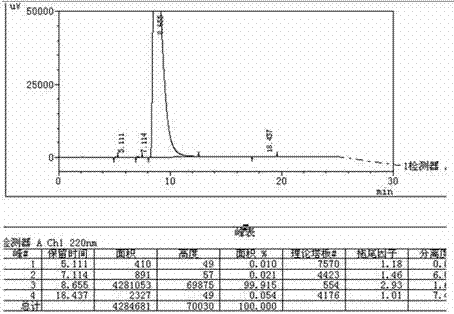
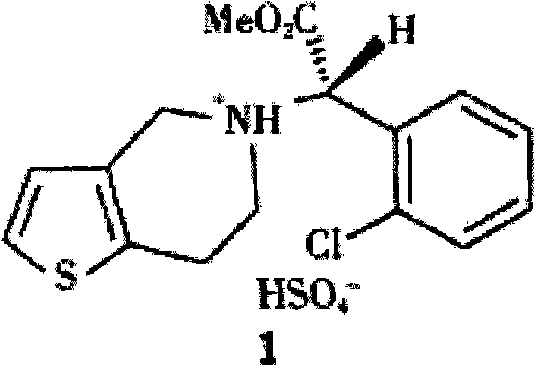
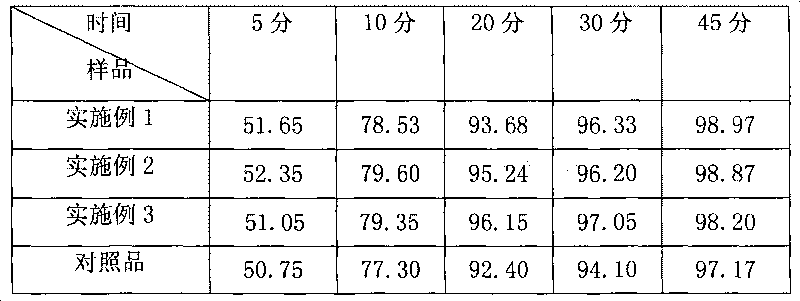
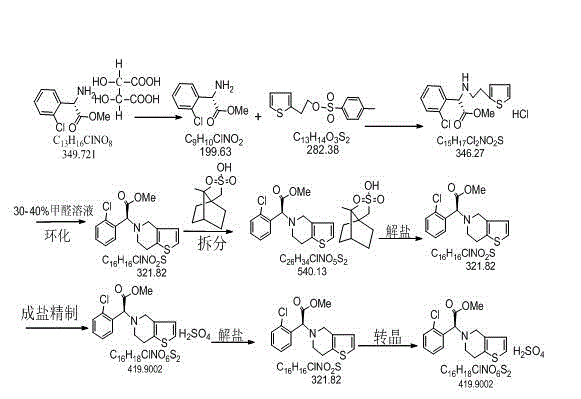
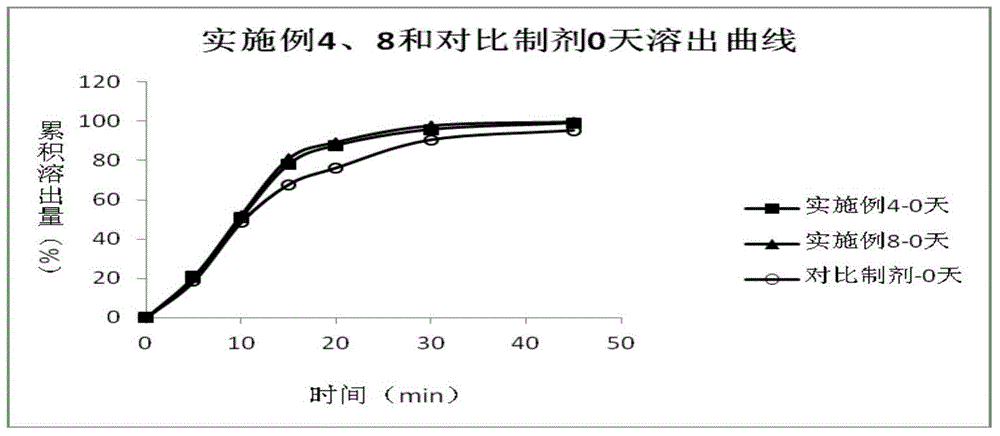
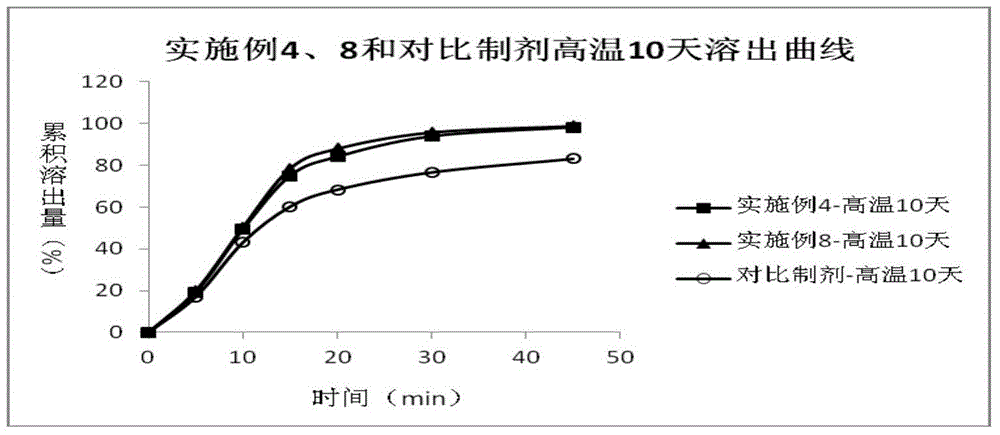
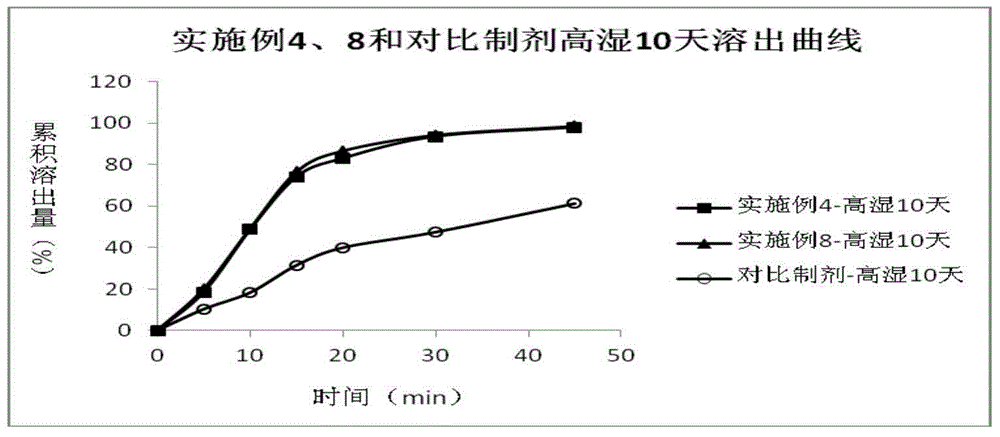
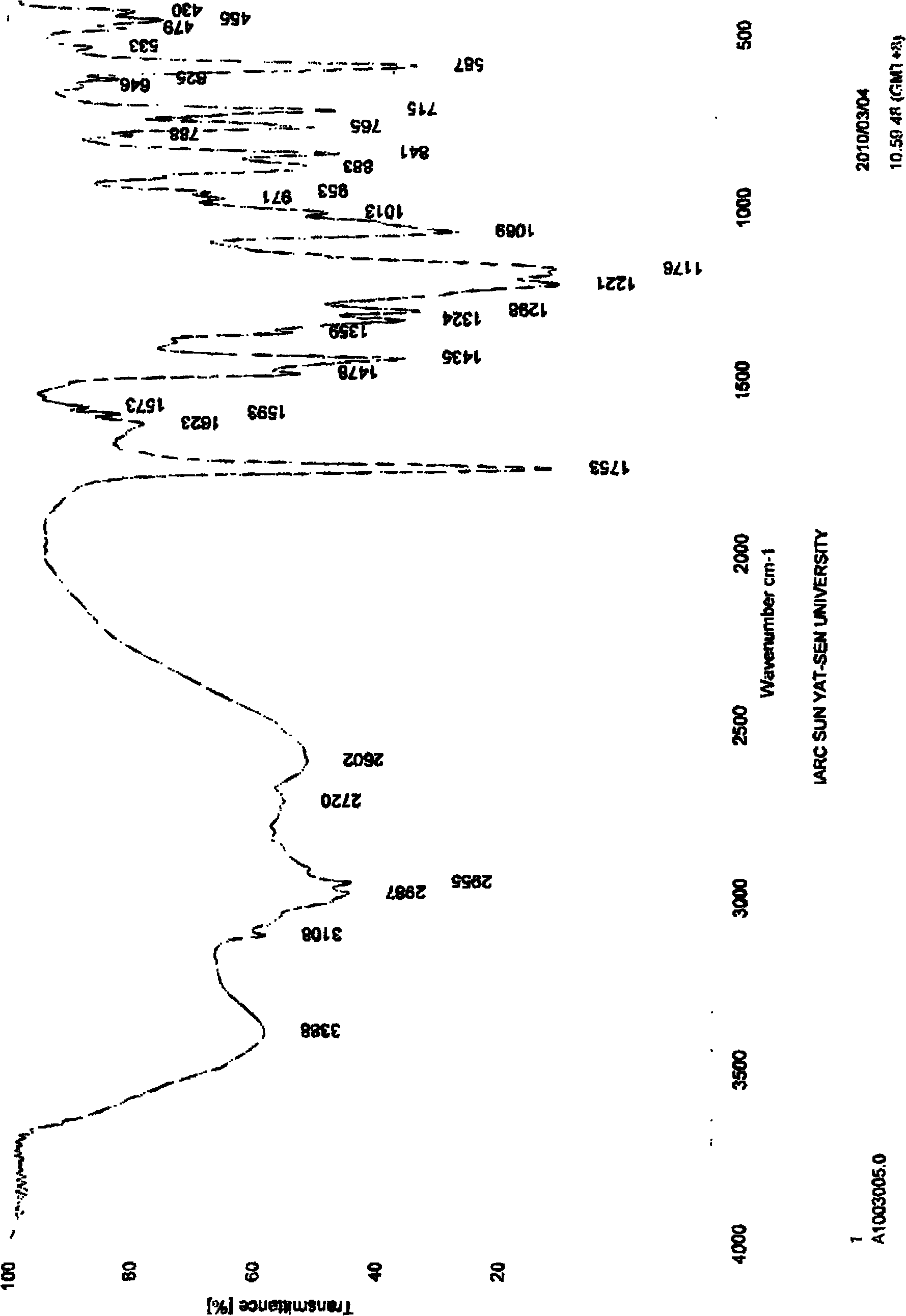
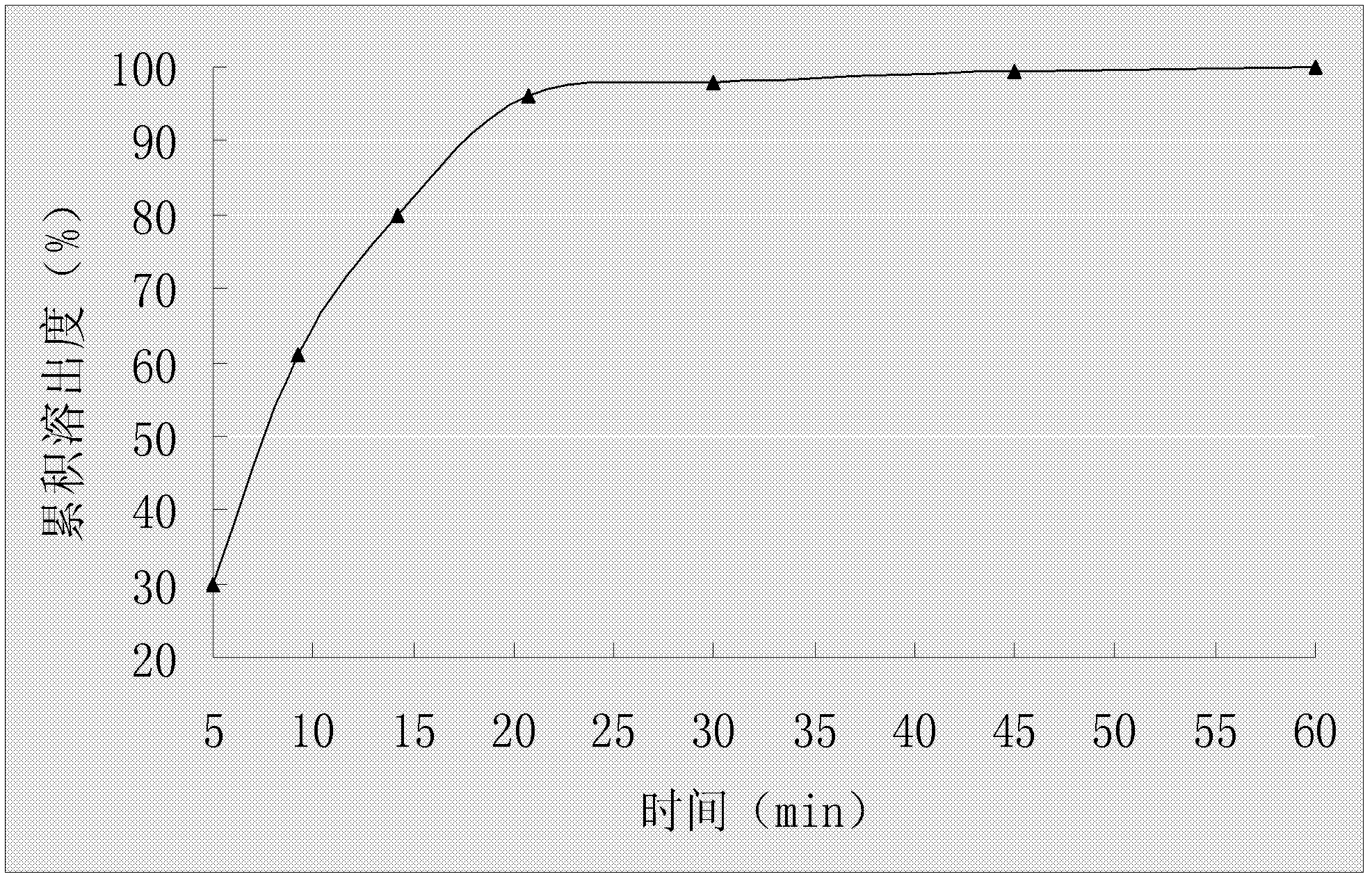Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
148 results about "Clopidogrel hydrogen sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
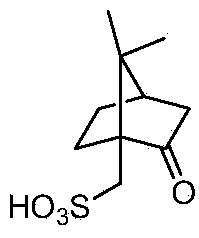
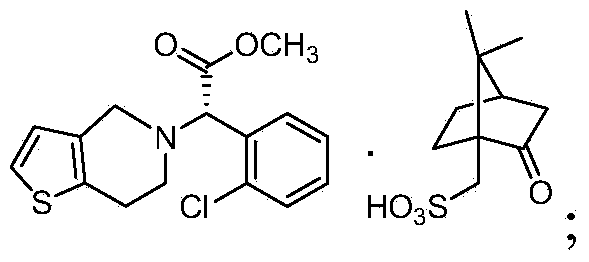
