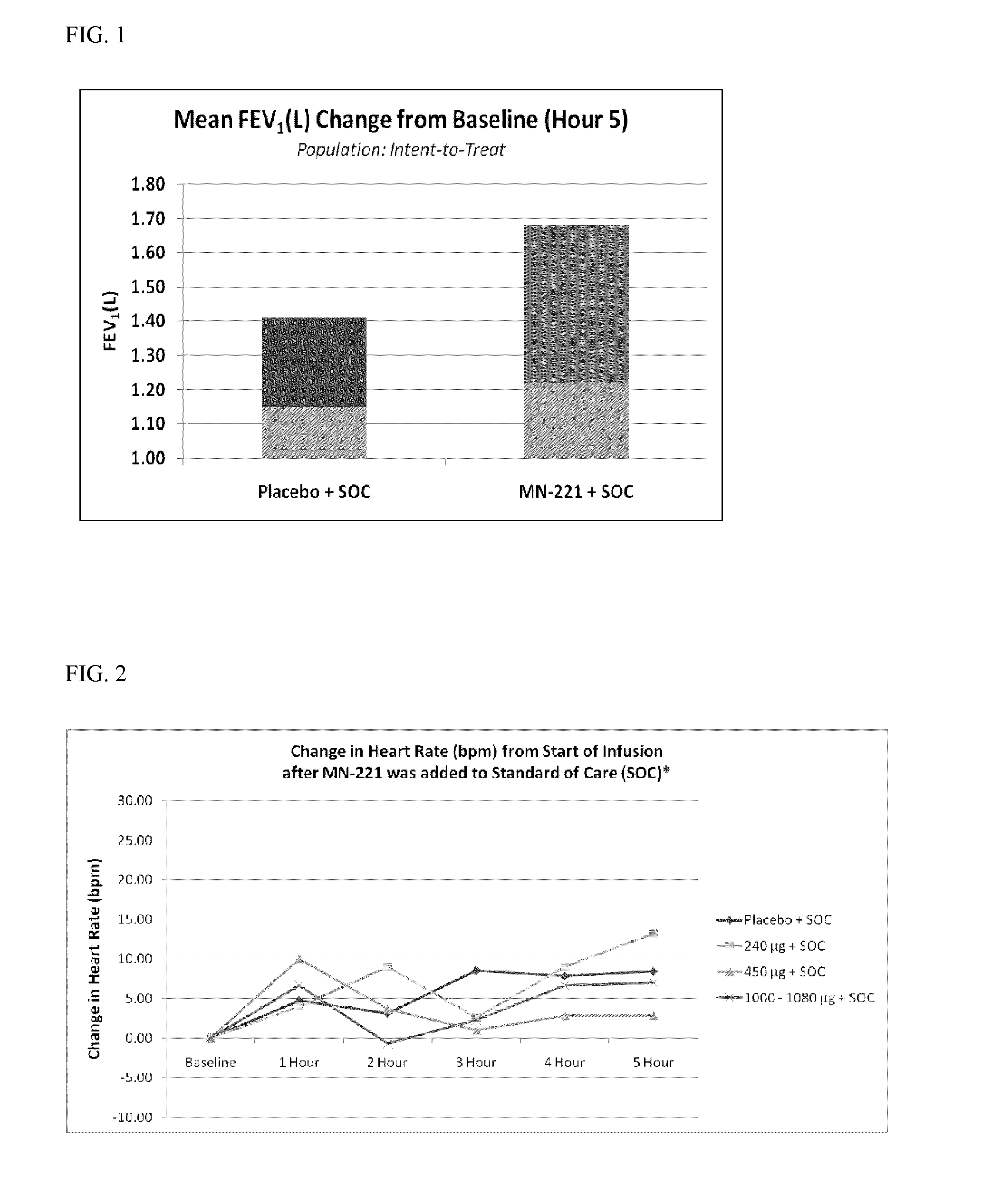
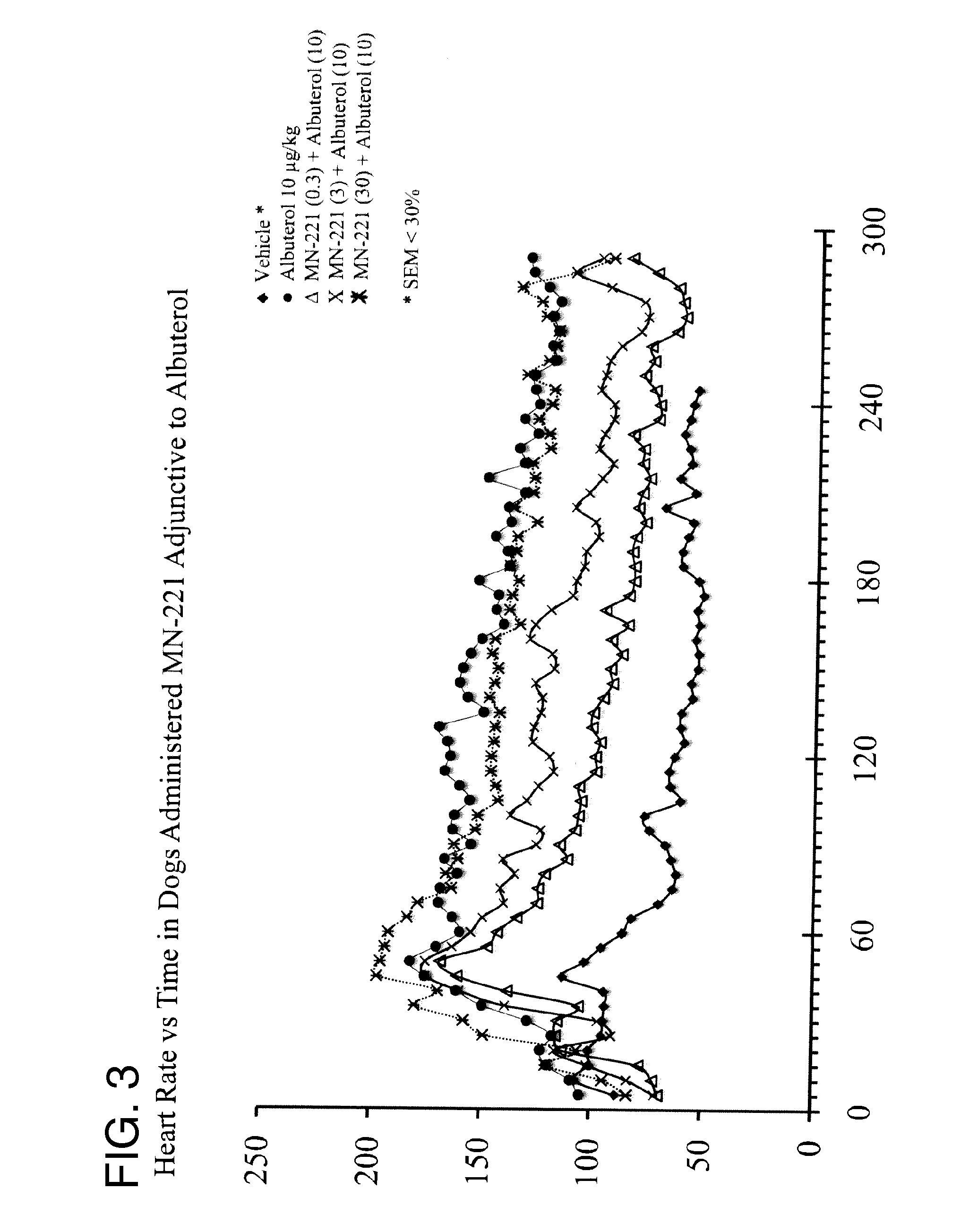
The invention provides a method of improving one or more clinical outcomes of an individual experiencing an acute respiratory attack. The acute respiratory attack may include acute reversible bronchospasm, severe acute bronchospasm, or acute exacerbation of asthma. The method includes administering to an individual suffering from an acute respiratory attack an effective amount of bedoradrine or a pharmaceutically acceptable salt thereof in combination with a standard of care (SOC) treatment regimen.
Owner:MEDICINOVA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com