Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65results about How to "Fast onset time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intranasal Formulation of Epinephrine for the Treatment of Anaphylaxis
ActiveUS20150005356A1Fast onset timeSuitable for intranasal useBiocidePharmaceutical delivery mechanismBronchospasmInjection epinephrine
This invention relates to pharmaceutical compositions of epinephrine for delivery to the nasal mucosa and methods of treating a subject in acute severe anaphylaxis, bronchospasm or during cardiopulmonary resuscitation (CPR). The composition further comprising agents, that either prevent localized degradation of epinephrine or enhance its absorption in the nasal mucosa to counter anaphylactic effects, symptoms or complications in a subject.
Owner:G2B PHARMA
Method for making fermenting bed for ecological pig breeding by using composite strain culture
InactiveCN101565684AShort degradation cycleStrong bacterial activityFungiBacteriaMicroorganismStart time
The invention discloses a method for making a fermenting bed for ecological pig breeding by using composite strain culture, in particular relates to a method for making the fermenting bed by using a plurality of microorganisms. The invention comprises the steps of preparing the culture and making the fermenting bed. In the invention, the culture is mainly prepared by microzyme, lactobacillus, actinomycetes, photosynthetic bacterium and mycelial fungus according to proportion. The culture after being activated is stirred uniformly with organic padding pro rata, piled, fermented, prepared into the fermenting bed, and then can be used for breeding live pigs. The method solves the problems of long fermenting starting time of the organic padding, unstable microbial inoculum, strict requirements on the fermenting conditions and materials as well as short service life in the existing ecological pig breeding method. The method uses the composite strain culture to build ecological colony house; the fermenting starting time of the organic padding is short, which can be 1 to 2 days in summer and 3 to 5 days in winter; the strain activity is strong; the decomposing process can be finished in 1 to 3 days; the effect lasts for long; and the fermenting bed can be continuously used for 5 to 8 years. Moreover, the method is green and environmental friendly.
Owner:千智伟
Clopidogrel and salt submicron emulsion injection thereof as well as preparation method of same
InactiveCN102697724AFast onset timeMedication convenienceOrganic active ingredientsEmulsion deliveryDrugEmulsion
The invention belongs to the technical field of medicine and relates to clopidogrel and salt submicron emulsion injection thereof as well as a preparation method of the clopidogrel and the salt submicron emulsion injection thereof. Particularly, the invention provides clopidogrel, salt submicron emulsion injection including clopidogrel, salt, oil for injection, assistant emulsifier, isoosmotic adjusting agents, pH adjusting agents and water for injection, and a preparation method of the clopidogrel and the salt submicron emulsion injection. 100 mL of the injection comprises clopidogrel, 0.05 g to 0.15 g salt (measured according to clopidogrel), 5 g to 30 g of oil for injection, 0.5 g to 3.4 g of emulsifier, 0 g to 1 g of assistant emulsifier, 2.0 g to 4.0 g of isoosmotic adjusting agents, and 7.0 g to 9.0 g of pH adjusting agents, and 100 mL of water for injection is added. The clopidogrel and the salt submicron emulsion injection thereof, provided by the invention, have the physical chemical property conforming to the requirement of drug for intravenous use and are suitable for clinical application.
Owner:SHENYANG PHARMA UNIVERSITY
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671AAccelerate time to peak blood concentrationImprove in vitro dissolutionOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method of preparing quick-acting jiuxin orally disintegrating tablet through 3D printing
ActiveCN105362320AWork quicklyTake fastAdditive manufacturing apparatusHydroxy compound active ingredientsOrally disintegrating tabletComputer printing
The invention provides a method of preparing a quick-acting jiuxin orally disintegrating tablet through 3D printing. The disintegrating tablet is formed by spraying a bonder through a nozzle of a 3D printer to bond medicinal powder, the medicinal powder comprises, by mass, 76-85% of, 12-18% of and 2-6% of, and 3D printing parameters include spraying radius of 2.5-10mm, spraying layer height of 0.1-0.5mm and spraying layer number of 10-50 layers. Heating is not needed in the preparation process, so that loss of active ingredients, such as borneol, which are unstable when being heated in conventional preparation methods is avoided; the disintegrating tablet prepared is high in medicine release speed, content of active ingredients meets requirements of the Chinese Pharmacopoeia, and the disintegrating tablet is especially suitable for acute diseases such as coronary heart disease and angina.