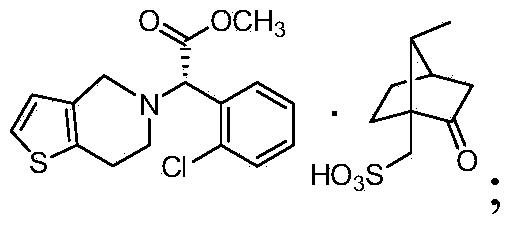
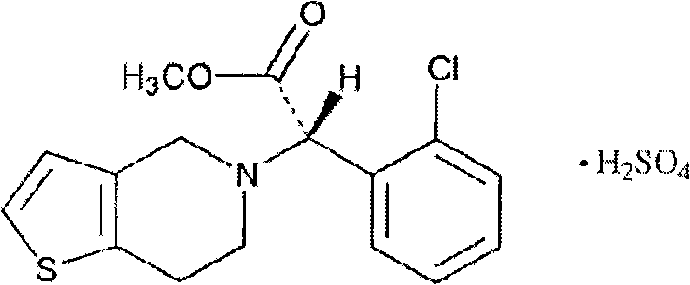
S-(+)-Clopidogrel Hydrogen Sulfate is the S enantiomer of clopidogrel and a P2Y12 receptor inhibitor.
Novel process for preparation of clopidogrel bisulfate polymorph - Form I
A process for making Clopidogrel Bisulfate Form I which comprises dissolving Clopidogrel Bisulfate Form II in a solublizing solvent at room temperature to form a solution; adding an anti-solvent to the said solution till turbid; stirring the said turbid solution; collecting the precipitated solid and drying the final solid product, form I.
