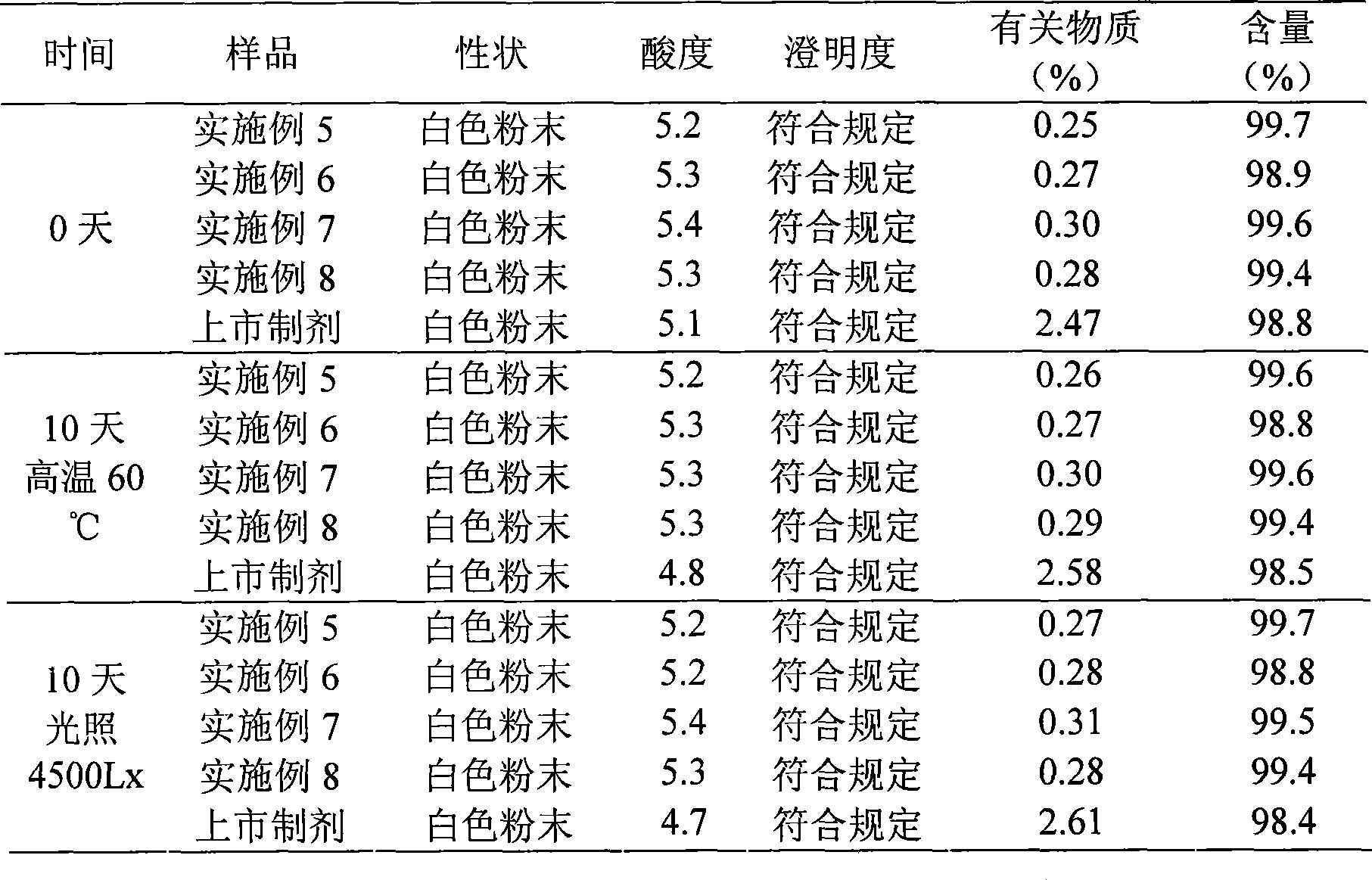
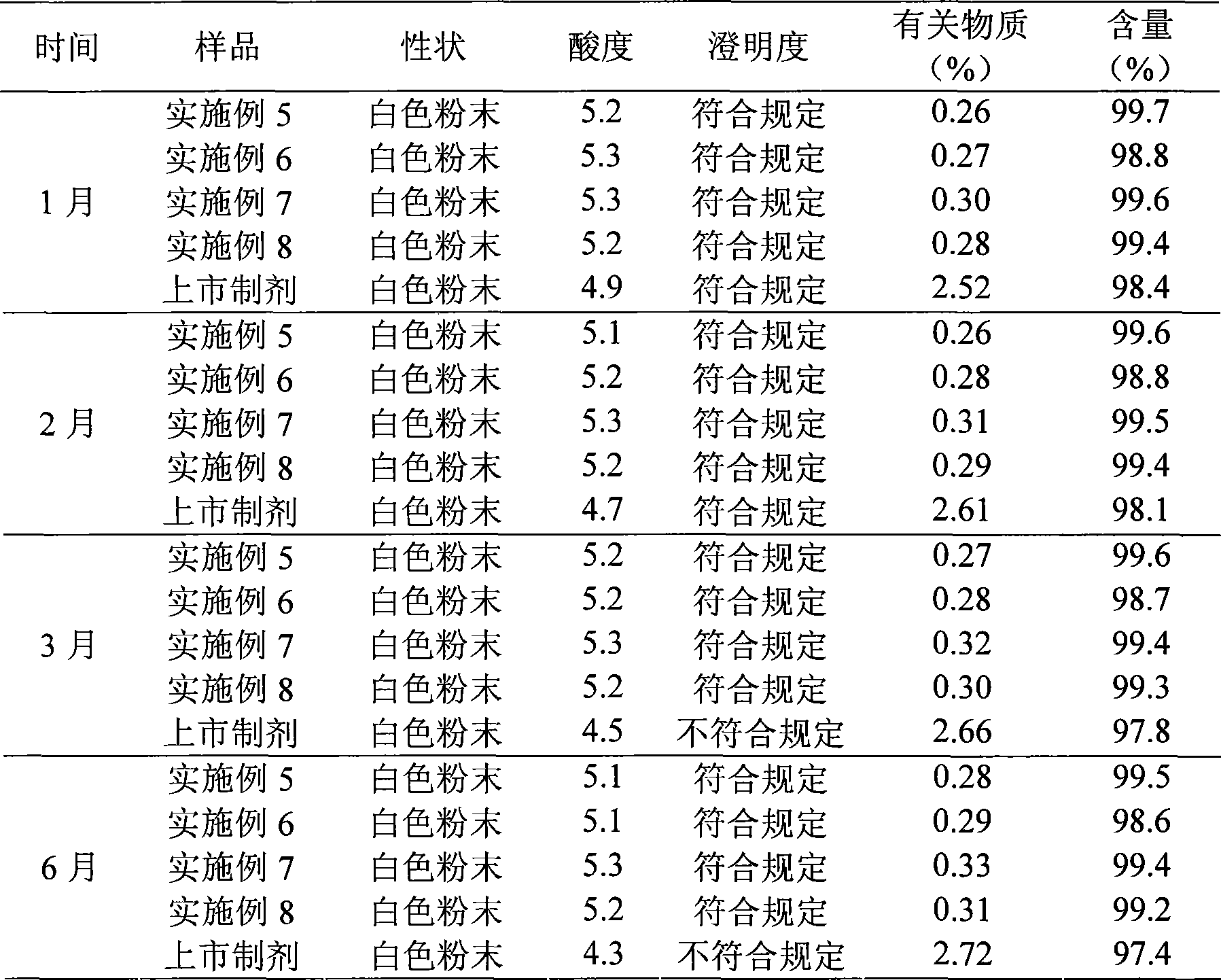
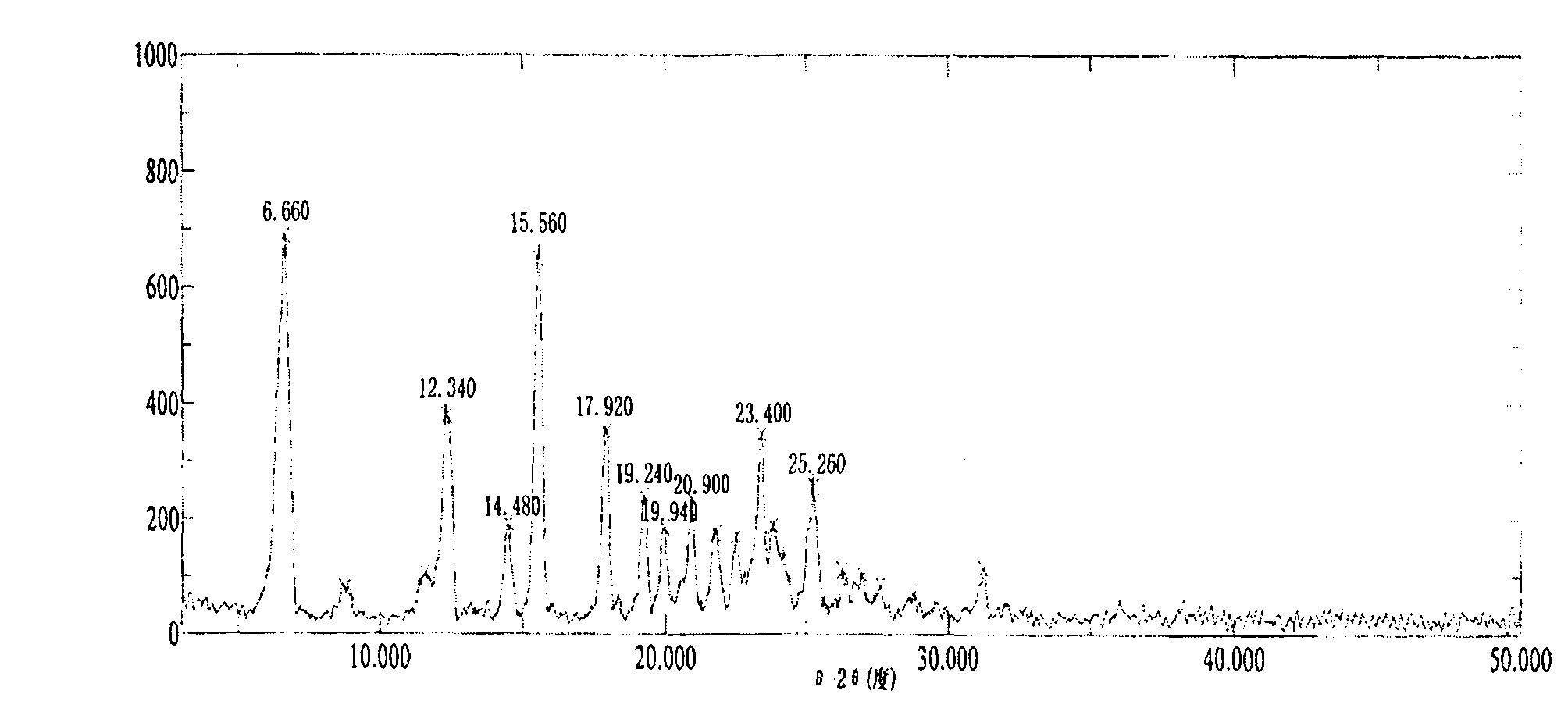
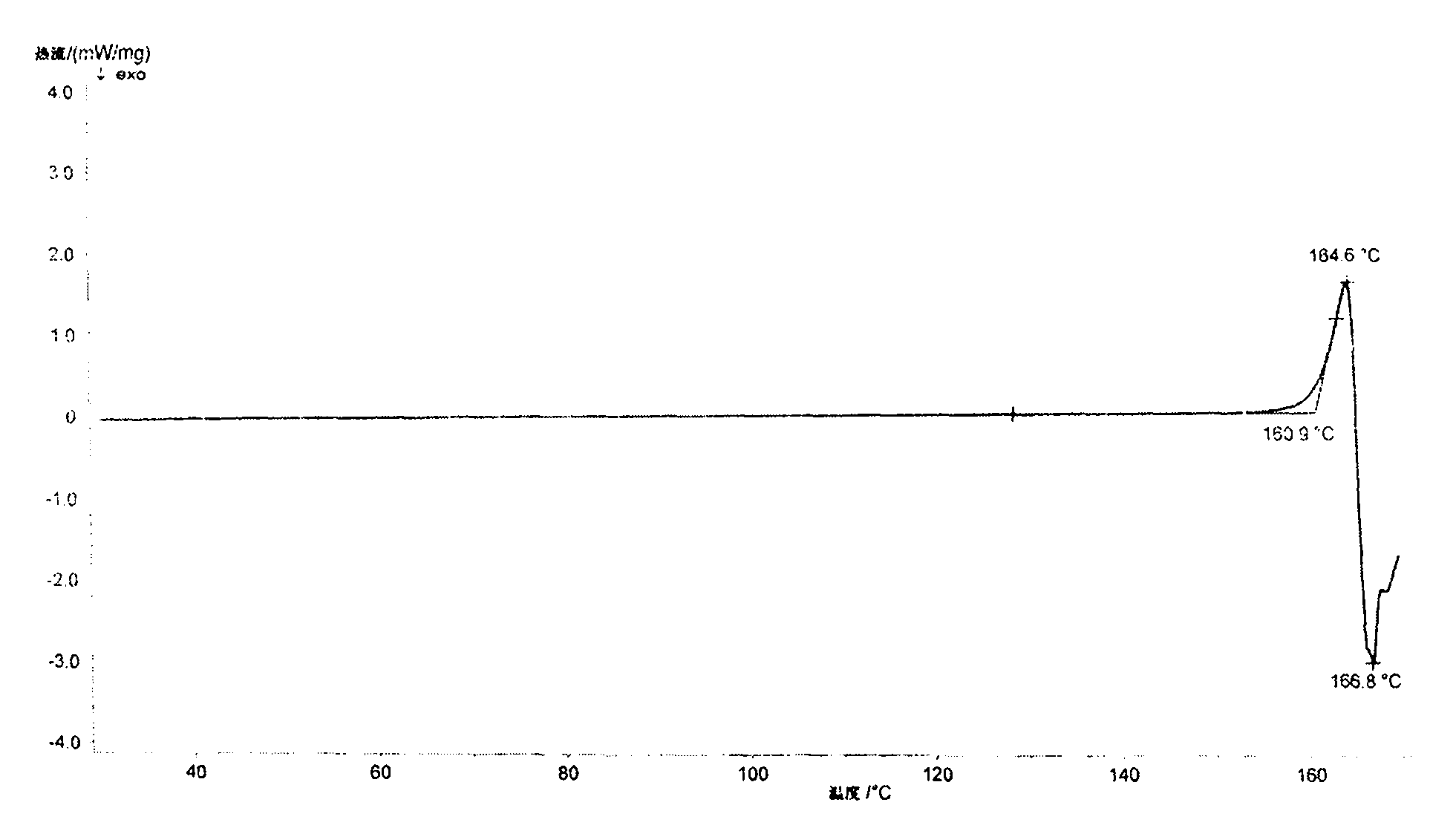
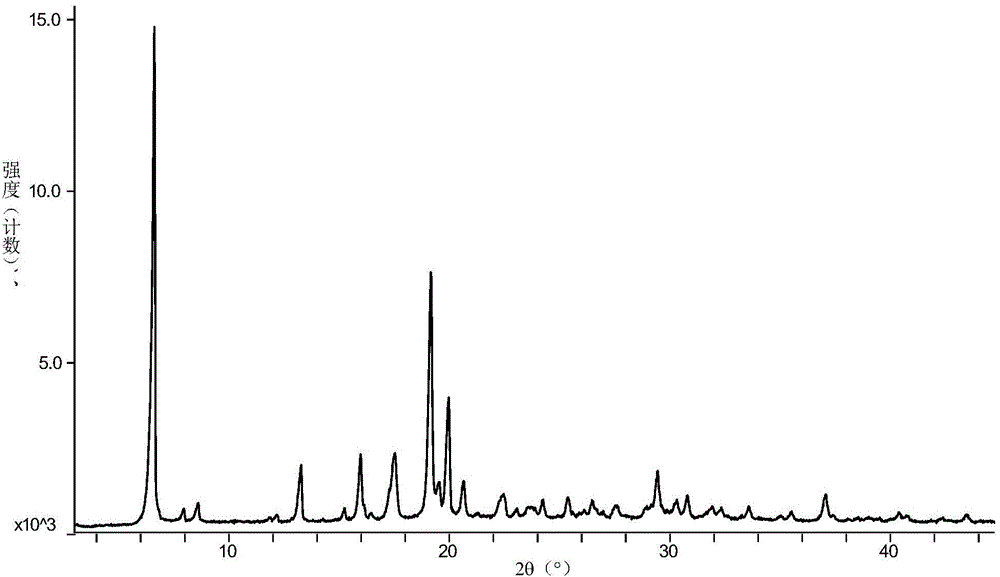
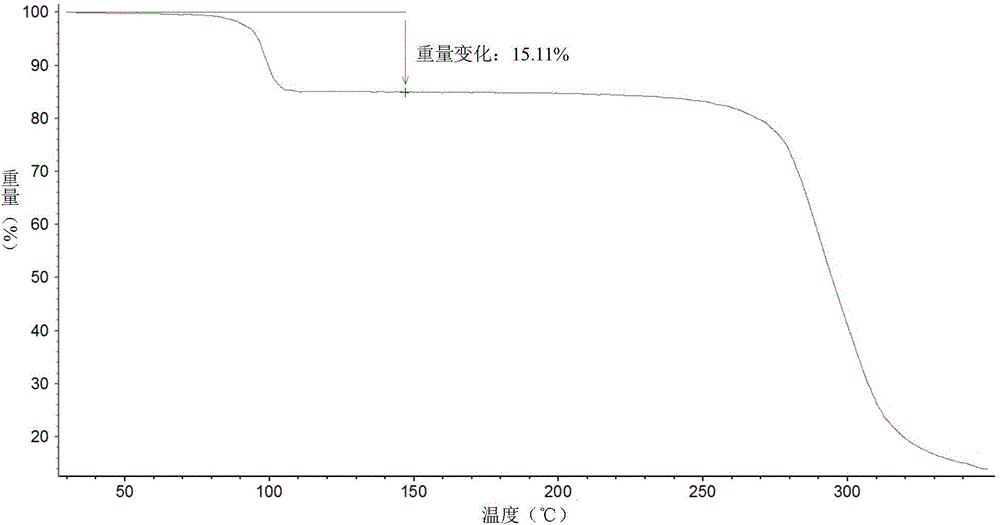
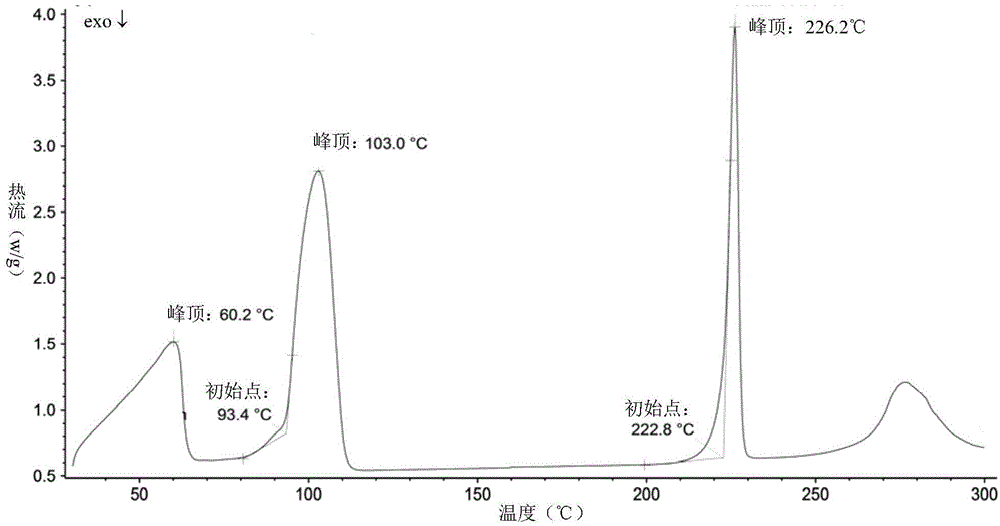
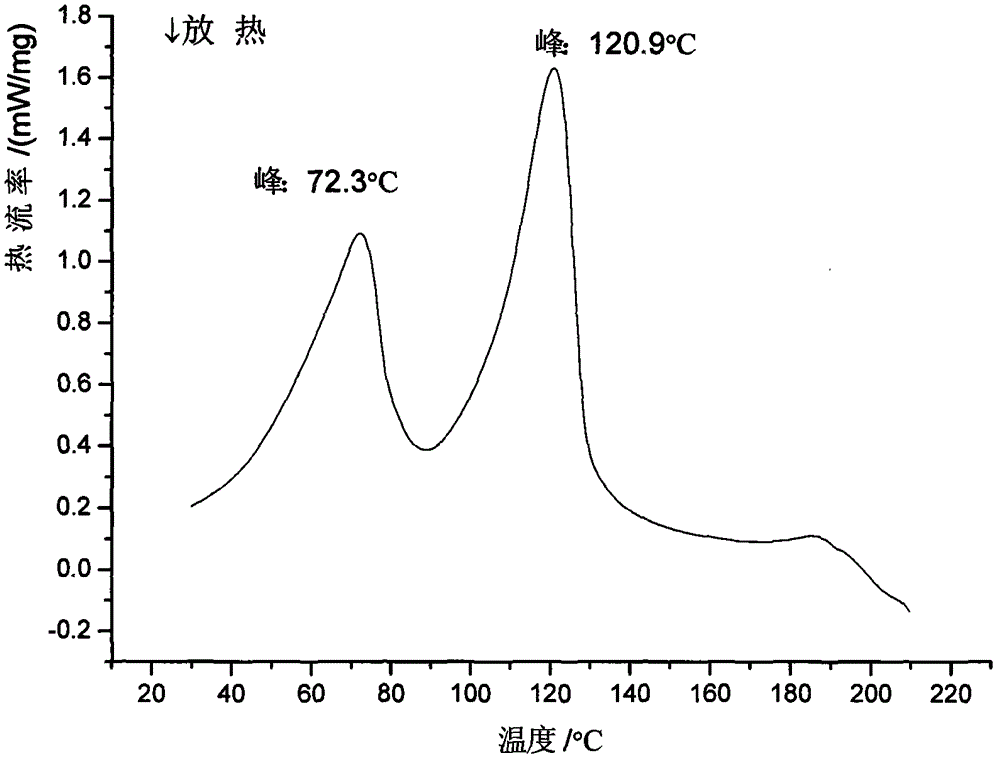
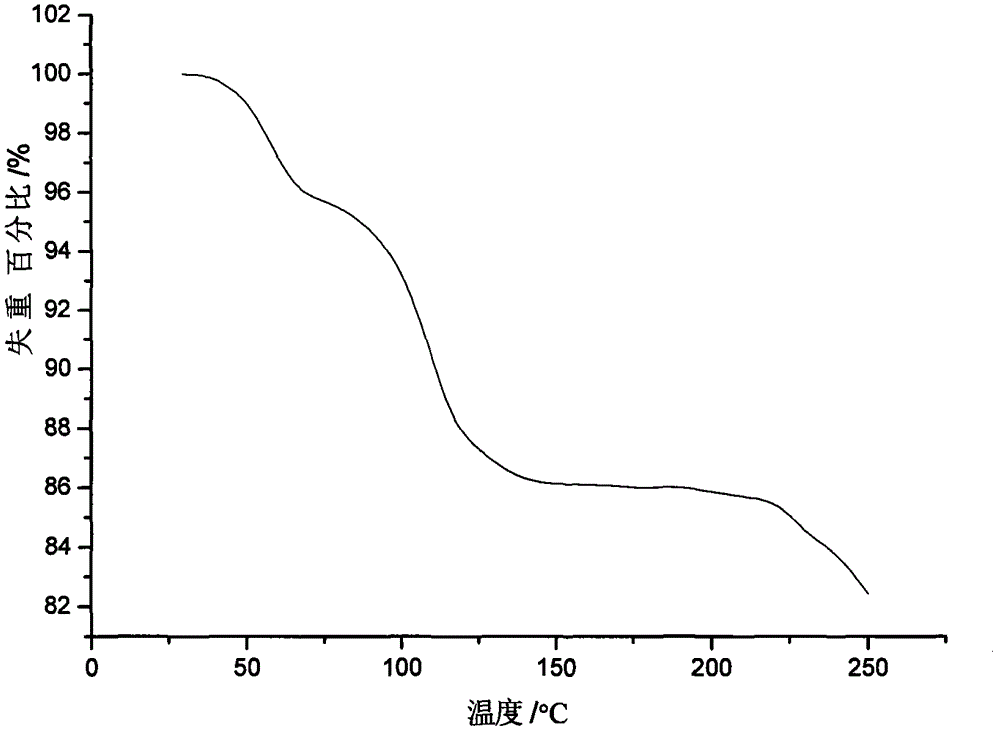
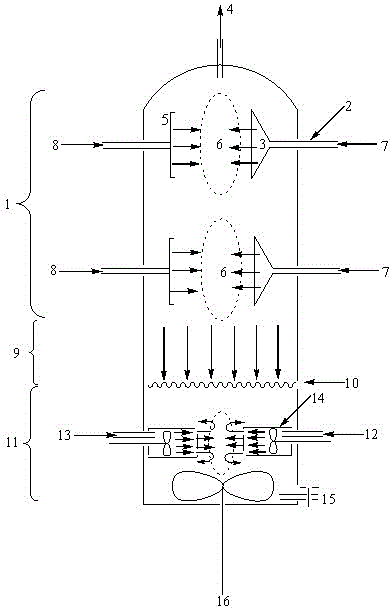
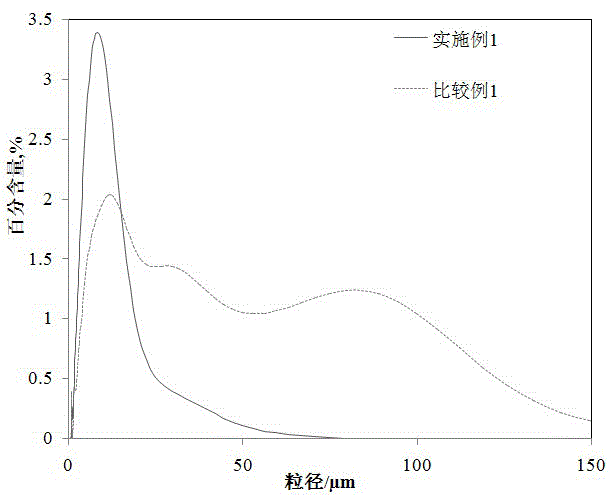
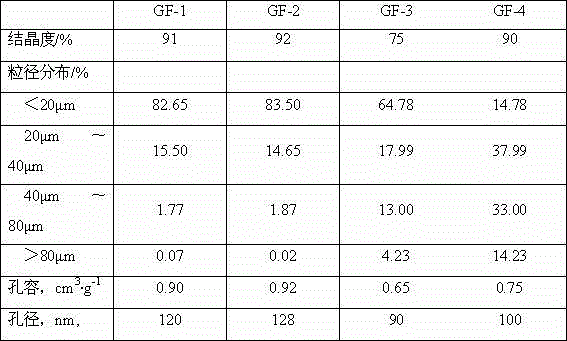
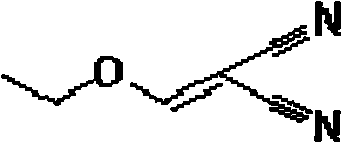Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
207results about How to "High crystal purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