Owner:GUANGDONG PHARMA UNIV
Traditional Chinese medicine composition for treating thrombophlebitis and preparation method thereof
ActiveCN101618161AUniform particle sizeWill not destroy active ingredientsAnthropod material medical ingredientsBlood disorderThrombophlebitisFiltration
The invention discloses a traditional Chinese medicine composition for treating thrombophlebitis. The invention also discloses a preparation method of the traditional Chinese medicine composition. The preparation method of the traditional Chinese medicine composition comprises the following steps: taking three kinds of animal class medicinal powder of leech, centipede and scorpio which are processed by a superfine pulverization technique and have the average particle diameter smaller than the fineness of 75mum and medicinal materials of which volatile oil is extracted, adding water firstly, extracting the volatile oil, taking the medicinal materials after extracting the volatile oil and other medicinal materials which are extracted by using the water, adding the water, decocting, extracting, and preparing water extractive paste by a water extract through filtration, concentration, ethanol transfer dissolution and concentration.
Owner:LUNAN HOPE PHARM CO LTD
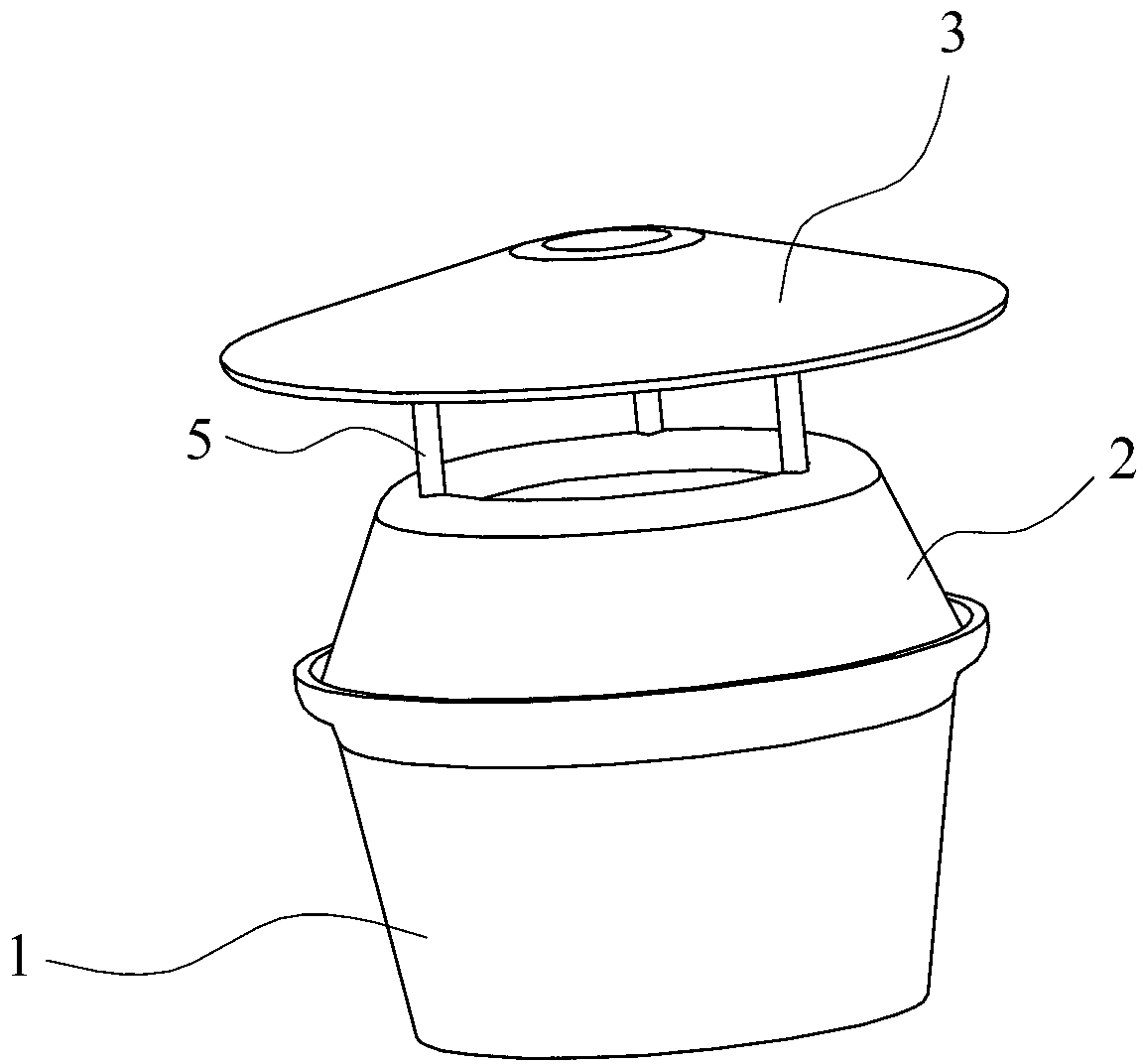
Agricultural land snail trapping and killing device and agricultural land snail trapping and killing method
InactiveCN102960321ASimple structurePrevent escapeInsect catchers and killersAgricultural landEnvironment effect
The invention relates to an agricultural land snail trapping and killing device, which comprises a barrel-shaped container with an upward opening, wherein an inverted-U-shaped barrel cover is arranged at the opening of the barrel-shaped container and is provided with a center hole. The invention simultaneously relates to an agricultural land snail trapping and killing method using the trapping and killing device, which comprises the steps of putting a trapping and killing agent into the bottom of the barrel-shaped container and then burying the barrel-shaped container of the trapping and killing device in soil, wherein the trapping and killing agent comprises a trapping material and a killing material. Compared with the prior art, the trapping and killing device disclosed by the invention has the advantages of simple structure and low cost; and the trapping and killing method using the trapping and killing device has the advantages that the environment is less influenced, a good trapping and killing effect is achieved and field snails can be steadily trapped and killed for long time.