Owner:SAWANT KAMLESH DIGAMBAR +2
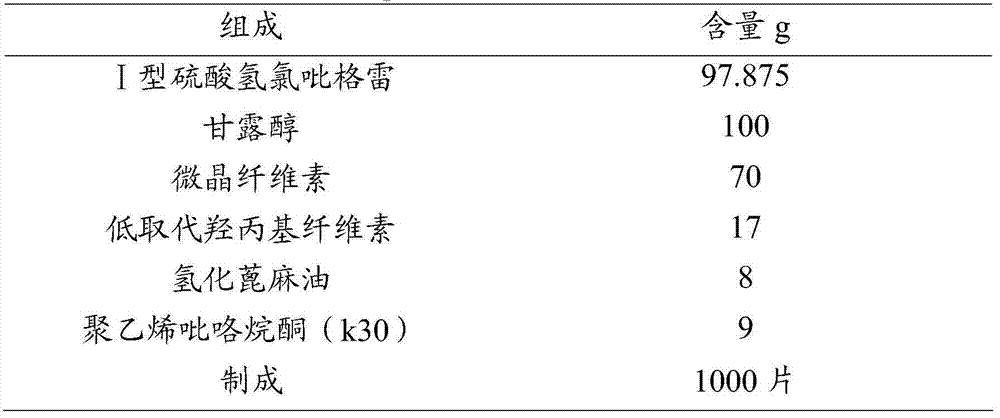
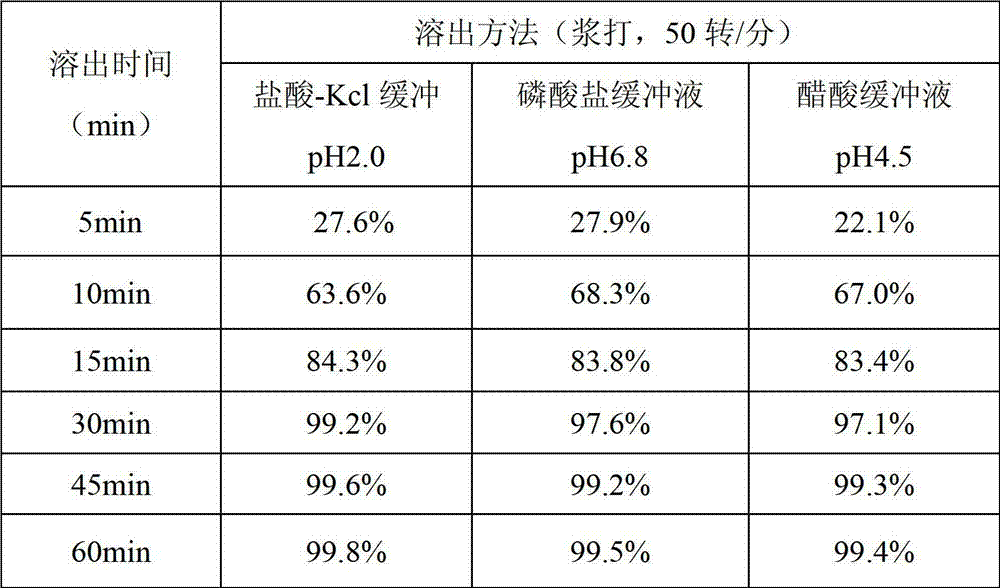
Solid medicinal composition of clopidogrel hydrogen sulfate
ActiveCN101721410AFast and efficient disintegrationFast and efficient dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsMedicineFiller Excipient
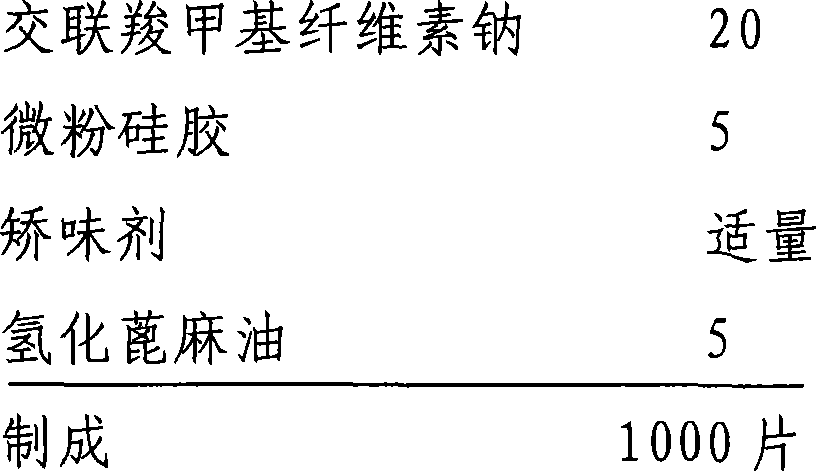
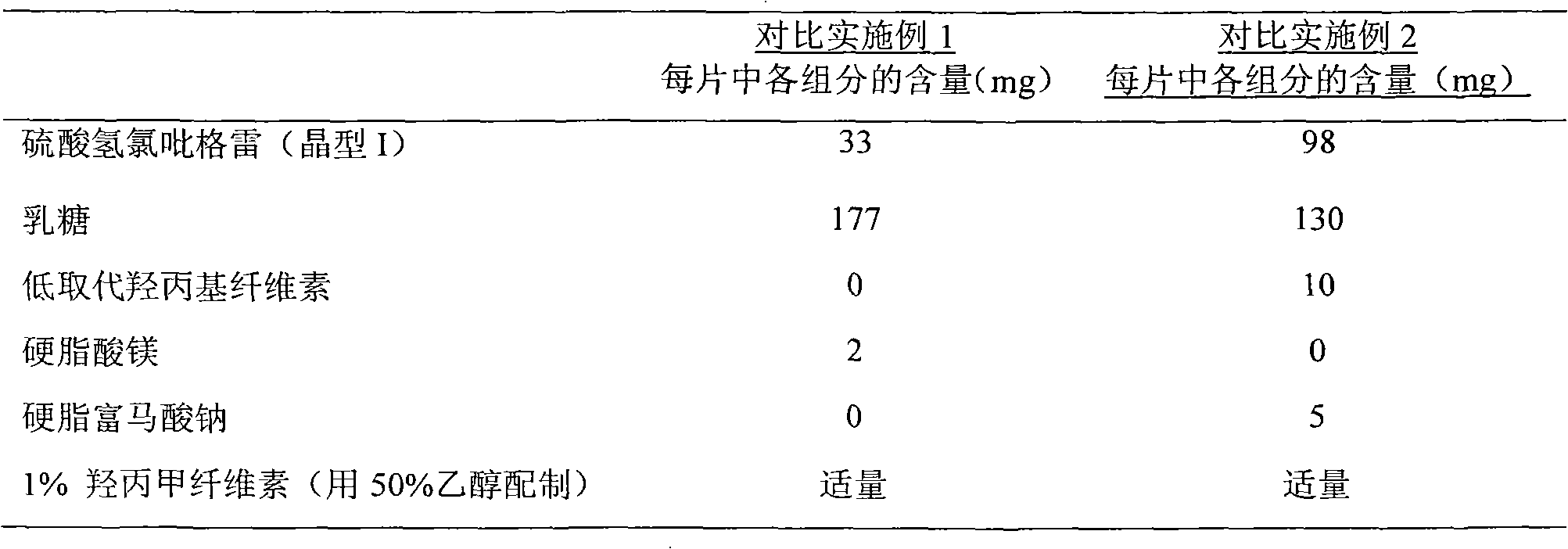
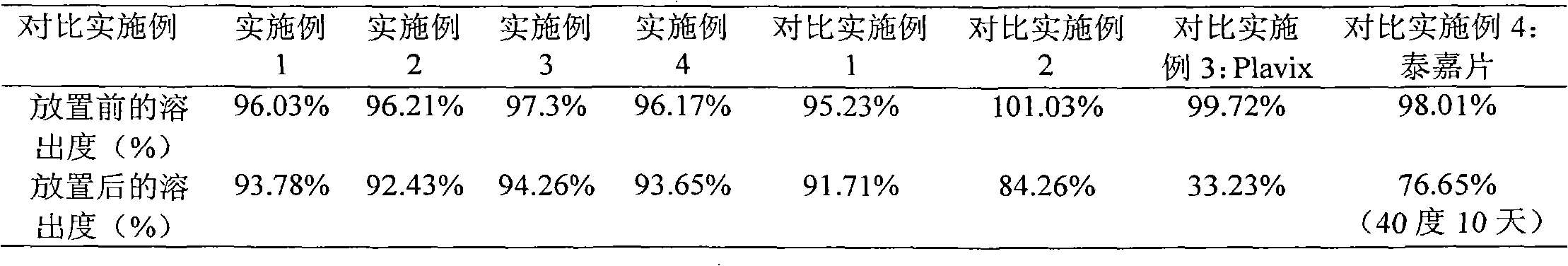
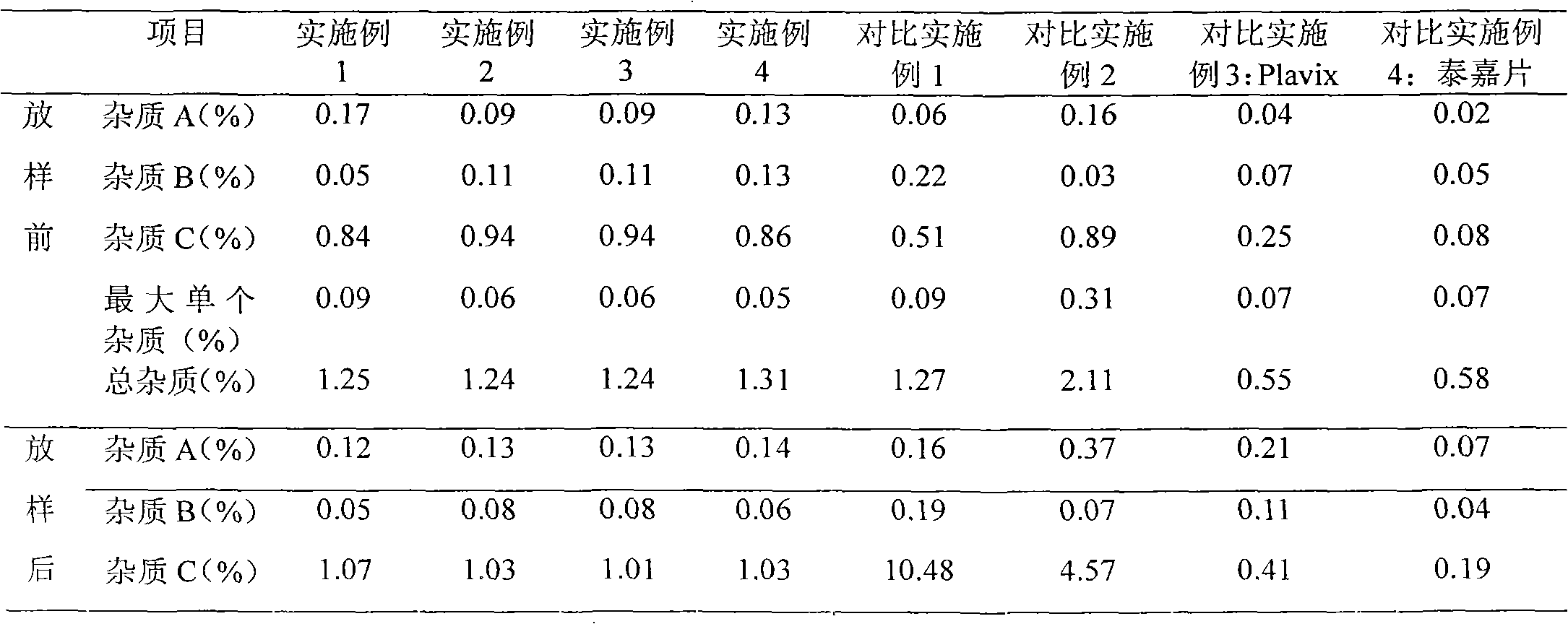
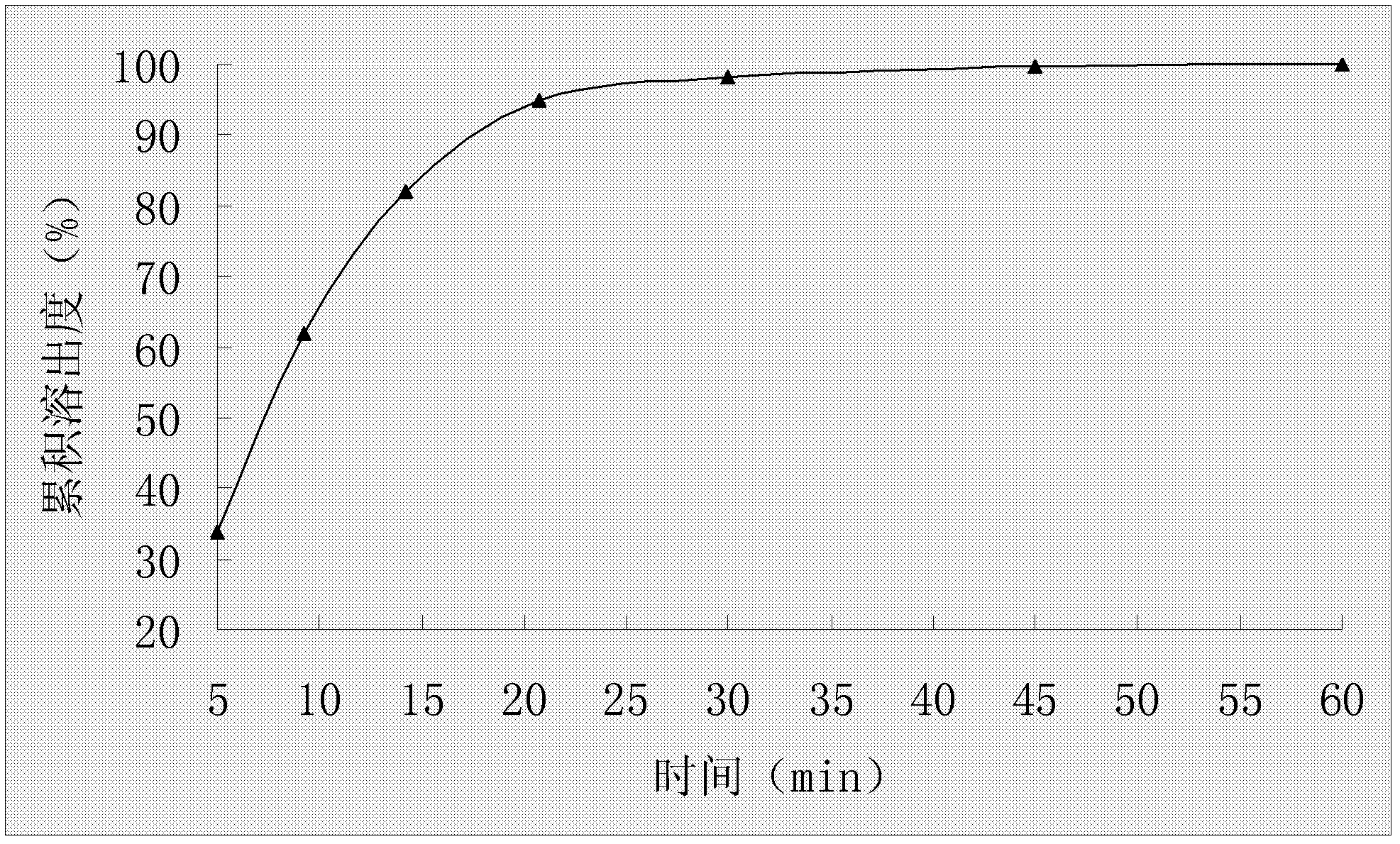
The invention relates to an oral medicinal composition containing I crystal-form clopidogrel hydrogen sulfate, polyethylene glycol and micro-powder silica gel, proper disintegrating agent and filling agent. The auxiliary materials of the composition are low in price and can be obtained easily; the preparation process is simple; the requirement for equipment and environment is not high; and the prepared tablet is stable in quality and good in reproducibility, and is applied to industrialized production. The prepared tablet has good stability in long-term storage and can be maintained stable after being placed for 6 months at the temperature of 40 DEG C, without appearance change and obvious degradation or crystal-form inversion phenomenon; the dissolution rate of the tablet is not reduced after the tablet is placed for a long time; and the dissolution rate of the tablet in 10 minutes can still reach more than 95 percent after the tablet is placed for 6 months at the temperature of 40 DEG C.
Owner:NANJING CHIA TAI TIANQING PHARMA
Clopidogrel hydrogen sulfate tablet and preparation method thereof
InactiveCN101590023AImprove stabilityImprove securityOrganic active ingredientsPill deliveryButylated hydroxyanisoleVitamin C
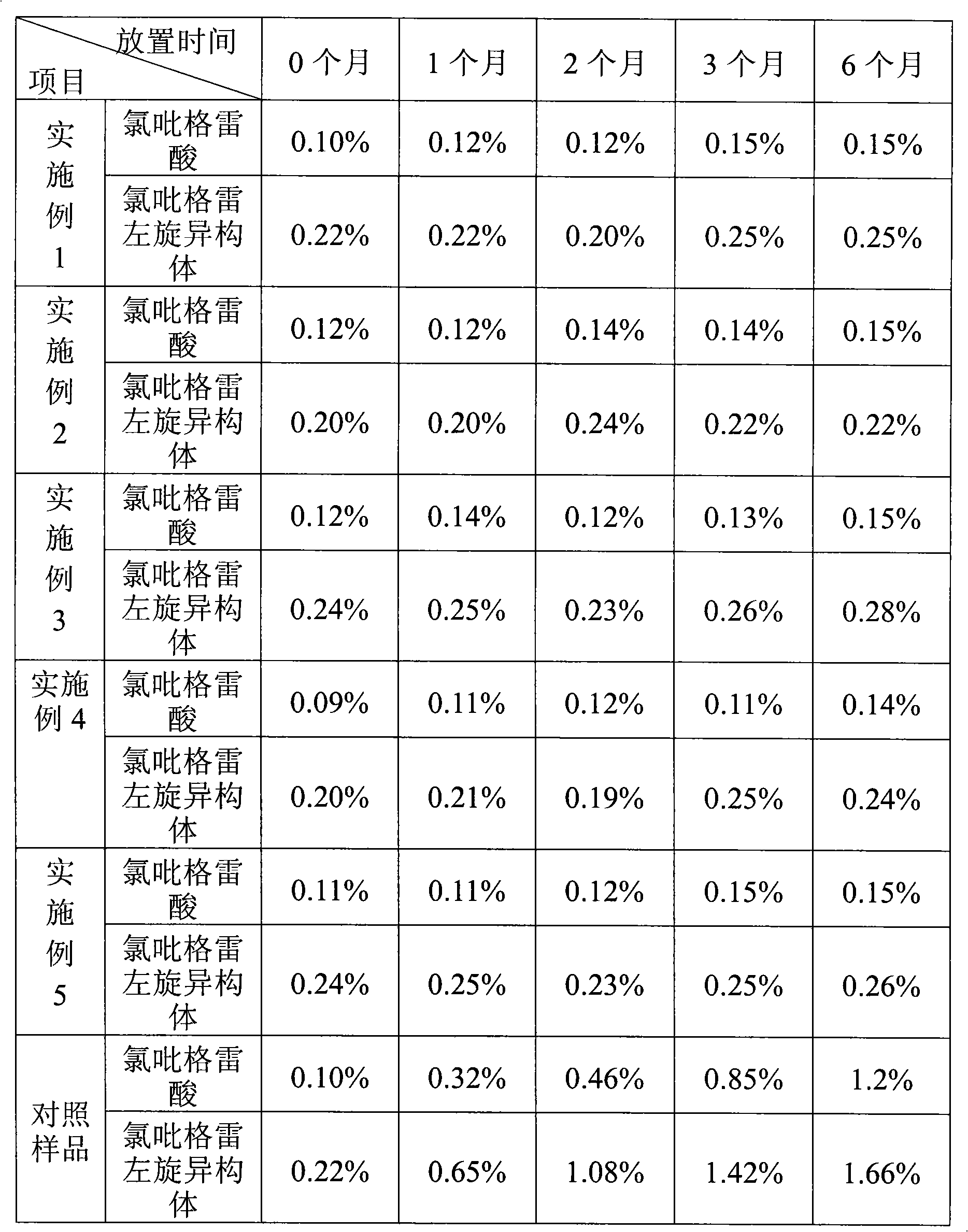
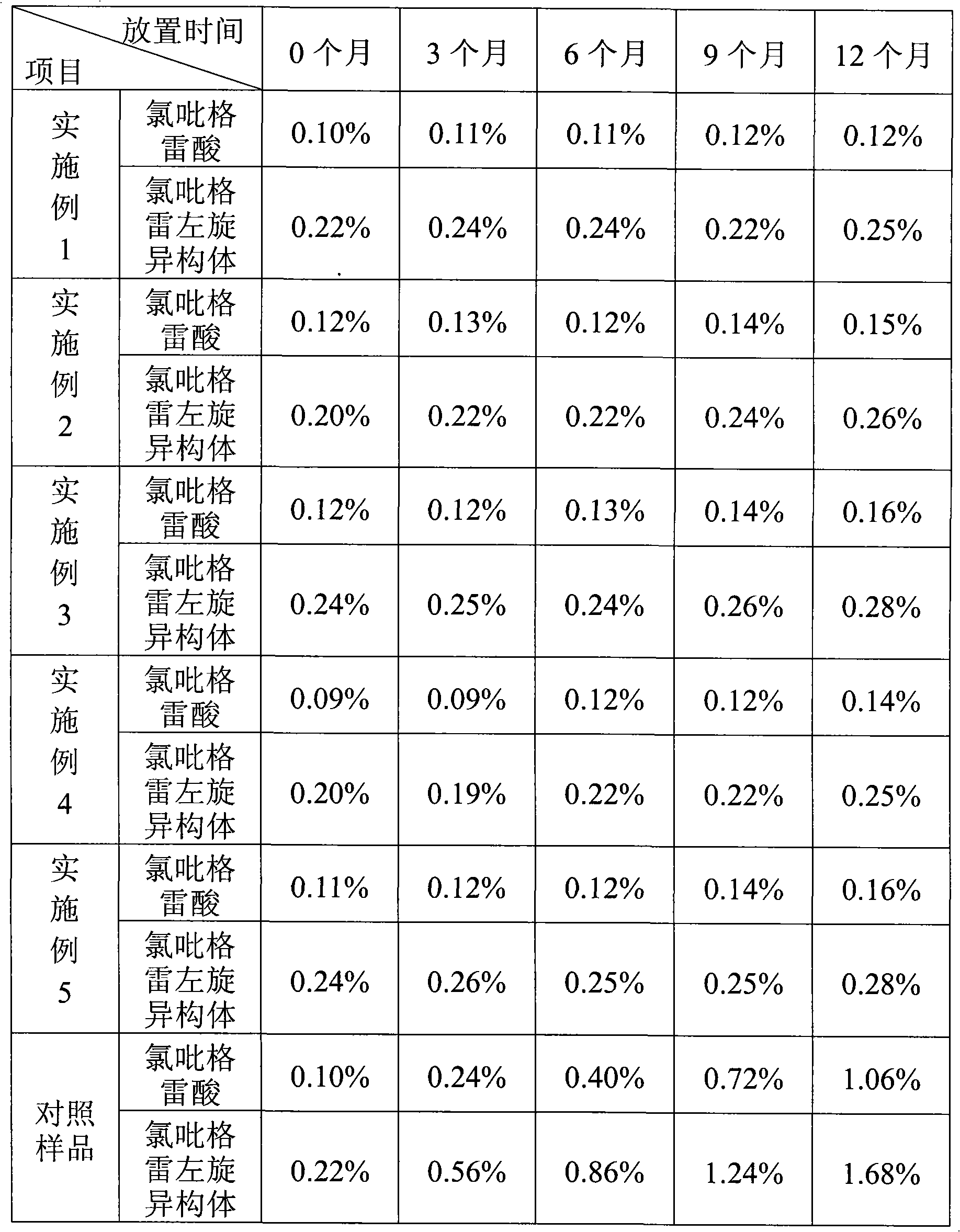