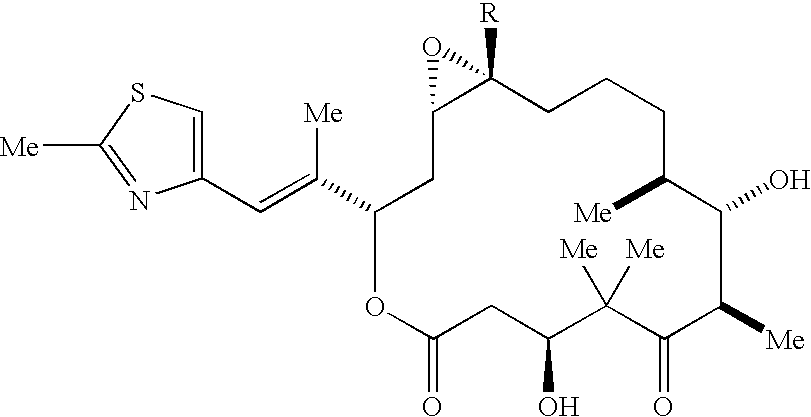
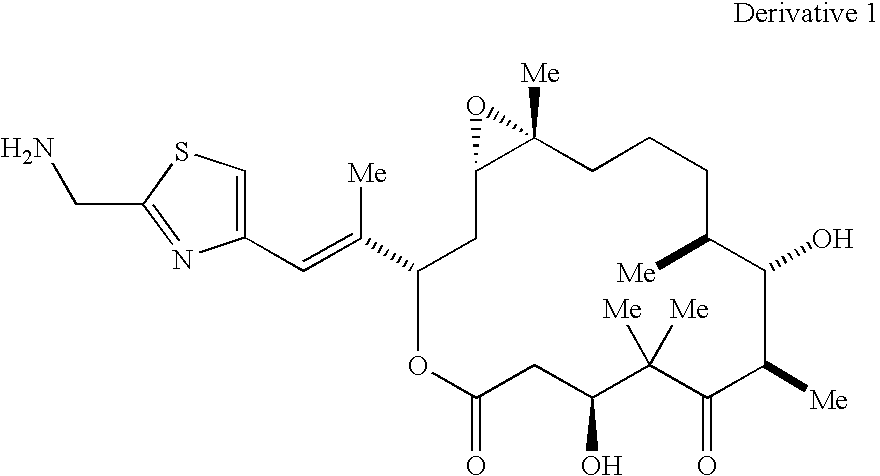
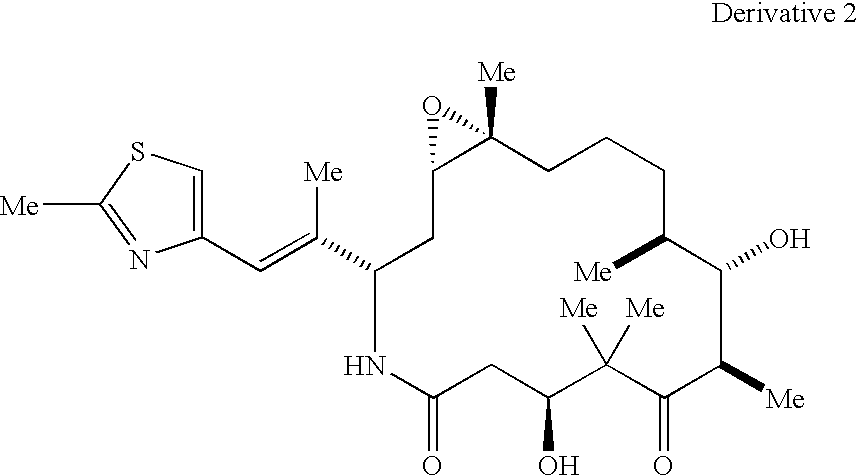
Methods for the preparation, isolation and purification of epothilone B, and x-ray crystal structures of epothilone B
ActiveUS20040132146A1Improve epothilone titerQuality improvementOrganic active ingredientsBiocidePurification methodsEpothilone B
The present invention relates to improved methods for the production, isolation and purification of epothilone B. These methods include, for example, a fermentation process for the production of epothilone B, isolation via adsorption onto a resin, and subsequent purification.
Owner:GESELLSCHAFT FUR BIOTECHNOLOGISCHE FORSCHUNG MBH GBF +1
Method for extracting amber acid from fermentation liquor
InactiveCN101486637AQuality improvementReduce contentUltrafiltrationElectrodialysisElectrolysisCross-flow filtration
The invention discloses a method for extracting succinic acid from a broth. The method comprises the following steps: a sodium succinate broth generated from microbial fermentation goes through a microfiltration membrane to conduct cross-flow filtration so as to remove thallus and other relatively large impurity particles; cross-flow filtration is carried out through a nanofiltration membrane to remove residual protein and pigment in the broth so as to get a clean filtrate; after the electrolysis, electrodialysis and electrolysis of the clean filtrate through a bipolar membrane, a succinic acid solution and a sodium hydroxide solution are obtained respectively; and the succinic acid solution is decompress-vaporized, cooled and crystallized, and therefore white succinic acid crystals are obtained. All working procedures in the method are closely connected, feed liquid always recycles under sealed condition, conditions through the whole technique process are mild, no organic solvent and any other organic compounds are added, the technique flow is short, steps are simple, the requirements on equipment are not high, the final product has high purity and the content of succinic acid is more than 99.0 percent through test.
