Superfine Iguratimod powder and quick released oral preparation
A technology of powder and speed, applied in the field of insoluble drug Iilamod crystalline micropowder, can solve the problems of fine crystals and too late to grow, and achieve the effect of reducing drug particle size, increasing surface area and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Airamod Crystalline Micropowder
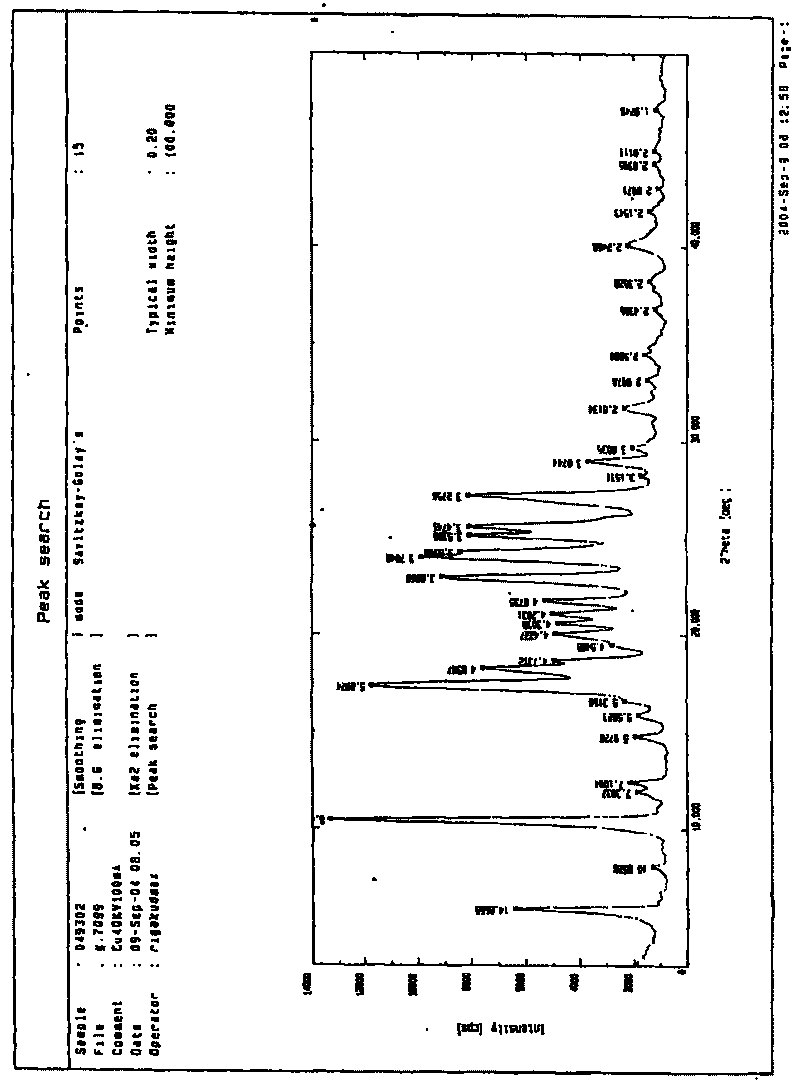
[0037] Take 1.0g of the raw material of iguratimod and add 17.5ml of dimethylformamide to dissolve it, slowly (or drop it) into 500ml of water, add dropwise and stir at the same time, the stirring speed is 800r / min, iguratimod will precipitate and disperse immediately In the water. Filter, stir wash three times with distilled water, filter, and dry at 60°C for 2 hours. The above-mentioned powder was taken for powder X-ray diffraction measurement and powder particle size inspection, and the average particle size of the above-mentioned powder was about 4 μm. The yield was 97.5%. Powder X-ray Diffraction Pattern See figure 1 .