The invention relates to a clopidogrel hydrogen sulfate tablet and a preparation method thereof. Clopidogrel is more easily converted into clopidogrel acid and a clopidogrel dextroisomer is more easily converted into a levoisomer in the prior clopidogrel hydrogen sulfate tablet. The clopidogrel hydrogen sulfate tablet takes clopidogrel hydrogen sulfate as a main medicine and is matched with filling agent, disintegrant, flow agent and lubricant, and a mixture with the weight percent of 0.05-5 of one or two of vitamin C and butylated hydroxyanisole are added into the clopidogrel hydrogen sulfate tablet. The vitamin C and / or the butylated hydroxyanisole can well inhibit the clopidogrel from being converted into the clopidogrel acid and inhibit the clopidogrel dextroisomer from being converted into the levoisomer, thereby improving the stability of the clopidogrel hydrogen sulfate tablet and the safety and the effectiveness of clinical medicines.
Owner:ZHEJIANG JINGXIN PHARMA
Clopidogrel hydrogen sulfate dispersible tablets and preparation method thereof
InactiveCN101396350AReduce manufacturing costGood disintegrationOrganic active ingredientsPharmaceutical non-active ingredientsAnti plateletDrug administration
The invention discloses an anti-platelet aggregation drug-clopidogrel hydrogen sulfate tablet dispersible tablet and a preparation method thereof. The clopidogrel hydrogen sulfate tablet dispersible tablet consists of 30 percent to 55 percent of clopidogrel hydrogen sulfate and 45 percent to 70 percent of auxiliary materials according to the weight percentage. The auxiliary materials comprise disintegrant, loading agent, lubricant and taste-modifying agent, wherein, the disintegrant accounts for 5 percent to 20 percent of the total weight percentage of the prescription. The preparation method of the clopidogrel hydrogen sulfate tablet dispersible tablet is wet granulation tabletting press method or direct powder pressing method. Compared with other forms of preparation, the invention has the advantages of good dispersing state, short disintegration time, fast drug dissolvability, convenient drug administration, low production cost, good portability, high stability, etc.