Owner:NINGBO ACAD OF AGRI SCI
Functional biochar-based nutrition medium for preventing and treating root rot and preparation method
ActiveCN107056409AFast growthHigh amount of laccaseFungiGrowth substratesTrametes trogiiTrichoderma asperellum
The invention discloses a functional biochar-based nutrition medium for preventing and treating a root rot and a preparation method. The functional biochar-based nutrition medium is characterized by being prepared from the following components in parts by weight: 10-30 parts of a forestry and agricultural waste carbonized material, 0.1-1 part of trichoderma asperellum CGM-10 conidial powder of which the preservation number is CCTCC No. M 2014001, 10-20 parts of a trametes trogii SEM-6 bacteria solution of which the preservation number is CCTCC No. M 2014002, 0-10 parts of humic acid, 0-50 parts of municipal dry sludge, 0-50 parts of dry biogas residue and 0-20 parts of plant ash; and the components are mixed evenly and baked until the moisture content is 20-30% to obtain the functional biochar-based nutrition medium for preventing and treating the root rot. The functional biochar-based nutrition medium has the effects of obviously promoting plant growth and improving the performance of soil, can be used as a fertilizer, a nutrient medium, nutrient soil and the like, and has wide application value in the aspect of agricultural crop planting.
Owner:ENERGY RES INST OF SHANDONG ACAD OF SCI
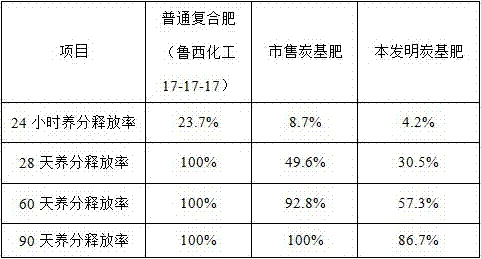
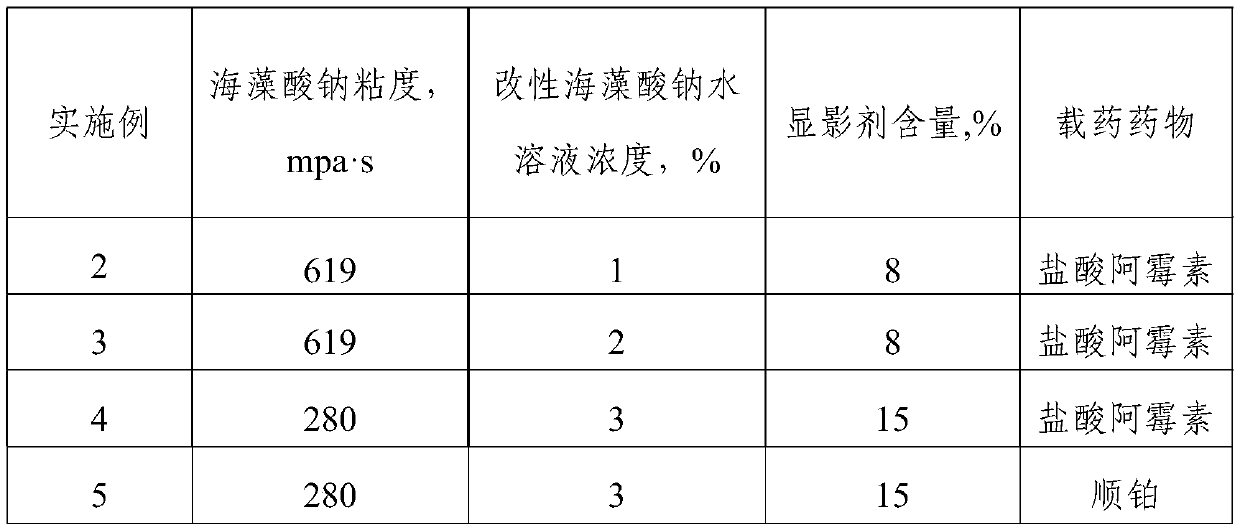
Modified sodium alginate self-developing embolization microsphere and preparation method and application thereof
ActiveCN111481734AImprove drug loading and drug loading rateDelayed release timeSurgical adhesivesPharmaceutical delivery mechanismTherapeutic embolizationDrug loading dose
The invention relates to the technical field of biological medicines, and particularly discloses a modified sodium alginate self-developing embolization microsphere and a preparation method and application thereof. The modified sodium alginate self-developing embolization microsphere comprises modified sodium alginate and a developing agent, wherein the modified sodium alginate is sodium alginatewhich is firstly subjected to branching modification to introduce a plurality of hydroxyl groups and then subjected to hydrophobic modification and sulfonation modification, and the branching substitution rate of branching modification is 22-81%; the hydrophobic substitution rate of the hydrophobic modification is 5-32%; and the sulfonation substitution rate of sulfonation modification is 57-266%.When the embolization microsphere disclosed by the invention is used as a drug carrier, the drug loading capacity and the drug loading rate of a negatively charged drug can be remarkably improved, the drug release time can be delayed, and the burst release of the drug is reduced.
