Iguratimod oral double-layer sustained-release preparation
A controlled-release preparation, double-layer technology, applied in anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., to achieve the effect of improving bioavailability and accelerating time to peak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
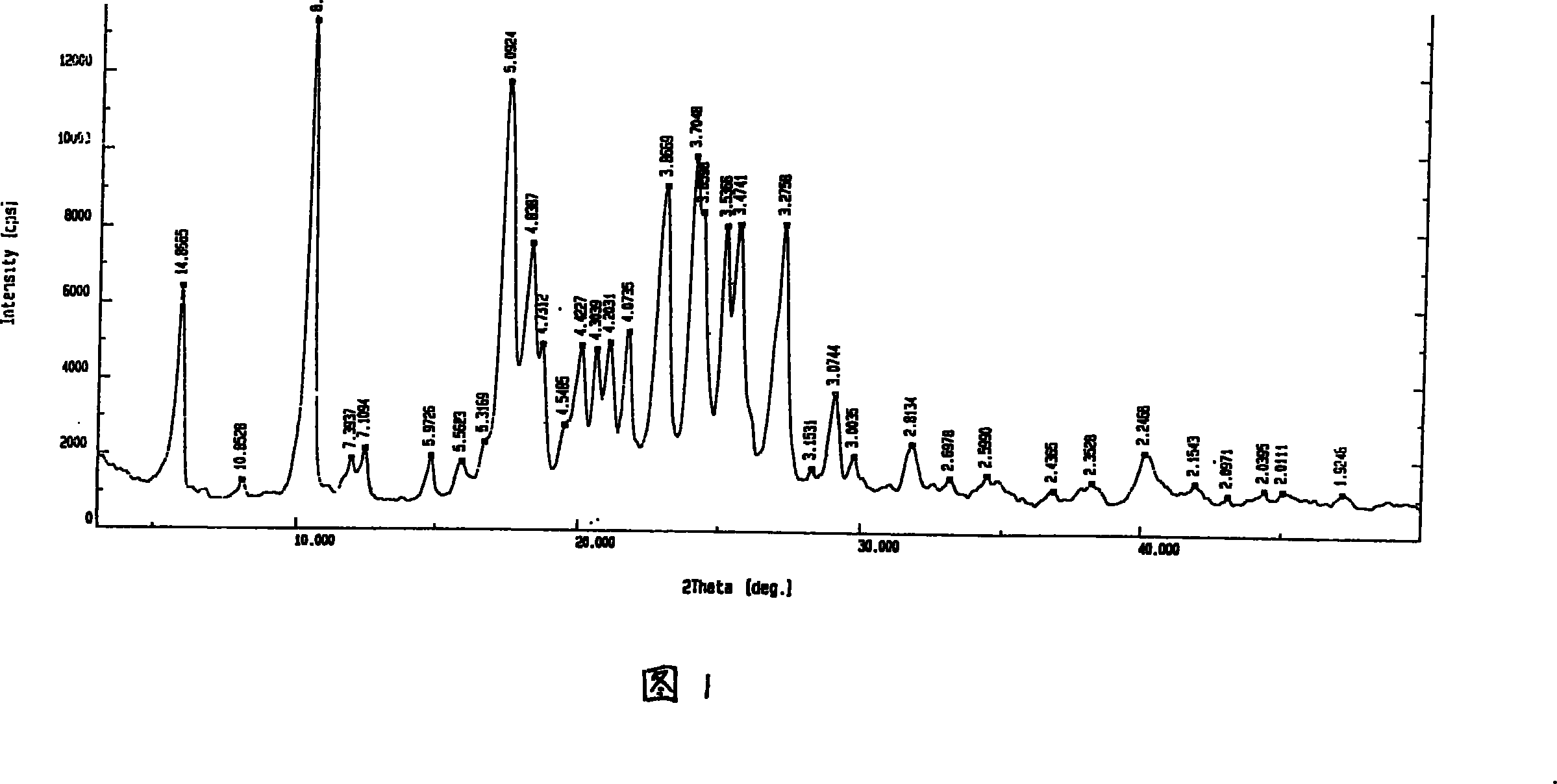
[0050] The preparation of embodiment 1 Airamod crystalline micropowder
[0051] Take 1.0g of the raw material of iguratimod and add 17.5ml of dimethylformamide to dissolve it, slowly (or drop it) into 500ml of water, add dropwise while stirring, the stirring speed is 800r / min, iguratimod will precipitate and disperse immediately In the water. Filter, stir wash three times with distilled water, filter, and dry at 60°C for 2 hours. The above-mentioned powder was taken for powder X-ray diffraction measurement and powder particle size inspection, and the average particle size of the above-mentioned powder was about 4 μm. The yield was 97.5%. The powder X-ray diffraction pattern is shown in accompanying drawing 1.