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Oral solid preparation containing clopidogrel hydrogen sulfate
ActiveCN101791309ARealize industrial productionAvoid elevationOrganic active ingredientsInorganic non-active ingredientsParaffin waxOral medication
The invention relates to an oral administration, in particular to a solid composite containing clopidogrel hydrogen sulfate; the solid composite containing clopidogrel hydrogen sulfate comprises the following ingredients by weight part: 100 parts of clopidogrel hydrogen sulfate and 5-100 parts of mixture of liquid paraffin and talcum powder; the composite can realize industrial production of the clopidogrel hydrogen sulfate and solve the problems that related substances of the tablets rises and the dissolution is reduced.
Owner:CHINA PHARM UNIV +1
Process for synthesizing I-clopidogrel hydrogen sulfate
The invention provides two methods for synthesizing Clopidogrel sulfate of single I crystal system, wherein the first method starts from indefinite form Clopidogrel sulfate, and the second method starts from Clopidogrel salts.
Owner:SHANGHAI INST OF TECH
Improved preparation method of II-type clopidogrel hydrogen sulfate crystal
InactiveCN103524528AInhibit side effectsGood lookingOrganic chemistryPhysical chemistryCombinatorial chemistry
The invention discloses an improved preparation method of a II-type clopidogrel hydrogen sulfate crystal, which is an improvement of the existing II-type clopidogrel hydrogen sulfate crystal form preparation technique. The method greatly enhances the yield on the premise of ensuring the product appearance (the average yield is up to 92%), and prepares the product with better appearance on the premise of obviously enhancing the product yield.