Owner:NKD PHARMA CO LTD
Lidocaine microemulsion-based gel and preparation method thereof
InactiveCN107595766AIncrease contentIncrease the amount of solubilizationHydroxy compound active ingredientsAerosol deliverySurgical operationSide effect
The invention discloses a lidocaine microemulsion-based gel. The lidocaine microemulsion-based gel comprises the components of, by mass, 2-6% of lidocaine, 0-0.8% of menthol, 3.75% of an oil phase, 8.75% of a surfactant, 82.1% of distilled water, 0.1% of a preservative and 0.4% of carbomer 980. A preparation method of the lidocaine microemulsion-based gel comprises the steps that the lidocaine andthe menthol are dissolved in the oil phase under 70 DEG C, and then the surfactant is added; then the distilled water is added drip by drip during stirring to form microemulsion; the preservative isadded; and after a mixture is subjected to still standing and cooled to the room temperature, the carbomer 980 is spread, and after full swelling, stirring, degassing and sterilization are conducted,the lidocaine microemulsion-based gel is obtained. The lidocaine microemulsion-based gel is a noninvasive local anesthetic, the toxic and side effects, caused by injection, for a whole body can be avoided, and the lidocaine microemulsion-based gel can be used for skin surgical operations, cosmetology, micro-plastic surgeries and the like.
Owner:三禾生物工程(广州)有限公司
Hydroxytyrosol pellets and preparation method thereof
InactiveCN102166195AControl releaseImprove bioavailabilityHydroxy compound active ingredientsAntinoxious agentsHydroxytyrosolSolubility
The invention relates to a simple, convenient and easy preparation method of hydroxytyrosol pellets. The invention comprises a technology and preparation method which avoids the problem of difficult form and bad stability of hydroxytyrosol in prior technology and provides a novel hydroxytyrosol preparation, pellets. The invention adopts a micronization technology of crushing the embedded hydroxytyrosol powder into micro powder, the average grain diameter of the micro powder is in general less than 15um, and the micro powder is mainly distributed in the range of 5-20um, so as to improve the active principle absorptivity and the bioavailability; in addition, the invention can raise the internal dissolvability of the phloretin greatly by using water-soluble adhesive and excipient, so as to improve bioavailability of the preparation and accelerate the initial time to become effective. The invention brought in pellets technology which developed rapidly in medical field, can produce the pellet-type product with controllable release in vivo and high bioavailability so as to carry out the preparation and pave the road for hydroxytyrosol application study.
Owner:天津市尖峰天然产物研究开发有限公司
Gamuzhuer medicinal latex preparation and preparation method thereof
InactiveCN102648922AHigh drug contentEasy to administerHeavy metal active ingredientsHydroxy compound active ingredientsPreservativeGlycerol
The invention provides a Gamuzhuer medicinal latex preparation and a preparation method thereof. The Gamuzhuer medicinal latex preparation comprises the following components by weight percentage: 10-40% of Gamuzhuer, 0.1-8% of bioadhesive agent, 0.1-5% of non-ionic emulsifier, 1-30% of glycerol, 0-30% of grease and the balance of water. The bioadhesive material is bond with the Gamuzhuer, so thatmedicines are dispersed and adhered to a focus part so as to achieve a therapeutic aim; the Gamuzhuer latex preparation has a higher content, is more convenient to administer and is not added with any irritant solvent; the acting time is prolonged; during preparation, a proper amount of emulsifier is added, so that dissolution of musk and borneol can be promoted; compared with the conventional powdery preparation, the latex preparation takes effect more quickly; a proper amount of grease and a proper amount of emulsifier are added to achieve a good lubricating effect; the grain diameter of the medicinal powder is optimized, so that 99 percent of the powder is smaller than 100 um and thus the stability of the medicinal powder is improved; and the medicines have antibacterial and antisepticfunctions, so that a preservative is not required.