Owner:CHANGMAO BIOCHEMICAL ENG CO LTD
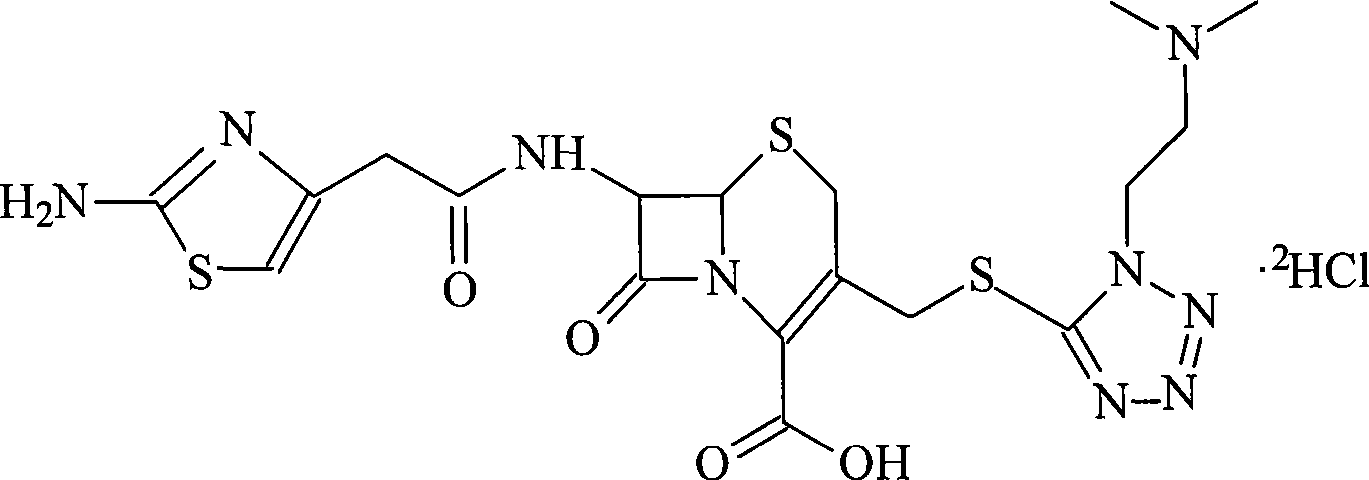
Cefotiam salt compound and pharmaceutical composition made therefrom
InactiveCN101544662APromote precipitationEasy to operateAntibacterial agentsPowder deliveryCefotiam HydrochloridePurification methods
The invention relates to a high-purity cefotiam hydrochloride compound prepared from crystal cefotiam or a crude product of crystal cefotiam salt. The invention also relates to a product obtained according to a purification method of the invention, in particular to a pure cefotiam hydrochloride and a pharmaceutical composition containing the product.
Owner:HAINAN LINGKANG PHARMA CO LTD
Ilaprazole crystal form and preparation method thereof
ActiveCN103172618AEasy to manufactureHigh crystal purityOrganic active ingredientsOrganic chemistryPhysical chemistryAnalytical chemistry
The invention discloses an ilaprazole crystal form X and a preparation method thereof. The ilaprazole crystal form X disclosed by the invention is easy to prepare. The ilaprazole crystal form X provided by the invention is high in purity and low in impurity content. The preparation method disclosed by the invention is low in required solvent amount and low in production cost. The preparation method is simple to operate, mild in reaction conditions, easy to control, and can obtain a target product crystal form in an extremely determined and good-reproducibility mode.
Owner:LIVZON PHARM GRP INC
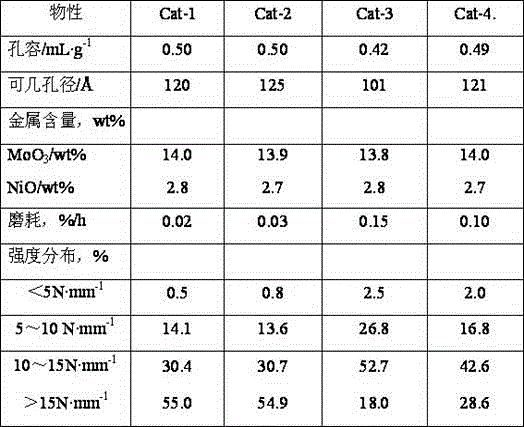
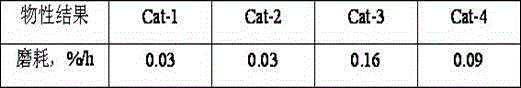
Preparation method for fluidized bed catalyst
ActiveCN104549528AAdvantages of preparation methodAdequate responseCatalyst carriersCatalyst activation/preparationAluminateGas phase
The invention discloses a preparation method for a fluidized bed catalyst. The preparation method comprises the following steps: (1) adding reaction liquid into an impinging stream reactor, enabling the reaction liquid to reach the bottom of the impinging stream reactor, heating and starting a stirring paddle at the bottom; (2) respectively injecting an alkali metal aluminate aqueous solution and CO2 gas flow through an acceleration pipe at the upper part of the impinging stream reactor, performing gas-liquid impinging stream reaction on the alkali metal aluminate aqueous solution subjected to atomization and the CO2 gas flow to generate an aluminum hydroxide crystal nucleus entering a settling region; (3) at the end of the gas-liquid impinging stream reaction, simultaneously and continuously adding an acidic aluminum salt aqueous solution and the alkali metal aluminate aqueous solution or an alkaline settling agent from feed inlets II and III, adjusting the pH value, and performing neutral reaction; (4) performing aging, filtering, washing and drying to obtain aluminium oxide dry glue; (5) uniformly mixing the aluminium oxide dry glue, small-hole SB powder and sesbania powder, adding an adhesive to form a plasticizer, performing extrusion and forming, drying and roasting to obtain an aluminum oxide carrier, immersing active metal, drying and roasting to obtain the fluidized bed catalyst. The impinging stream reactor is adopted in the method, so that the transfer characteristic between the gas phase and the liquid phase as well as between one liquid phase and another liquid phase is enhanced, so that the prepared fluidized bed catalyst is high in intensity and low in abrasion; furthermore, the pore volume and the aperture of the catalyst are larger.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparing method for vortioxetine hydrobromide alpha crystal form
ActiveCN105367515AHigh crystal purityModerate granularityOrganic chemistryDesolvationSec-butyl alcohol
The invention discloses a preparing method for a vortioxetine hydrobromide alpha crystal form. The preparing method comprises the following step that sec-butyl alcohol is removed from a vortioxetine hydrobromide-sec-butyl alcohol complex to obtain the vortioxetine hydrobromide alpha crystal form. The method is low in desolvation temperature, and the prepared vortioxetine hydrobromide alpha crystal form is high in purity, suitable in particle size and suitable for industrial production.
Owner:BEIJING BEILU PHARM CO LTD
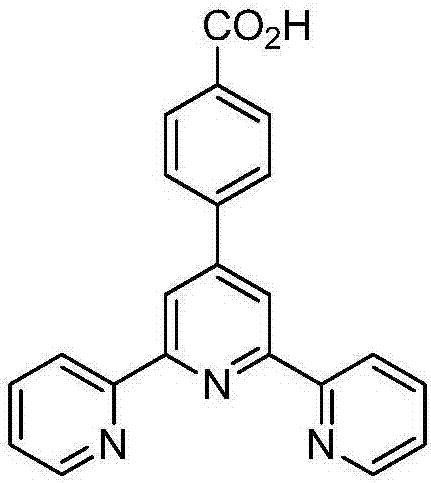
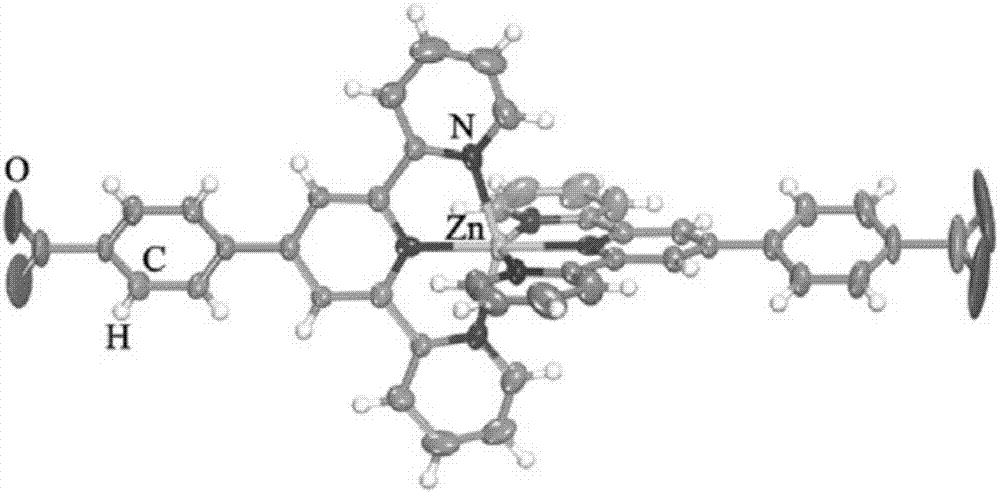
Terpyridine zinc benzoate complex and preparation condition thereof
ActiveCN106854175AImprove thermal stabilityHigh crystal purityOrganic chemistryLuminescent compositionsFluorescenceDecomposition
Belonging to the field of fluorescent solid materials, the invention in particular relates to a terpyridine zinc benzoate complex [Zn(tpb)2(Htpb)2] and its preparation condition. The terpyridine zinc benzoate complex prepared by the invention has a coordination structure shown as formula (1) in the specification, can emit yellow fluorescence under 365nm ultraviolet irradiation, and has high thermal stability, and the skeleton starts decomposition at a temperature above 300DEG C, so that the complex can be used as a zinc based fluorescent solid material in various fields, and specifically can be applied to ink anti-counterfeiting technology, like anti-counterfeiting of bills, currencies, securities and other high-end products. According to the preparation condition of the terpyridine zinc benzoate complex [Zn(tpb)2(Htpb)2] provided by the invention, the operation is simple, the crystallization purity is high, and the yield is up to 69%. (formula (1)).