Owner:吉林省博大伟业制药有限公司
Preparation method of clopidogrel hydrogen sulfate I crystal form spherical crystal
InactiveCN105061459AReduce claddingSimple removal processOrganic active ingredientsOrganic chemistry methodsSolvent2-Butanol
The invention overcomes the technological difficulty that a single solvent is different to prepare a spherical crystal. The single solvent 2-butanol is adopted, and the amount and grain diameters of added clopidogrel hydrogen sulfate I crystal form spherical crystal are controlled, so that clopidogrel hydrogen sulfate is stably separated out from a solution system in a spherical crystal form in a specific powder form range, the obtained clopidogrel hydrogen sulfate spherical crystal has the characteristics of specific powder and has superior state in the aspects of solvent residue, bulk density and mobility, and the preparation method is beneficial for the realization of powder vertical compression preparation technology. The invention also further discloses a medicine composition containing the clopidogrel hydrogen sulfate I crystal form spherical crystal prepared and obtained through the technology.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD +2
Clopidogrel hydrogen sulfate preparation method
ActiveCN104370935AShort synthetic routeRaw materials are easy to getOrganic chemistryOrganic reactionAcetaldehyde
The present invention relates to a clopidogrel hydrogen sulfate preparation method. According to the clopidogrel hydrogen sulfate preparation method, 2-thiophene aldehyde is adopted as a basic starting material, the 2-thiophene aldehyde and o-chlorophenylglycine methyl ester are subjected to a condensation reaction to produce the corresponding imine intermediate, the imine intermediate is reduced into the corresponding secondary amine intermediate by adopting sodium borohydride or by directly adopting sodium cyanoborohydride intermediate, the secondary amine intermediate and formaldehyde are subjected to reaction ring closure to obtain clopidogrel, and the clopidogrel is acidified with sulfuric acid to obtain the target compound clopidogrel hydrogen sulfate. According to the present invention, the provided synthesis route is short, the related reactions are classical organic reactions, the reaction conditions are mild, the operation is simple, the total yield is high, the cost is low, and the preparation method is suitable for industrial production.
Owner:HENAN PURUI PHARMA TECH CO LTD
Process for preparing I-clopidogrel hydrogen sulfate
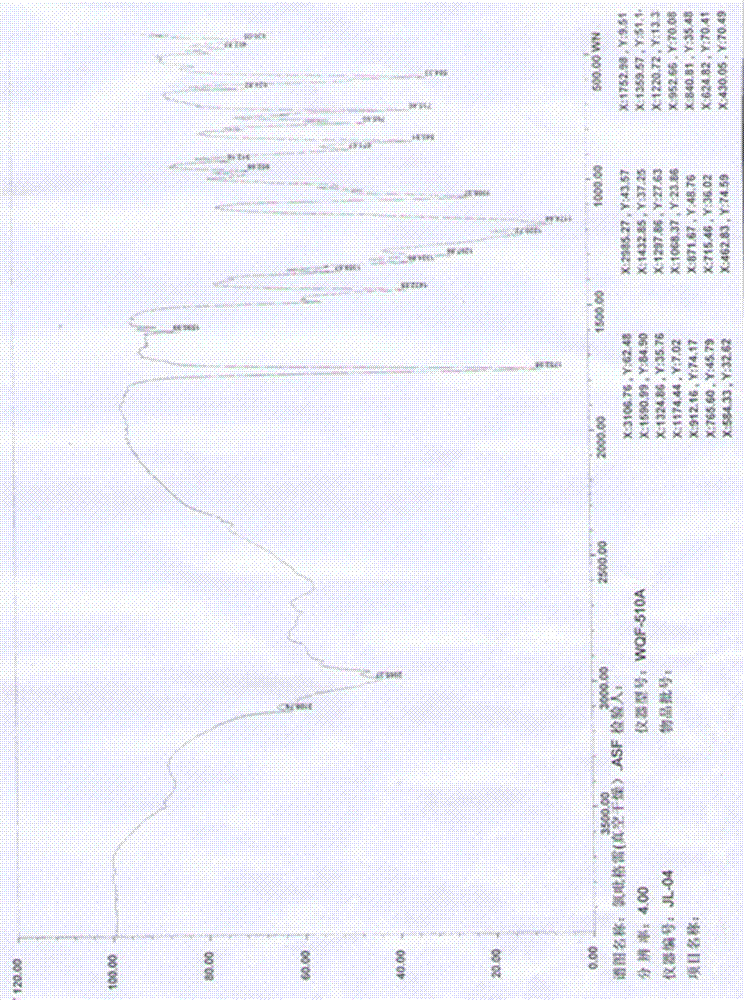
InactiveCN1690060AConvenient and stable preparationStable in natureOrganic chemistryCrystal systemInfrared
The invention relates to a method for producing sulfuric acid chlorogray of styleó±, containing the following steps: under the protection of inert gas and cooled with iced water, adding aqueous solution with sodium carbonate / potassium into organic solvent comprising chlorogray salt until the pH is higher than 9; and demixing the liquid, extracting the water layer with the same organic solvent, after combining with organic phase, drying and concentrating it to get free chlorogray alkali; then dissolving it in solvent, dropping in stoichiometric sulfate liquor, and the chlorogray formed, with the temperature controlled between minus 20 Deg.C to 5 Deg.C; then filtering the mixed solution, vacuum drying it, and then the sulfuric acid chlorogray of styleó±can be collected. The sulfuric acid chlorogray produced in such way is proved to beó±morph crystal system confirmed by X-ray diffraction, infrared spectrum and DSC spectrogram, and the fusible point is 181~186 Deg.C, and the specific rotation is 52.0í½55.0 deg. (c=1, carbinol). The method can produce sulfuric acid chlorogray of styleó±steadly.
Owner:上海开特生物科技有限公司
Tablet containing clopidogrel hydrogen sulfate and preparation method thereof
ActiveCN102302465ASolve the problem of accelerated clopidogrel degradationImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsVegetable oilPolyethylene glycol
The invention discloses a tablet containing clopidogrel hydrogen sulfate. The tablet comprises clopidogrel hydrogen sulfate micropills which are prepared into tablets through coating; the micropills are prepared by clopidogrel hydrogen sulfate, a diluent and an adhesion agent; the diluent is mannitol or / and microcrystalline cellulose; the adhesion agent is an anhydrous ethanol solution of hydroxypropylcellulose or povidone; and an lubricating agent is one or more of magnesium stearate, zinc stearate, talc, polyethylene glycol 6000, stearic acid, sodium stearyl fumarate, sodium lauryl sulfate and hydrogenated vegetable oil.
Owner:ZHEJIANG ANGLIKANG PHARMA
Process for the preparation of polymorphic forms of clopidogrel hydrogen sulfate
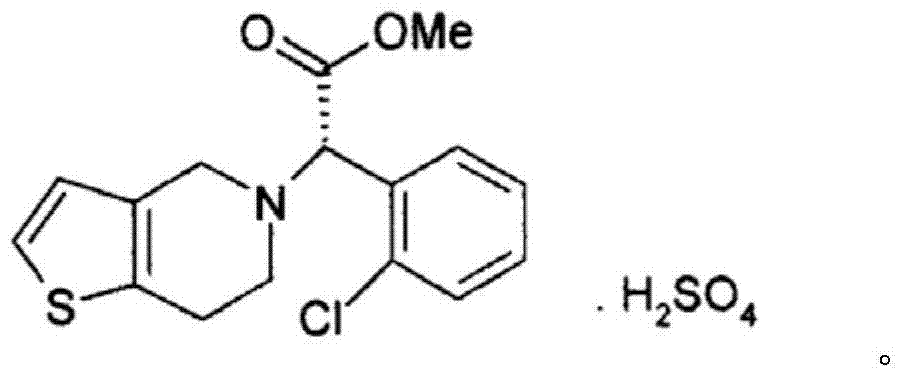
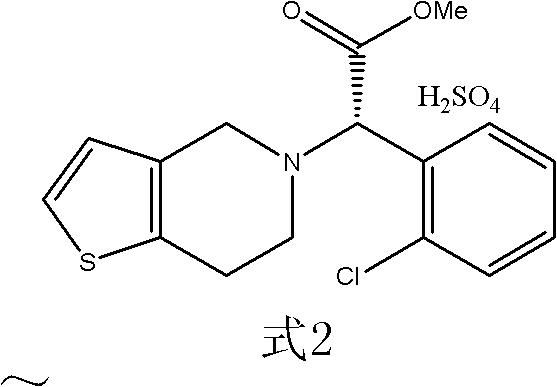
The present invention relates to a novel process for the preparation of polymorphic forms of clopidogrel hydrogen sulfate, namely methyl (+)-(S)-a-(o-chlorophenyl)-6,7-dihydrotliieno [3,2-c] pyridine-5(4H)-acetate hydrogen sulfate of formula (I). Particularly the present invention relates to the process for the preparation of form (I) and amorphous clopidogrel hydrogen sulfate.