Owner:SHENZHEN JIAXUAN MEDICAL TECH DEV +1
Method for preparing Weigan Futai capsule for treating liver complaint
InactiveCN1868504AShort disintegration timeAdd qualitative identificationDigestive systemAntiviralsActive componentCurative effect
A process for preparing the Chinese medicine 'Weiganfutai capsule', an improvement to its tablet, features that the extracts of schisandra fruit and Taihe chicken are dried in vacuum condition and the ginsenoside and conk polyose are dried by spray for retaining their active components to maximum.
Owner:上海祥鹤药业有限公司
Compound traditional Chinese medicine superfine powder dispersion preparation and preparation method thereof
ActiveCN102961582AWon't breakUniform particle sizeDigestive systemFood preparationVegetationCurative effect
The invention discloses a compound traditional Chinese medicine superfine powder dispersion preparation and a preparation method thereof. The compound traditional Chinese medicine superfine powder dispersion preparation has the effects of supplementing Qi and nourishing Yin, and restoring pulse and promoting the secretion of saliva or body fluid. A superfine powdering technology is adopted to crush three vegetation medicinal materials, namely, ginseng, radix ophiopogonis and fructus schisandrae, in order to overcome the defects of a conventional crushing technology and a conventional preparation technology; and the industrialization of the crushing fineness superfine powdering technology for medicines of preparation and the superfine powdering technology of traditional Chinese medicines can be realized. According to the compound traditional Chinese medicine superfine powder dispersion preparation and the preparation method thereof, the superfine powder pulse activating dispersion preparation is directly used as medicine in form of superfine powder of traditional Chinese medicines, so that the loss of effective components can be avoided, the absorption of the medicine and the bioavailability can be improved, the effect is obviously improved, and the clinical effect is improved; and as the clinical effect inspection test shown, the superfine powder pulse activating dispersion preparation is superior to the pulse activating products processed by a general crushing technology and a traditional preparation technology.
Owner:LUNAN PHARMA GROUP CORPORATION
Eye drop for treating xerophthalmus and preparation method of eye drop
ActiveCN105012404AAvoid damageIncrease irritationSenses disorderInorganic boron active ingredientsEye dropBorax
The invention discloses an eye drop for treating xerophthalmus and a preparation method. The eye drop is prepared from the following raw materials and auxiliary materials in parts by weight: 130-170 parts of an extract containing bear gall powder, 1.5-3.3 parts of borneol, 1-2 parts of a bacteriostatic agent, 6-10 parts of a solubilizer, 40 parts of sodium borate and 10 parts of boric acid. According to the physical and chemical properties of chemical components contained in the medicinal material in the formula, use of volatile oil is removed; the dosage of the borneol and the bear gall powder is adjusted; the borneol and the bear gall powder are added in different manners; muddy opalescence and the like caused by a conventional bear gall powder adding method are reduced; the medicinal composition is ensured; meanwhile, the preparation directly acts on the medication part; the dosage is greatly reduced; the onset time of the medicine is quickened; and the curative effect is improved.
Owner:SHANDONG JINHE DRUG RES DEV
Scopolamine transdermal patch and preparation method thereof
InactiveCN102038664AFast onset timeShorten the dissolution timeDigestive systemPharmaceutical non-active ingredientsPressure sensitiveDrug
The invention provides a scopolamine transdermal patch, composed of a back lining, a drug layer and an anti-sticky layer which are sequentially laminated, wherein the drug layer comprises scopolamine, skin penetration enhancer, pressure-sensitive adhesive and polyacrylic resin in the weight ratio of 15: (16-64): (150-420): (50-100). The invention also provides a preparation method of the scopolamine transdermal patch. The invention has the advantages that the scopolamine transdermal patch is simple in structure, has quick effect and can be pasted one hour before taking vehicle transportation, thus reducing pasting time for patients; and the preparation method is simple, thus reducing production time, improving production capacity and reducing energy consumption.