Owner:CHONGQING NORMAL UNIVERSITY
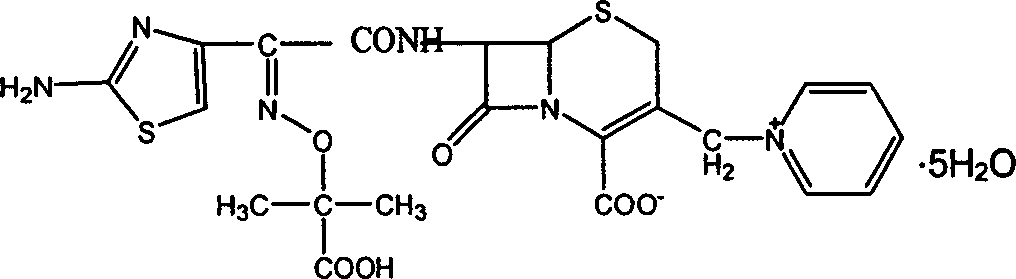
Ceftazidime pentahydrate purifying method
The method relates to a method for purifying ceftazidime, more concretely relating to a method for preparing high quality ceftazidime pentahydrate by impure, high-polymer impurity content ceftazidime through crystallization. It prepares impure, high-polymer impurity content ceftazidime pentahydrate, ceftazidime Hclú¼ceftazidime hydrobromide or ceftazidime overdue or recovered from the market, into a ceftazidime water solution, then regulates the pH value of the ceftazidime water solution to 1.5-2.5 by alkali or acid, here the impurity ceftazidime polymer is separated out with a little ceftazidime, and the separated-out matter is filtered, and the pH value of the filtrate is regulated to 3.5-4.8 by alkali so that the ceftazidime pentahydrate crystals are separated out. The method is simple and convenient to operate, low-cost, good-safety and high-yield. The obtained ceftazidime pentahydrate crystals are high purity and the polymer is low-content, reaching pharmacopoeia specified requirements.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD +1
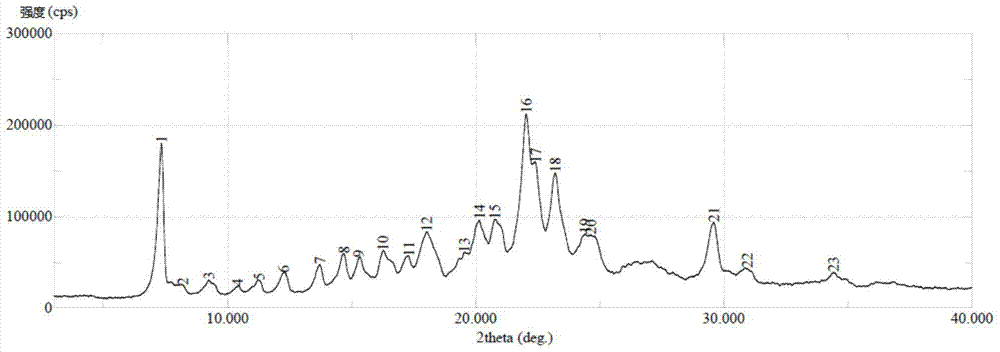
Crystal form of sorafenib tosylate, and preparation method thereof
InactiveCN104761492AHigh crystal purityHigh crystallinityOrganic chemistry methodsSulfonic acids salts preparationX-rayPowder diffraction
The invention provides a crystal form of sorafenib tosylate, and a preparation method thereof. The X-ray powder diffraction pattern of sorafenib tosylate of crystal form B has diffraction peaks when the values of 2theta are about 7.34DEG, 18.03DEG, 20.15DEG, 20.77DEG, 22.04DEG, 22.43DEG and 23.21DEG; and the X-ray powder diffraction pattern of sorafenib tosylate of crystal form C has diffraction peaks when the values of 2theta are about 7.61DEG and 13-33DEG. The sorafenib tosylate of crystal form C is prepared through reduced pressure heating of the sorafenib tosylate of crystal form B. The invention also provides a preparation method of amorphous crystals of sorafenib tosylate through reduced pressure heating of the sorafenib tosylate of crystal form B or C. The sorafenib tosylate crystals prepared in the invention have the advantages of good stability, high crystallization purity, simple preparation process, and suitableness for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing barium titanate monocrystal nano particles of six-pin structural perovskite
InactiveCN102691105AHigh crystal purityGood dispersionPolycrystalline material growthFrom normal temperature solutionsBarium titanatePotassium hydroxide
The invention discloses a method for preparing barium titanate monocrystal nano particles of six-pin structural perovskite, and a two-step hydrothermal method is adopted. The method comprises the following steps of: depositing tetrabutyl titanate which serves as a precursor by use of oxyhydroxide of titanium prepared from ethylene glycol monomethyl ether; performing hydrothermal reaction by utilizing potassium hydroxide a mineralizer, so as to obtain a K2Ti6O13 nano line; and performing secondary hydrothermal reaction on the prepared K2Ti6O13 nano line which serves as a titanium source, barium acetate which serves as a barium source and KOH which serves as the mineralizer, so as to obtain the barium titanate monocrystal nano particles of the six-pin structural perovskite. The process is simple and easy to control; pollution is avoided; the cost is low; large-scale production is realized; and the barium titanate monocrystal nano particle crystal of the six-pin structural perovskite is high in purity and high in dispersion.
Owner:ZHEJIANG UNIV
Prepn process of hydroprocessing catalyst
ActiveCN101088610AHigh pore volumeHigh surface areaMetal/metal-oxides/metal-hydroxide catalystsPlastic materialsActive component
The present invention relates to preparation process of hydrocarbon hydroprocessing catalyst, which has gamma-Al2O3 as carrier, VIB and VIII metal as the active component, and Ti, etc as the active assistant, with the assistant Ti being introduced in the pH swinging carbonization process for producing aluminum hydroxide. The process of preparing the catalyst is one complete mixing and kneading process including the steps of introducing Ti to aluminum hydroxide powder via adding Ti salt solution, adding Mo and / or W containing alkaline solution, mixing and kneading until the aluminum hydroxide powder is wetted completely by the alkaline solution, adding Co and / or Ni containing acid solution, mixing and kneading to obtain plastic material, extruding, drying, and roasting to obtain the catalyst. The catalyst has homogeneously distributed Ti component and obviously raised performance.