Embodiment 2
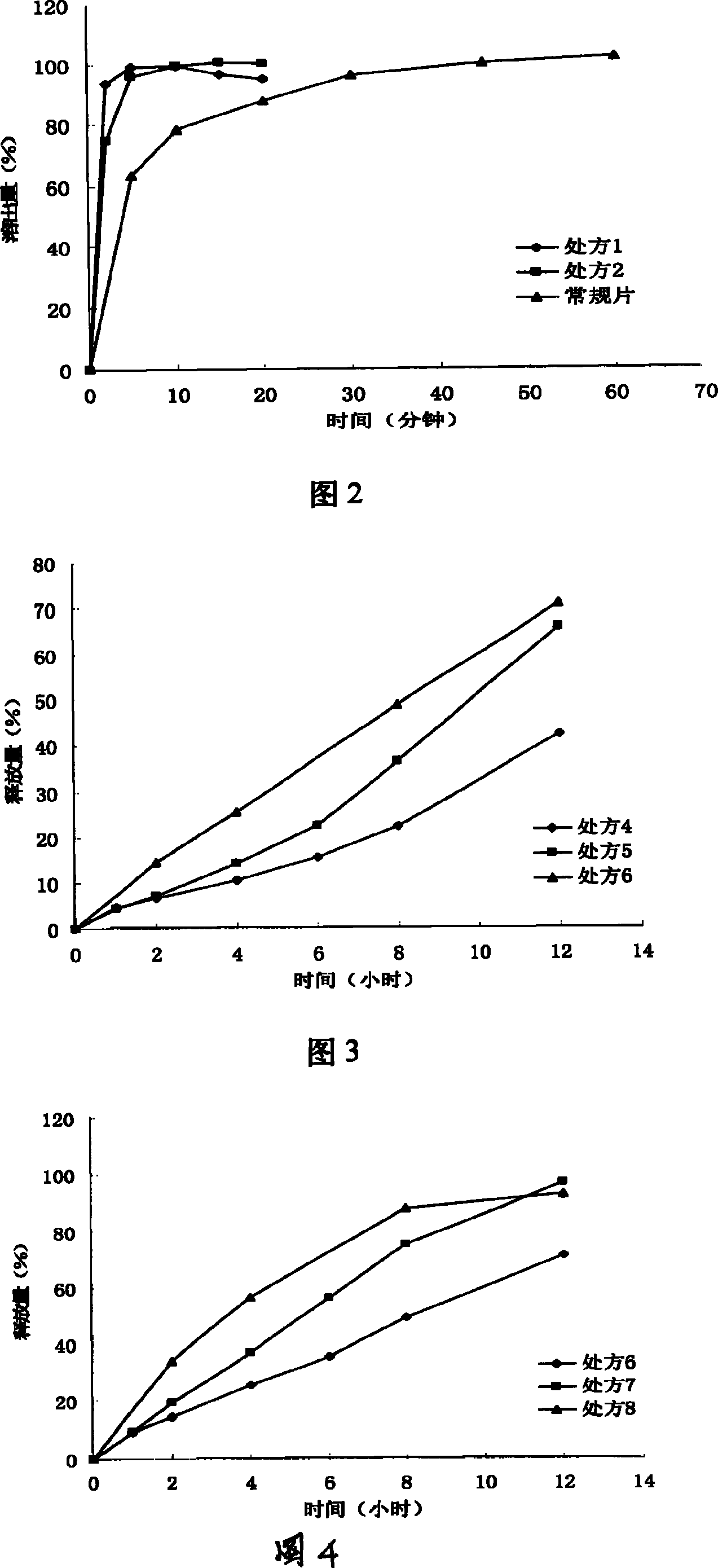
[0052] Embodiment 2 Alamode quick-release layer process investigation (1000):
[0053] prescription composition
[0054] making process:
[0055] (1) Crush the raw materials and auxiliary materials and pass through a 200-mesh sieve respectively, and set aside.
[0056] (2) In addition to povidone K30 and magnesium stearate, take the prescription amount according to the prescription, mix evenly with a 60-mesh sieve, add 10% povidone K30 solution to make soft materials, granulate with a 30-mesh sieve, and dry at 60°C for 2 Hour.
[0057] (3) Get the above granules, add the prescribed amount of magnesium stearate, and granulate through a 20-mesh sieve. Compress the tablet, control the pressure to 3-4 kg.
[0058] Dissolution Determination:
[0059] According to the requirements of the Chinese Pharmacopoeia in 2000, phosphate buffer (pH is 7.8) is used as the dissolution medium, the slurry method, the rotating speed is 100 rpm, and the ultraviolet spectrophotometric ...