Owner:蚌埠丰原涂山制药有限公司
Candesartan cilexetil double-release capsule and preparation method thereof
InactiveCN102885810APromote dissolutionShort onset timeOrganic active ingredientsPharmaceutical delivery mechanismMedicineActive component
The invention relates to a candesartan cilexetil double-release capsule and a preparation method thereof, belonging to the technical field of pharmaceutical formulations and solving the technical problems of low dissolution speed of a candesartan cilexetil formulation, short effectiveness time and poor blood drug concentration continuity in the prior art. Each capsule of the candesartan cilexetil double-release capsule comprises quick release particles and slow release particles, wherein the weight ratio of the active components of candesartan cilexetil in the quick release particles and the slow release particles is 1.0: (0.5-2.0). The preparation method comprises the steps of: uniformly mixing the quick release particles and the slow release particles; filling into the capsule body of a capsule shell; and covering a capsule cover to obtain the candesartan cilexetil double-release capsule. The candesartan cilexetil double-release capsule has high dissolution speed, the effectiveness time is quickened, the stable blood drug concentration effect is realized, and the corresponding preparation method has the advantages of simplicity in process and easiness in operation.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
Common lamiophlomis root chewable tablet and preparation thereof
ActiveCN101564424ADefinite curative effectLow priceAntipyreticAnalgesicsDosage formAdditive ingredient
The invention relates to a common lamiophlomis root chewable tablet and preparation thereof. The chewable tablet is a novel dosage form of common lamiophlomis root prepared from a common lamiophlomis root, a filler, a corrigent, a lubricant, and a binder. It is proposed that the medicament is made into chewable tablets with consideration on inconvenience in taking the medicament by patient and fear of medicament admission of the aged and the children. Comparing with the ordinary tablets and capsules, the chewable tablet can be chewed and swallowed, having great disintegrating degree. The chewable tablet is not only beneficial to absorption of effective ingredients, shortening affecting time, but also better in mouth feeling comparing with ordinary tablet and capsule, making the aged and the children eager to take, thus adaptability of patient in taking the medicament is enhanced. And the chewable tablet is suitable for large scale production because of no special requirement for the apparatus.
Owner:KANGYA OF NINGXIA PHARMA
Pharmaceutical composition for treating acetyl cholinergic urticaria
ActiveCN103690555AWeak anti-allergic effectImprove anti-allergicOrganic active ingredientsDermatological disorderPharmaceutical drugTherapeutic effect
The invention belongs to the technical field of treatment medicines for urticaria, and specially relates to a pharmaceutical composition for treating acetyl cholinergic urticaria and application of the pharmaceutical composition in preparation of a medicine for preventing or treating the acetyl cholinergic urticaria, aiming at overcoming the shortage that the existing medicine for treating the acetyl cholinergic urticaria has poor treatment effect and cannot act for a long time in the prior art. The pharmaceutical composition for treating or preventing the acetyl cholinergic urticaria consists of levocetirizine and isopropylidene sedoheptuiosan. The pharmaceutical composition shows a good effect on treatment of acetyl cholinergic urticaria and is remarkably worthy of clinical popularization.
Owner:启东市清汉农副产品专业合作社
Phloretin pellet and preparation method thereof
InactiveCN102166193AImprove bioavailabilityMeet needsAntinoxious agentsKetone active ingredientsSolubilityAdhesive
The invention relates to a preparation method of a phloretin pellet, which is simple, convenient and easy to operate. The preparation method overcomes the shortcomings in the prior art, and phloretin is prepared into a pellet type product with controllable release in the body and high bioavailability, so as to better meet the demands of general consumers. In the preparation method, the phloretin is ground into fine powder by adopting micronization technology, and the average particle size of the fine powder is generally less than 15 um and mainly distributed in the range of 5-20 um. By adopting the preparation method, the absorption rate of effective ingredients is increased, and the bioavailability is improved; and by adopting water-soluble adhesive and excipient, the solubility of the phloretin in the body is greatly enhanced, and as the solubility of the micron-sized effective ingredients in the gastrointestinal tract is significantly increased, the bioavailability of the micron-sized effective ingredients is increased, and the onset time of the micron-sized effective ingredients is shortened.
Owner:天津市尖峰天然产物研究开发有限公司
Dapoxetine hydrochloride orally disintegrating tablet, preparation method thereof an application of orally disintegrating tablet
InactiveCN110833530AFast absorptionEasy to useOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletTraditional medicine
The invention discloses a dapoxetine hydrochloride orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet comprises dapoxetine hydrochloride, fillers, disintegrating agents, dried binding agents, penetration promoting flavoring agents and lubricating agents. The orally disintegrating tablet is prepared by a direct tablet pressing method. The orally disintegrating tablet has the advantages that the orally disintegrating tablet is simple in preparation process, low in cost and convenient to take and has high effect on male premature ejaculation. The orally disintegrating tablet contains 0.1%-2% of penetration promoting flavoring agents, the taste of the orally disintegrating tablet is improved, stability of the orally disintegrating tablet is improved, absorption of the orally disintegrating tablet is further improved, the maximum plasma concentration (Cmax) is achieved within 0.5-1 hour after the orally disintegrating tablet is taken, effect taking time becomes earlier, and bioavailability is remarkably improved.