Owner:CHINA PETROLEUM & CHEM CORP +1
Extraction technology of glutamic acid recovered by combining crystal transformation and ion exchange
InactiveCN101671705AAvoid Yield ImpactHigh crystal purityOrganic compound preparationClimate change adaptationHigh concentrationMycoprotein
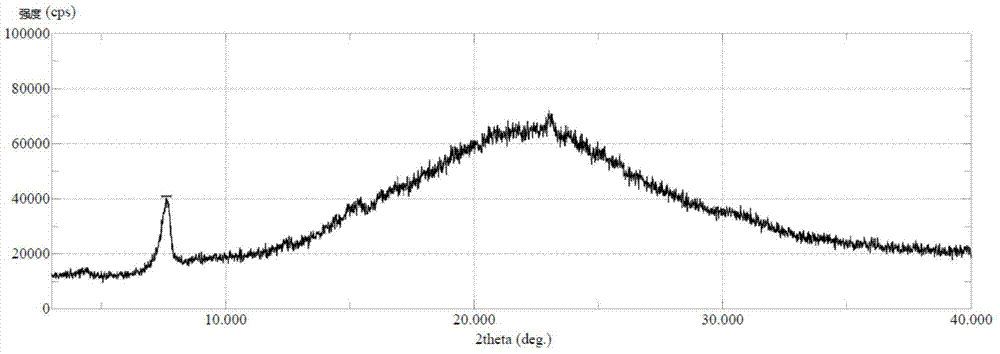
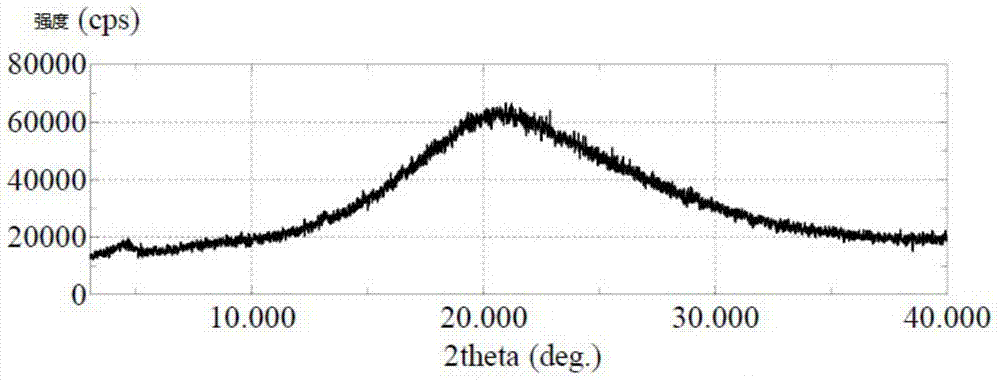
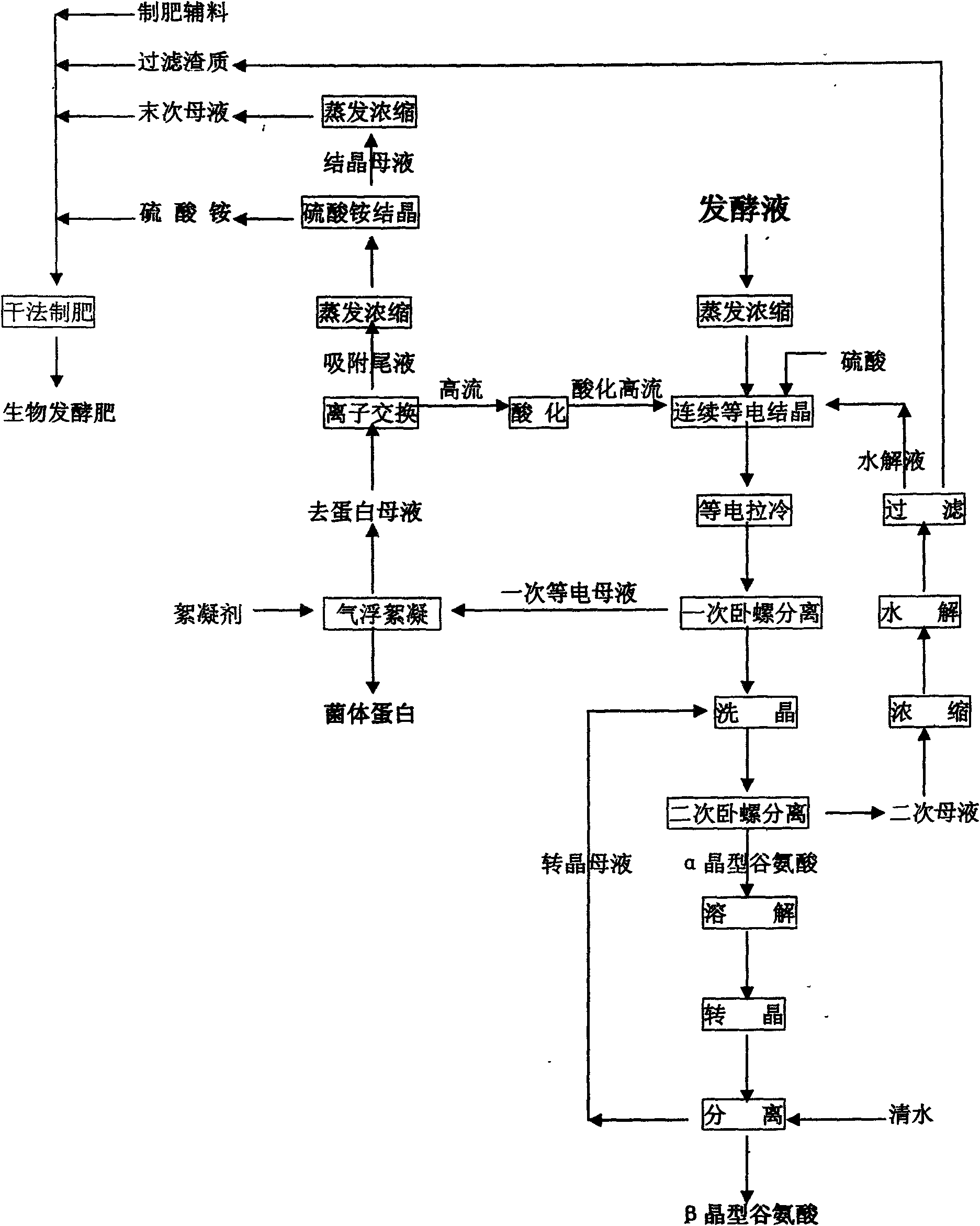
The invention relates to an extraction technology of a glutamic acid recovered by combining crystal transformation and ion exchange, belonging to the technical field of food industry. The extraction technology comprises the following technical steps: using the fermentation liquor of the glutamic acid, and heating, evaporating and concentrating the fermentation liquor; cooling the fermentation liquor to 25-50 DEG C after the fermentation liquor is evaporated and concentrated so that the glutamic acid is crystallized and separated out in an alpha form; obtaining an alpha form glutamic acid crystal, a first isoelectric separation mother liquor and a secondary separation mother liquor; enabling the glutamic acid to be crystallized and separated out in a beta crystal form, calling crystal transformation; separating feed liquor to obtain a beta crystal form glutamic acid crystal after the crystal transformation; after the secondary separation mother liquor is concentrated, adding a sulphuricacid to hydrolyze the concentrated secondary separation mother liquor, separating to obtain hydrolysis liquid and solid filter residues, i.e. humus; returning the first isoelectric separation motherliquor to a continuous crystallizing tank, and then using the first isoelectric separation mother liquor as an acid for isoelectric crystallization. The invention achieves the total extraction yield of 97-98 percent, greatly enhances the crystallization purity, greatly reduces the consumption of the sulphuric acid and liquid ammonia, recovers the mycoprotein and thoroughly eliminates the pollutionof high-concentration waste water.
Owner:FUXIN HAOSEN BIOSCI
Method for preparing amorphous atorvastatin calcium
The invention discloses a method for preparing amorphous Atorvastatin calcium. The current method uses more solvent, so the cost is over high; and the residual quantity of the solvent in a product is large, thereby causing big influence on the quality of the product and causing serious environmental pollution. In the method, alcohol solvent and water are combined into mixed alcohol-water solvent which dissolves Atorvastatin calcium containing one or more crystal forms completely; proper temperature is kept for ensuring that the Atorvastatin calcium is not precipitated; and the amorphous Atorvastatin calcium is precipitated by a spray drying method. The method uses the mixed alcohol-water solvent which is removed from the product easily, has little organic residue and causes less influence on drug quality; the usage amount of the solvent is reduced greatly; the concentration of the Atorvastatin calcium in the solution is high; and the purity of the prepared product of the amorphous Atorvastatin calcium is high.
Owner:ZHEJIANG JINGXIN PHARMA
Crystallization-type ilaprazole sodium and preparation method thereof
The invention discloses a crystallization-type Ilaprazole sodium and a preparation method thereof. The Ilaprazole sodium with a novel crystalline form provided by the invention is easy to prepare, high in purity and low in impurity content. The preparation method provided by the invention needs low solvent amount, has advantages of low production cost, simple operation and mild reaction condition, is easy to control, and can produce the target product crystalline form doubtlessly and well-repeatedly.