Embodiment 2
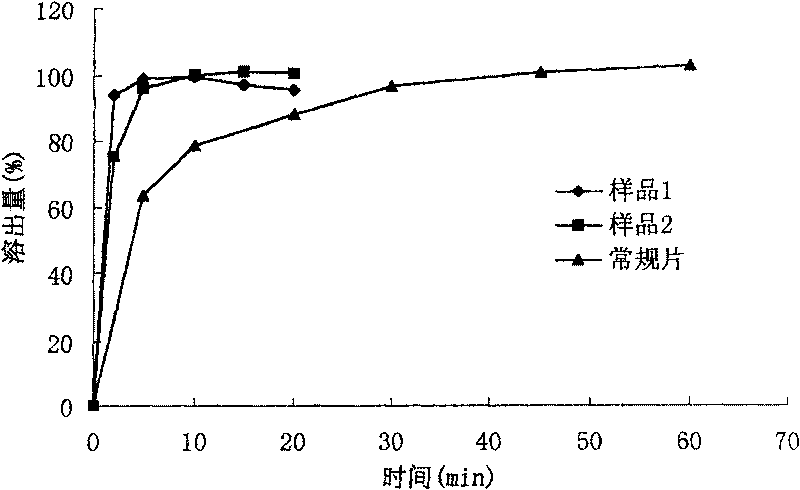
[0039] Preparation of Aguratimod Immediate Release Tablets (1000 Tablets):
[0040] Formulation composition Sample 1 Sample 2 Sample 3
[0041] Alamode Micronized Powder 25g 25g 25g
[0042] Lactose 50g 25g 50g
[0043] Microcrystalline Cellulose 50g 50g 25g
[0044] Mannitol / 37.5g /
[0045] Croscarmellose Sodium 20g 20g 24g
[0046] Sodium lauryl sulfate 4.5g 5g /
[0047] Povidone K30 appropriate amount appropriate amount
[0048] Magnesium Stearate 3g 3g 2.3g
[0049] Preparation Process:
[0050] (1) Crush the raw materials and auxiliary materials and pass through a 200-mesh sieve respectively, and set aside.
[0051] (2) In addition to povidone K30 and magnesium stearate, weigh the prescription amount according to the prescription, mix evenly with a 60 mesh sieve, add 10% PVP-K30 solution to make soft materials, granulate with a 30 mesh sieve, and dry at 60°C for 2 hours .
[0052] (3) Get above-mentioned granule, add the magnesium stearate of recipe quantity...
Embodiment 3
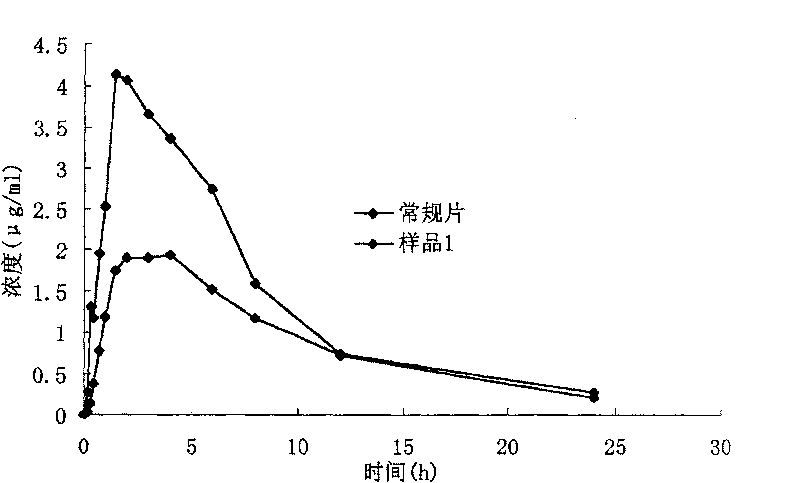
[0058] A pharmacokinetic study in Beagle dogs compared the in vivo release profile of Sample 1 with conventional tablets.
[0059] The trial employed 6 individuals and blood samples were collected at regular intervals and analyzed for iguratimod. The result is as follows:
[0060]
[0061] Results: The above pharmacokinetic parameters showed that the peak concentration (Cmax) and area under the curve (AUC) of sample 1 were significantly higher than those of conventional tablets, and the statistically significant difference was significant, and the bioavailability was significantly improved; the time to peak (Tpeak) was 1 Hours, but statistically insignificant; elimination rate constant (Ke) and elimination half-life (T 1 / 2Ke ) There was no difference between the two formulations.
[0062] The (Beagle dog) drug concentration-time curves of sample 1 and conventional tablets are shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com