Embodiment 3
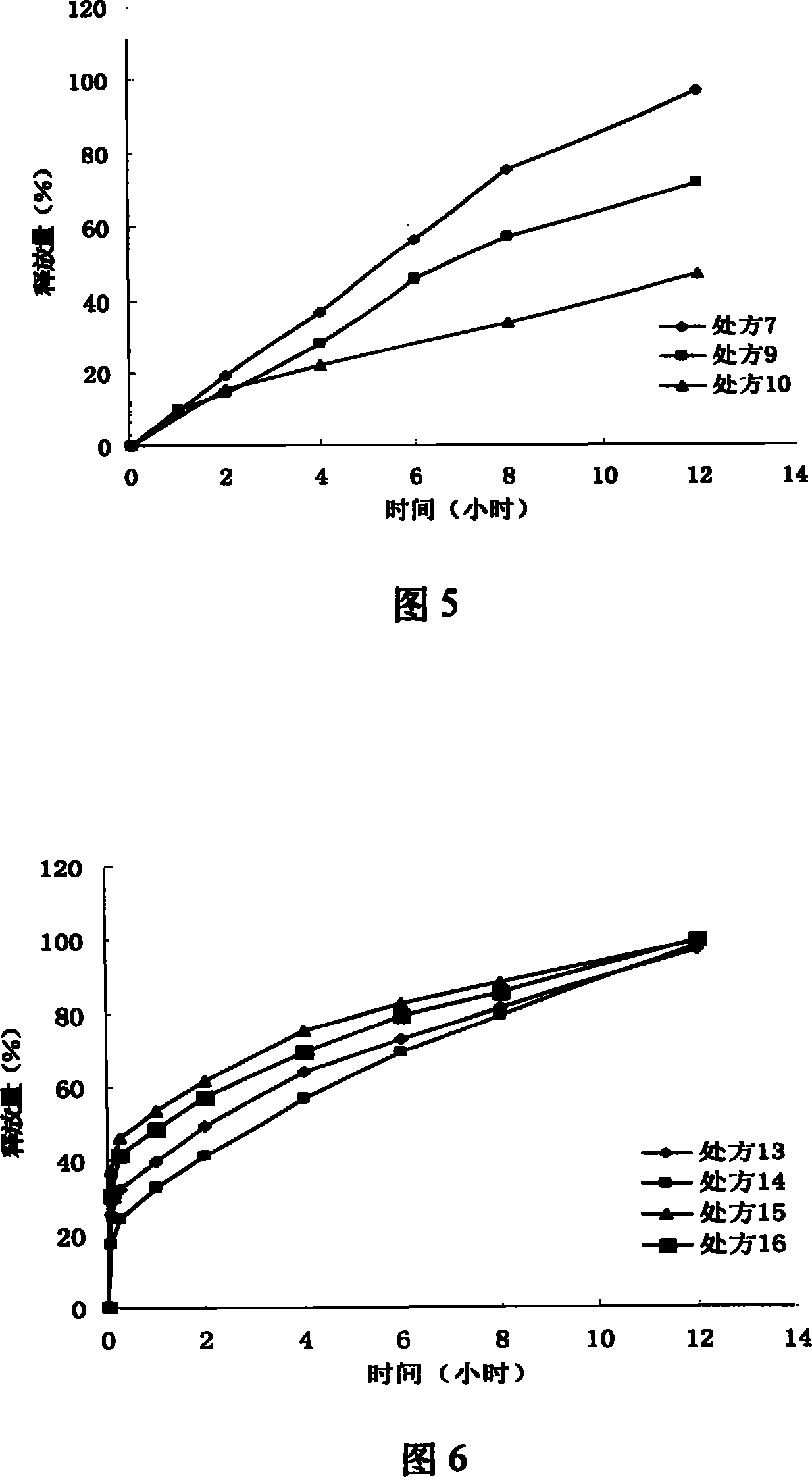
[0062] The impact of embodiment 3 different HPMC dosages on the release of iguratimod sustained-release layer (1000 tablets)
[0063] prescription composition
Prescription 4
Prescription 5
Prescription 6
Alamode micronized powder
50g
50g
50g
HPMC (K4M)
50g
35g
24g
80g
80g
80g
20g
20g
20g
Povidone K30
Appropriate amount
Appropriate amount
Appropriate amount
2%
2%
2%
[0064] Pass HPMC (K4M), lactose, and microcrystalline cellulose through a 80-mesh sieve, fully mix with micronized iguratimod, add an appropriate amount of 50% ethanol solution with a concentration of 10% povidone K30 to make a soft material, and make a 30-mesh sieve Granules, wet granules were dried at 60°C for 2 hours and sieved through a 20-mesh sieve, added magnesium stearate, mixed evenly, and compressed into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com