Owner:盖天力医药控股集团制药股份有限公司
A kind of biological deodorant and preparation method thereof
ActiveCN111514740BGood removal effectFast onset timeGas treatmentDispersed particle separationBiotechnologyOrganic synthesis
Owner:BEIJING SHUANGLONG AMMS TECH +1
Processing method for improving drug effect of tendril-leaved fritillary bulb powder and product and use
InactiveCN108014258AIncreased peak concentrationAverage dwell time increasesPowder deliveryPharmaceutical non-active ingredientsPhosphateTherapeutic effect
The invention discloses a processing method for improving drug effect of tendril-leaved fritillary bulb powder and a product and use, and belongs to the field of medicinal material processing. The processing method for improving the drug effect of the tendril-leaved fritillary bulb powder in preventing and treating of bronchial diseases such as cough and asthma is disclosed based on innovation ofmodern traditional Chinese medicine processing method starting from the theory and practice of the traditional Chinese medicine. The processing method comprises the following steps in turn: taking fresh tendril-leaved fritillary bulb, washing, chopping, adding of Vc, Vc sodium, Vc phosphate and tea polyphenols as protective agents, coarse crushing, enzymatic hydrolysis, ultrafine crushing, low temperature microwave vacuum drying, fine crushing and the like. The method is easy to operate and has a short production cycle. The dissolution of active substances is increased, the loss of active ingredients is reduced, bioavailability is improved, and the tendril-leaved fritillary bulb powder has better drug and treatment effect for the bronchial diseases such as cough and asthma. At the same time, the tendril-leaved fritillary bulb powder is uniform in color, good in smell, and aseptic, and the method has a broad application prospect in the processing area of the tendril-leaved fritillary bulb powder.
Owner:四川德仁堂中药科技股份有限公司
Pharmaceutical composition for treating acute urticaria
InactiveCN103585160AGood synergyImprove anti-allergicOrganic active ingredientsImmunological disordersAcute urticariaSide effect
The invention discloses pharmaceutical composition for treating acute urticaria and an application of the pharmaceutical composition to preparation of drugs for preventing or treating the acute urticaria, and aims at overcoming the defect that drugs for treating the acute urticaria are poor in effect and non-sustainable in action at present, the pharmaceutical composition for preventing or treating the acute urticaria is low in treatment cost, convenient to take by a patient, quick in curative effect and free of side effects and comprises levocetirizine and triptolide. The pharmaceutical composition has a good treatment effect in treating the acute urticaria and obvious clinical promotional values.
Owner:李伟丽
Traditional chinese medicine compound for relieving eczema, and slow-release agent as well as preparation method and application
InactiveCN112546101APerformance changePromote dissolutionAerosol deliveryOintment deliveryRelease timeDissolution
The invention relates to the technical field of traditional chinese medicines, in particular to a traditional chinese medicine compound for relieving eczema, and a slow-release agent as well as a preparation method and application. The traditional chinese medicine compound is prepared from the following raw materials of scutellaria baicalensis, coptis chinensis, divaricate saposhnikovia root and atractylodes lancea. According to the traditional chinese medicine compound for relieving eczema, and the slow-release agent as well as a preparation method and application, after a traditional chinesemedicine is nanocrystallized, the bioavailability of the medicine can be improved, the onset time of the medicine is advanced, and the medicine effect of the original medicine is enhanced; a nano-scale molecular material is used as a carrier material to carry the medicine, so that the medicine is adsorbed or coated in the nano-carrier, the release time of the medicine can be delayed, and the medicine can be automatically released at a preset speed within preset time; and by increasing the dissolution rate of active ingredients of the traditional chinese medicine, the variety of pharmaceuticaldosage forms can be increased, and the development of multiple products such as gel paste, mist and emulsion is facilitated; and by using a proper gel carrier, the traditional chinese medicine compound can be promoted to permeate into corresponding epidermal cells to enter targets, the medicine effect for relieving eczema is improved, and the unstable medicine effect, caused by uneven dissolution, for relieving eczema is reduced.