Owner:IND SWIFT LAB
Preparation method of type I clopidogrel hydrogen sulfate
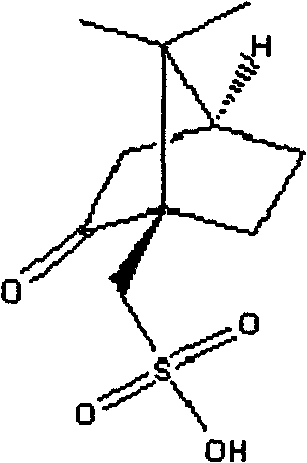
The invention relates to a preparation method of type I clopidogrel hydrogen sulfate. The preparation method comprises the following steps of: generating (S) clopidogrel free alkali from the raw material (S)-alpha-[(2-thiophene)ethylamino]-alpha-(2-chlorphenyl) methyl acetate hydrochloride; reacting L-camphorsulfonic acid with the (S)-clopidogrel free alkali, and removing impurities to obtain (S)-alpha-(2-chlorphenyl)-6,7-dichlorothieno-[3,2-c] pyridine-5(4H)-methyl acetate-(L)-camphosulfonate; performing refining and impurity removal on the (S)-alpha-(2-chlorphenyl)-6,7-dichlorothieno-[3,2-c] pyridine-5(4H)-methyl acetate-(L)-camphosulfonate by using acetone-petroleum ether to obtain (S)-clopidogrel-(L)-camphosulfonate; removing L-camphorsulfonic acid from the (S)-clopidogrel-(L)-camphosulfonate and dropwise adding concentrated sulfuric acid to the type I clopidogrel hydrogen sulfate. The preparation method provided by the invention is capable of obtaining high-purity type I clopidogrel hydrogen sulfate.
Owner:SICHUAN EMEISHAN PHARMA
Preparation method of crystal form I of clopidogrel hydrogen sulfate
The invention provides a preparation method of a crystal form I of clopidogrel hydrogen sulfate. The preparation method comprises the following steps of (1) dropwise adding concentrated sulfuric acid or 2-methyltetrahydrofuran solution of the concentrated sulfuric acid into 2-methyltetrahydrofuran solution of clopidogrel free base to obtain mixed solution, wherein the temperature of the mixed solution is controlled to range from -10 DEG C to 5 DEG C when the dropwise adding is performed; and (2) heating the mixed solution to the temperature of 10-35 DEG C, performing crystal precipitation with stirring, separating precipitated crystals, and drying the precipitated crystals to obtain the crystal form I of the clopidogrel hydrogen sulfate. The preparation method is simple, easy to implement, good in reproducibility and suitable for industrial production. The prepared crystal form I of the clopidogrel hydrogen sulfate has the advantages of being high in crystal form purity and high-performance liquid chromatography (HPLC) purity, low in cost, good in stability, high in solvent recovery, environment-friendly and the like.
Owner:ZHEJIANG HISOAR PHARMA
I type clopidogrel hydrogen sulfate particles and preparation method thereof as well as I type clopidogrel hydrogen sulfate solid preparation and preparation method thereof
The invention belongs to the field of medicaments and in particular relates to I type clopidogrel hydrogen sulfate particles and a preparation method thereof as well as an I type clopidogrel hydrogen sulfate solid preparation and a preparation method thereof. The preparation method of the I type clopidogrel hydrogen sulfate particles comprises the following steps: a) mixing a crystal form stabilizer and a solvent to obtain a crystal form stabilizer solution; and mixing I type clopidogrel hydrogen sulfate and adjuncts to obtain a mixture; and b) sequentially carrying out mixing, granulation and drying on the crystal form stabilizer solution and the mixture to obtain the I type clopidogrel hydrogen sulfate particles. The I type clopidogrel hydrogen sulfate particles prepared by the preparation method are prepared into the solid preparation; the stability of the solid preparation is tested, and results show that in long-term stability experiments and accelerated stability experiments, the I type clopidogrel hydrogen sulfate in the solid preparation can not be converted to II type clopidogrel hydrogen sulfate.
Owner:BEIJING COLLAB PHARMA
Crystallization method for preparing high-purity I-type clopidogrel hydrogen sulfate
The invention discloses a method for preparing high-purity I-type clopidogrel hydrogen sulfate, belonging to the technical field of finding and preparing of drug crystal forms. The method comprises the following four steps of: (A) transforming a clopidogrel salt into free alkali thereof at a lower temperature; (B) dropwise adding sulfuric acid into aqueous alkali at 20 to 25 DEG C, reacting and crystallizing; (C) growing crystals for 1 to 2 hours at the same temperature; and (D) washing an obtained solid by using ethyl acetate, and performing vacuum drying. An I-type clopidogrel hydrogen sulfate crystal prepared by using the method is determined to be the high crystal form purity I-type clopidogrel hydrogen sulfate after being subjected to X-ray powder diffraction, infrared spectrum and thermal analysis. An appropriate amount of seed crystal is added during crystallization, the crystallization speed is obviously increased, and the crystallization can be completed within about 5 hours.
Owner:JIANGNAN UNIV
Preparation method of clopidogrel hydrogen sulfate I
InactiveCN102070648AHigh purityOvercome the external conditions of synthesisOrganic chemistryOrganic solventDrug compound
The invention relates to a preparation method of a drug compound, in particular to a preparation method of clopidogrel hydrogen sulfate I. The method provided by the invention comprises the following steps: 1, mixing clopidogrel salt with an organic solvent and water, adding an acid binding agent to react to generate free alkali of clopidogrel, extracting the water layer with the organic solvent, combining the organic phases and carrying out concentration; and 2. adding the organic solvent into the free alkali of clopidogrel obtained after concentration, dropwise adding sulfuric acid into the solution for reaction based on the molar ratio of the sulfuric acid to the free alkali of clopidogrel, filtering the reactant and drying the filtrate to obtain the crystal form of clopidogrel hydrogen sulfate I, wherein the molar ratio is (0.8-1.05):1.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Clopidogrel hydrogen sulfate tablet and preparation method thereof
InactiveCN102247333AImprove liquiditySimple processOrganic active ingredientsBlood disorderAdhesiveMedicine
The invention relates to a preparation for preventing and treating adverse events of atherosclerosis and cardio-cerebrovascular embolism as well as the complications thereof, i.e. a clopidogrel hydrogen sulfate tablet and a preparation method thereof. The tablet comprises clopidogrel hydrogen sulfate, a filler, a disintegrating agent, a lubricating agent, an adhesive, and a film coating premixing agent. The preparation method is characterized by the steps of: weighing the filler, the disintegrating agent in a proportion specified in the prescription, mixing them well, making wet granules with the right amount of the adhesive, drying the wet granules at a temperature of 40-60DEG C, sieving the granules through a sieve of 24 meshes and finishing the granules, adding clopidogrel hydrogen sulfate and the lubricating agent and mixing well, then conducting tabletting, coating and packaging, thus obtaining the tablet. The tablet of the invention has the characteristics of simple prescription and process, good stability, high bioavailability, low cost, high production efficiency and the like.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Clopidogrel hydrogen sulfate tablet and preparation method thereof
ActiveCN102961355AReduce stickinessSolve the problem of easy stickingOrganic active ingredientsOil/fats/waxes non-active ingredientsPolyethylene glycolLactose
The invention discloses a clopidogrel hydrogen sulfate tablet and a preparation method thereof. The tablet comprises the following components in parts by weight of: 39.44 parts of clopidogrel hydrogen sulfate, 31.53 parts of lactose, 15 parts of pregelatinized starch, 10 parts of microcrystalline cellulose, 2.7 parts of polyethylene glycol 6000, 1.33 parts of hydrogenated castor oil and 3 parts of lagging cover thin film. According to the invention, the pregelatinized starch and the polyethylene glycol 6000 are added by an inner adding method and an outer adding method in the process of pelletizing, so that the sticking of drug in the process of tabletting can be reduced. Furthermore, after the dry granulation is adopted, the problem that the drug is easy to stick in the process of tabletting can be further solved. The clopidogrel hydrogen sulfate tablet prepared by the invention is short in disintegration time, and quick in dissolving-out speed, and the hardness of the tablet can reach 70-80N. The invention is simple to operate, and easy to industrially popularize.
Owner:HENAN RUNHONG PHARMA
Synthesis method of high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate
The invention discloses a synthesis method of high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate, which comprises the following steps: 1) mixing an acetone solution of L(-)-camphorsulfonic acid and an acetone solution of (+)-(S)-clopidogrel crude product to carry out salification reaction for 6-10, filtering after the reaction finishes, and treating the filter cake to obtain (+)-(S)-clopidogrel L(-)-camphorsulfonate; 2) dissolving the product obtained in the step 1) in a mixture of dichloromethane or ethyl acetate and water, regulating the pH value to 7-8, stirring for 10-60 minutes, standing to stratify, and treating the organic layer to obtain a (+)-(S)-clopidogrel pure product; and 3) dissolving the product obtained in the step 2) in an organic solvent, adding a crystal seed, stirring, adding an organic solvent solution of sulfuric acid, heating to 50-60 DEG C, stirring for 1-3 hours, cooling to room temperature, filtering, and treating the filter cake to obtain the high-purity I-type (+)-(S)-clopidogrel hydrogen sulfate. The method has the advantages of simple technique, high product yield and high product purity, and is suitable for industrial production.
Owner:SHANGHAI MODERN HASEN SHANGQIU PHARMA
Clopidogrel dispersible tablet and preparation method thereof
InactiveCN101744780AShort disintegration timeGood dispersionOrganic active ingredientsPill deliveryTreatment effectClopidogrel
The invention discloses a clopidogrel dispersible tablet and a preparation method thereof, in particular relates to a clopidogrel hydrogensulfate preparation and a preparation method thereof. The clopidogrel hydrogensulfate preparation is used for curing various thrombosis induced by platelet aggregation. The clopidogrel hydrogensulfate preparation and the preparation method with short disintegration time, excellent dispersed state, quick drug dissolution and convenience and flexibility of taking have the characteristics of improving the medication compliance of the patients, ensuring the therapeutic effect of the drug and the like.
