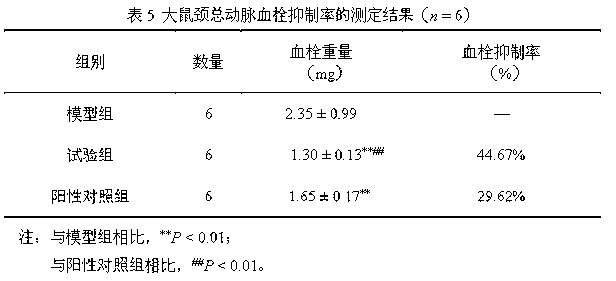
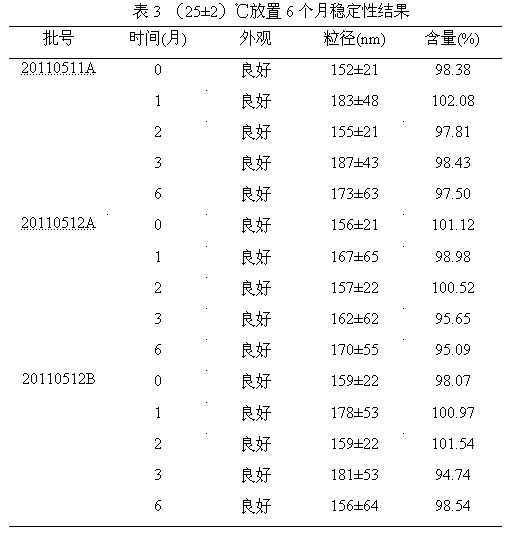
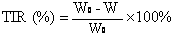Clopidogrel and salt submicron emulsion injection thereof as well as preparation method of same
A technology of clopidogrel and injection, which is applied in the field of medicine, can solve the problems such as the preparation method of clopidogrel and its salt submicroemulsion injection, and achieve the effects of avoiding and covering up bad smell, low solubility and convenient medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] prescription
[0052] Clopidogrel bisulfate (calculated as clopidogrel) 0.075 g Medium Chain Fatty Acid Triglycerides for Injection 5.0 g Soybean Oil for Injection 5.0 g egg yolk lecithin 1.2 g Poloxamer F-68 0.2 g Tween-80 0.2 g Glycerin for Injection 2.5 g Water for Injection to 100 mL
[0053] Preparation:
[0054] Step 1: Dissolve the formula amount of egg yolk lecithin in medium-chain fatty acid triglycerides for injection and soybean oil for injection, then add the formula amount of clopidogrel bisulfate, heat in a water bath to 60-80°C, and stir to make it Completely dissolved to obtain an oil phase;
[0055] Step 2: Dissolve the prescribed amount of glycerin for injection, poloxamer F-68, and Tween-80 in water for injection, heat in a water bath to 60-80°C, and stir to completely dissolve to obtain an aqueous phase;
[0056] Step 3: Mix the oil phase and the water phase at a speed of 16,000 rpm under th...
Embodiment 2
[0060] prescription
[0061] Clopidogrel bisulfate (calculated as clopidogrel) 0.075 g Medium Chain Fatty Acid Triglycerides for Injection 5.0 g Soybean Oil for Injection 5.0 g egg yolk lecithin 1.2 g Poloxamer F-68 0.2 g Tween-80 0.2 g Vitamin E 0.002 g Glycerin for Injection 2.5 g Water for Injection to 100 mL
[0062] Preparation:
[0063] Step 1: Dissolve the formula amount of vitamin E and egg yolk lecithin in medium-chain fatty acid triglycerides for injection and soybean oil for injection, then add the formula amount of clopidogrel hydrogen sulfate, heat in a water bath to 60-80°C, and Stir to dissolve completely to obtain an oil phase;
[0064] Step 2: Dissolve the prescribed amount of glycerin for injection, poloxamer F-68, and Tween-80 in water for injection, heat in a water bath to 60-80°C, and stir to completely dissolve to obtain an aqueous phase;
[0065] Step 3: Mix the oil phase and the water pha...
Embodiment 3
[0069] prescription
[0070] Clopidogrel bisulfate (calculated as clopidogrel) 0.075 g Medium Chain Fatty Acid Triglycerides for Injection 5.0 g Soybean Oil for Injection 5.0 g egg yolk lecithin 2.0 g Vitamin E 0.002 g Glycerin for Injection 2.25 g Water for Injection to 100 mL
[0071] Preparation:
[0072] Step 1: Dissolve the formula amount of vitamin E and egg yolk lecithin in medium-chain fatty acid triglycerides for injection and soybean oil for injection, then add the formula amount of clopidogrel hydrogen sulfate, heat in a water bath to 60-80°C, and Stir to dissolve completely to obtain an oil phase;
[0073] Step 2: Dissolve the formulated amount of glycerin for injection in water for injection, heat in a water bath to 60-80°C, and stir to completely dissolve it to obtain an aqueous phase;
[0074] Step 3: Mix the oil phase and the water phase at a speed of 16,000 rpm under the stirring of a high-shear disperser, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com