Owner:河南缘时商贸有限公司
Method for making pollution-free ecological pig-raising fermentation bed
InactiveCN106718965AShort degradation cycleStrong bacterial activityAnimal housingPhotosynthetic bacteriaPollution
The invention relates to a method for making a pollution-free ecological pig-raising fermentation bed. The method is characterized by including the steps of preparing a fermenting agent and making the fermentation bed. The fermenting agent is prepared by mixing saccharomycetes, lactic acid bacteria, actinomycetes, photosynthetic bacteria and filamentous fungi in a certain ratio, and then mixed evenly with organic filler in a matching ratio after being activated, the fermentation bed is made after stacking fermentation is completed, and then pigs can be raised. The method solves the problems that in an existing ecological pig-raising method, time spent by the organic filler on starting the fermentation is long, bacterium agents are not stable, requirements for fermenting conditions and raw materials are strict and the service life is short. According to the method, the composite strain fermenting agent is used for constructing an ecological pig house, time spent by the organic filler on starting the fermentation is short, that is, 2-3 days are spent in summer and 3-5 days are spent in winter, the strains are high in activity, the degradation process can completed within 3-5 days, the effect is lasting, and the fermentation bed can be used continuously for 6-10 years, and is green and environmentally friendly.
Owner:刘振宇
Acetylcysteine granule and preparation method thereof
ActiveCN112206209AImprove stabilityNo pollution in the processOrganic active ingredientsPharmaceutical non-active ingredientsMentholFlavouring agent
The invention relates to an acetylcysteine granule and a preparation method thereof. The acetylcysteine granule comprises a raw material acetylcysteine and auxiliary materials; and the auxiliary materials comprise a filler, menthol, a flavouring agent and essence, wherein the weight percentage of the menthol is 0.8%-1.5%. According to the acetylcysteine granule provided by the invention, the menthol is used; and furthermore, a fluidized bed one-step granulation process is adopted, so that the prepared acetylcysteine granule is better in stability and higher in bioavailability.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Preparation method of anti-allergy plaster
InactiveCN109758573AAnti-inflammatoryHas anti-allergic propertiesPeptide/protein ingredientsAntipyreticReflux extractionAlcohol
The invention relates to the technical field of preparation of plasters, and discloses a preparation method of an anti-allergy plaster. Coarse material in a prescription are smashed and soaked in ethyl alcohol, reflux extraction is carried out, and concentration is carried out to obtain extractum; fine materials in the prescription are smashed to obtain fine powder; 5 parts of honey is taken, heating and stirring are carried out, the heating temperature is 100-120 DEG C, when the water content in the heated honey is reduced to 3-15%, the extractum is added, heating and stirring are continued,heating is stopped until water vapor in a mixture is reduced to less than 2%, 2-3 parts of fish oil and fish roe protein by weight are added into the mixture, mixing is carried out, finally, the finepowder is added, uniform stirring is carried out, the stirred mixture is placed on non-woven adhesive tape, and the anti-allergy plaster is obtained. The anti-allergy plaster is suitable for all skins, especially for people with the sensitive skin.
Owner:孟召阳
Xiaobuling vaginal expansion suppository and preparation method thereof
ActiveCN105012335BImprove stabilityIncrease irritationAntibacterial agentsWeighing by removing componentGellan gumGynecology
The invention relates to a Xiaobuling vaginal expansion suppository and its preparation method and detection method. The expansion suppository includes a drug-containing matrix and an expansion carrier formed by nonoxynol ether, povidone iodine and the matrix; the drug-containing matrix also includes ester Calcium acyl lactate, gellan gum and heparin sodium, etc.; the expansion suppository has high stability, uniform dispersion of active ingredients, and less vaginal irritation; and the provided Xiaobuling vaginal expansion suppository adopts six original leading It has the beneficial effects of preventing the outflow of medicinal liquid, quick onset of action, and prevention of secondary infection.
Owner:哈尔滨田美药业股份有限公司
A kind of lidocaine microemulsion gel and preparation method thereof
InactiveCN107595766BIncrease contentIncrease the amount of solubilizationHydroxy compound active ingredientsAerosol deliverySurgical operationLocal anaesthetic
The invention discloses a lidocaine microemulsion-based gel. The lidocaine microemulsion-based gel comprises the components of, by mass, 2-6% of lidocaine, 0-0.8% of menthol, 3.75% of an oil phase, 8.75% of a surfactant, 82.1% of distilled water, 0.1% of a preservative and 0.4% of carbomer 980. A preparation method of the lidocaine microemulsion-based gel comprises the steps that the lidocaine andthe menthol are dissolved in the oil phase under 70 DEG C, and then the surfactant is added; then the distilled water is added drip by drip during stirring to form microemulsion; the preservative isadded; and after a mixture is subjected to still standing and cooled to the room temperature, the carbomer 980 is spread, and after full swelling, stirring, degassing and sterilization are conducted,the lidocaine microemulsion-based gel is obtained. The lidocaine microemulsion-based gel is a noninvasive local anesthetic, the toxic and side effects, caused by injection, for a whole body can be avoided, and the lidocaine microemulsion-based gel can be used for skin surgical operations, cosmetology, micro-plastic surgeries and the like.
Owner:三禾生物工程(广州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com