Owner:BEIJING HOPE HUGE PHARM SCI
Production technique of clopidogrel hydrogen sulfate
The invention relates to a production technique of clopidogrel hydrogen sulfate, which comprises the following steps: (1) preparation of amide hydrochloride, (2) preparation of levo-camphorsulfonate, (3) preparation of clopidogrel hydrogen sulfate crude product and (4) preparation of I-type clopidogrel hydrogen sulfate. The technique is simple, reduces the synthesis steps, lowers the consumption of toxic solvents, enhances the drug safety, facilitates the recovery and treatment of the waste liquid and lowers the production cost. The purity of the clopidogrel hydrogen sulfate obtained by the method is high, and the yield is 6.5% higher than other methods.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Clopidogrel hydrogen sulfate tablet medicine composition and preparation method thereof
ActiveCN104523627AImprove liquidityPrevent deliquescenceOrganic active ingredientsInorganic non-active ingredientsMedicineFluidized bed
The invention provides a clopidogrel hydrogen sulfate tablet medicine composition and a preparation method thereof. Magnesium aluminum silicate is adopted in a prescription, so that the material flowability can be improved, deliquescence of medicines can also be effectively prevented, a risk that clopidogrel is degraded to form clopidogrel acid due to moisture absorption can be reduced, and more stable quality can be achieved. The preparation method comprises the following steps: firstly preparing pill cores, and then directly spraying a clopidogrel hydrogen sulfate solution on the pill cores by virtue of fluidized bed coating for performing granulation. By adopting the clopidogrel hydrogen sulfate tablet medicine composition provided by the invention, the mixing uniformity of the medicines can be significantly improved; and moreover, the sticking problem of clopidogrel can be effectively solved, the appearance characteristics of products can be improved, the product yield can be improved, and the requirements for industrial large-scale production can be better met.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Preparation method of I type clopidogrel hydrogen sulfate
InactiveCN101805354AHigh crystal purityGood repeatabilityOrganic chemistryReaction temperatureNitrogen
The invention discloses a preparation method of I type clopidogrel hydrogen sulfate. The method comprises the following steps: (1) dissolving clopidogrel free base in tetrahydrofuran, and adding concentrated sulfuric acid at the temperature of minus 14-0 DEG C; (2) leading the mixture to react for 1-2 hours, then slowly increasing the reaction temperature to 20-40 DEG C, continuing the reaction for 10-12 hours, and fully precipitating crystals; and (3) filtering after obtaining the crystals, washing a filter cake with the tetrahydrofuran, draining, vacuum-drying and obtaining the I type clopidogrel hydrogen sulfate. The I type clopidogrel hydrogen sulfate prepared through the method is not doped with II type clopidogrel hydrogen sulfate and has high crystal phase purity, and the preparation process does not need the steps of nitrogen production and the like, thereby reducing the production cost, leading the operation method to be simple and easy, realizing high repeatability and being easy to realize mass production.
Owner:SUN YAT SEN UNIV
Method for determining content of dimethyl sulfate in clopidogrel hydrogen sulfate
InactiveCN111579689AHigh detection sensitivityImprove stabilityComponent separationPhysical chemistryMass spectrometry
The invention discloses a method for determining the content of dimethyl sulfate in clopidogrel hydrogen sulfate, and belongs to the technical field of medicines. The method adopts a gas chromatograph-mass spectrometer to determine the content of dimethyl sulfate in clopidogrel hydrogen sulfate, and comprises the following steps: firstly, preparing a test solution, and storing the prepared solution in an environment below 0 DEG C for later use; then setting detection conditions of a gas chromatograph-mass spectrometer; after the sample introduction condition is met, starting sample introduction, completing the detection process, and rcording a chromatogram. The method for detecting the dimethyl sulfate in the clopidogrel hydrogen sulfate is high in detection sensitivity, the linear range of the dimethyl sulfate is within the range of 5%-500% of the limit concentration, and the linear relation between the concentration and the peak area is good. According to recovery rate, repeatabilityand sample injection precision tests, the precision and accuracy of the method are good. A reference substance solution stability test shows that the reference substance solution has good stability after being placed at 0 DEG C or below for 6 hours.
Owner:JIANGSU LIANHUAN PHARMA
Method for preparing (+)-(S-)-clopidogrel hydrogen sulfate 1 crystal form
InactiveCN102796114AProcess reaction conditions are mildSuitable for the needs of industrial scale-up productionOrganic chemistryTemperature controlOrganic solvent
The invention provides a new method for preparing a (+)-(S-)-clopidogrel hydrogen sulfate 1 crystal form. The method comprises the following steps of: dissolving (+)-(S-)-clopidogrel (free alkali) into an organic solvent, stirring, controlling the temperature to be between 15 and 40 DEG C, adding seed crystal, dripping 5 to 40 percent concentrated sulfuric acid solution, stirring for certain time at a constant temperature, filtering, washing, drying, and thus obtaining the crystal form. The preparation method is mild in reaction conditions and suitable for temperature change of any season, does not require special temperature control equipment, avoids caking in the sulfuric acid dripping process, and is easy to operate, high in controllability, short in process time and suitable for the requirement for industrialized mass production; and the yield of the crystal form is over 85 percent, the crystal form is high in purity and high in quality stability, and the content of the 1 crystal form can reach over 99 percent.
Owner:ZHEJIANG JINGXIN PHARMA
Type I clopidogrel hydrogen sulfate salt preparation method
The invention discloses a type I clopidogrel hydrogen sulfate salt preparation method which comprises the following steps: (1) mixing clopidogrel salt with an organic solvent, adding water, adding an acid binding agent or an acid binding agent water solution to the solvent, stirring for reaction to generate clopidogrel free alkali, standing for layering, extracting aqueous phase with the same organic solvent, combining the organic phase, washing with water, recovering the solvent to dry; and (2) adding an organic solvent into the concentrated clopidogrel free alkali, stirring to fully dissolve the clopidogrel free alkali, cooling, adding seed crystal, according to the molar ratio of sulfuric acid to clopidogrel free alkali of 0.9-1.05:1, dropwise adding sulfuric acid solution into the solution, standing, filtering, and drying to obtain the type I clopidogrel hydrogen sulfate. The type I clopidogrel hydrogen sulfate obtained by the method has the advantages of high purity and stable property.
Owner:YABAO PHARMA GRP CO LTD
Recovering and applying method of clopidogrel resolving agent
InactiveCN101786970ASolve wasteHigh optical purityOrganic chemistryOrganic compound preparationClopidogrel HydrochlorideSodium bicarbonate
The invention discloses a recovering and applying method of camphorsulfonicacid for resolving clopidogrel, which comprises the following steps: taking resolving mother solution of racemic clopidogrel hydrochloride as a raw material; reacting the raw material with sodium bicarbonate to generate salt; reacting the salt with sulfuric acid to obtain the camphorsulfonicacid with qualified optical rotation; and resolving the clopidogrel again. The invention recovers and applies the camphorsulfonicacid in the reaction waste liquid of clopidogrel hydrogen sulfate, greatly reduces the cost, has simple industrial operation and is beneficial for industrial production on a large scale.
Owner:TIANJIN CENT PHARM CO LTD
Tablet capsule containing clopidogrel hydrogen sulfate salt tablets and acetylsalicylic acid tablets
InactiveCN101584680AWill not happenNo chemical transformationOrganic active ingredientsCapsule deliverySalicylic acidBULK ACTIVE INGREDIENT
The invention provides a tablet capsule with various active ingredients which do not contact mutually. The tablet capsule comprises a capsule and a tablet in the capsule, wherein the capsule comprises an upper capsule body and a lower capsule body; the capsule internally contains at least one clopidogrel hydrogen sulfate salt tablet and one acetylsalicylic acid tablet; the active ingredients of the two tablets have pharmaceutic proper forms of containing raw materials or auxiliary materials which do not have physical or chemical reaction with the two tablets, and preferably a blank tablet containing no active components is between the two tablets. Compared with the prior art, the invention has the following advantages that no chemical change or side product is generated among all the ingredients so that the stability of the product is improved. The product of the invention is very suitable for commercial process and enables a sufferer to have good compliance.
Owner:HEILONGJIANG FUHE HUAXING PHARMA GROUP
Preparation method of type I clopidogrel hydrogen sulfate
The invention relates to a preparation method of type I clopidogrel hydrogen sulfate, belonging to the technical field of preparation of medical substances. The preparation method of the type I clopidogrel hydrogen sulfate comprises the following steps of: reacting clopidogrel hydrogen sulfate with an alkali in an organic solvent and water to obtain clopidogrel alkali; adding clopidogrel alkali into a mixed solvent of isopropyl ether and isopropanol, and acidifying to obtain a suspension; and filtering and drying to obtain the type I clopidogrel hydrogen sulfate. The method has the advantages of mild reaction conditions, small damages of used solvent to human bodies, safety, reaction yield of 85-93 percent and suitability for large-scale industrial production.
Owner:SHANDONG QIDU PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com