Owner:LIVZON PHARM GRP INC
Preparation method of aluminium oxide dry glue
InactiveCN104649307AAdvantages of preparation methodAdequate responseAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationAluminateSaline solutions
The invention discloses a preparation method of aluminium oxide dry glue. The preparation method comprises the following contents: (1) adding bottom water into the bottom of an impinging stream reactor and heating to a certain temperature, starting stirring blades at the bottom; (2) starting an accelerator and respectively injecting an alkali metal aluminate aqueous solution and CO2 airflow by accelerating tubes at the upper part of the impinging stream reactor, atomizing the alkali metal aluminate aqueous solution by a pressure atomizing nozzle, carrying out a gas-liquid impinging stream reaction between the atomized alkali metal aluminate aqueous solution and the CO2 airflow in a gas-liquid impinging area so as to generate aluminium oxide crystal nucleus, and letting the aluminium oxide crystal nucleus enter a settling zone after settlement; (3) simultaneously and continuously adding an acidic aluminium saline solution and the alkali metal aluminate aqueous solution or an alkali precipitant into feed inlets II and III at two ends of the bottom of the impinging stream reactor after the end of the gas-liquid impinging stream reaction, adjusting pH value, carrying out a neutralization reaction and stabilizing for a period of time; and (4) shutting down the impinging stream reactor after the end of the stabilization, aging, filtering, washing and drying to obtain the aluminium oxide dry glue. The aluminium oxide dry glue can be used for preparation of a hydrogenation catalyst carrier.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing high-purity all-trans lycopene crystal
ActiveCN101417917AEasy to dehydrateGood to miscellaneousMicroorganism based processesWaste based fuelForeign matterIsomerization
The invention discloses a preparation method of high-purity all-trans lycopene crystal, and comprises the following steps: filtering the ferment material liquid, thus obtaining mycelium; adding polar organic solvent to the mycelium to grind and break the wall, and then filtering and removing the organic solvent; taking the mixture of mycelium and low-boiling petroleum based organic solvent as degreaser to remove mycelium oily consistituent and the foreign matter such as little amount of Beta-carotene; taking the mixture of mycelium and low-boiling petroleum based organic solvent as extractingagent to liquid-solid extract the lycopene in mycelium; cooling down the extract liquor for crystallization; filtering and drying in vacuum, thus obtaining high-purity all-trans lycopene crystal. Thetechnique adopted by the method can avoid lycopene from retrogradation and isomerization owing to heat and oxidation. The lycopene crystal prepared by the method has high purity, the content of lycopene exceeds over 97 percent, and the content of the all-trans structure exceeds over 90 percent.
Owner:JIANGSU ALAND NOURISHMENT
Quantum dot, preparation method thereof and application thereof
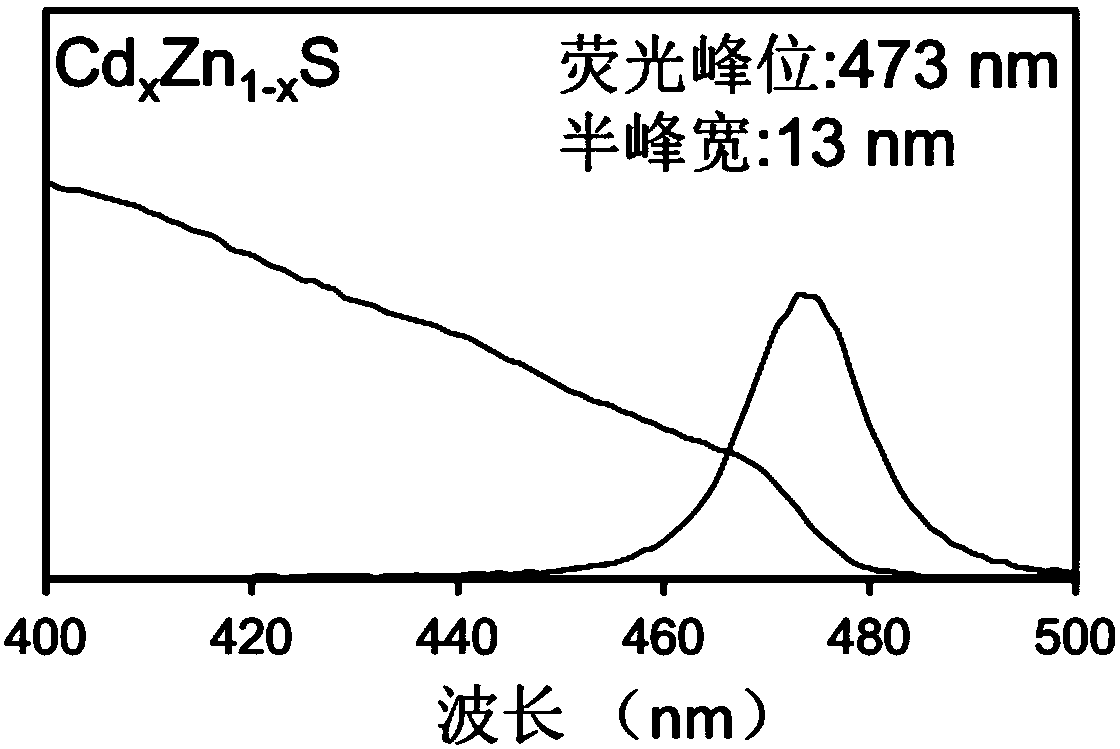
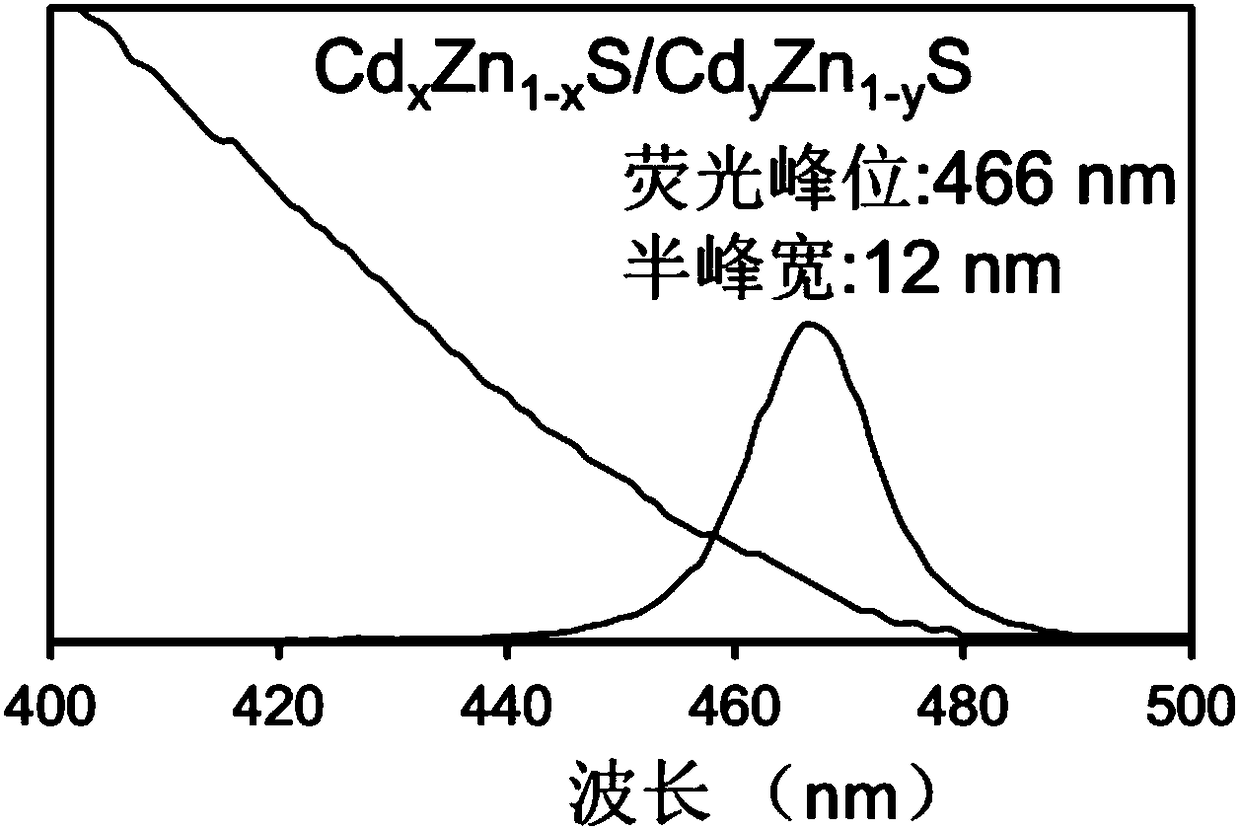
ActiveCN108410467AHalf maximum widthPrevent proliferationNanoopticsLuminescent compositionsSulfurFluorescence
The invention provides a quantum dot, a preparation method thereof and application thereof. The preparation method comprises the following steps: S1, providing a precursor mixture of a zinc-containingprecursor and a cadmium-containing precursor, wherein a molar ratio of zinc in the precursor mixture is greater than and equal to 10; S2, reacting first sulfur precursor with the precursor mixture toobtain a CdS / ZnS core-shell quantum dot system; S3, performing alloying treatment on the CdS / ZnS core-shell quantum dot system to obtain a quantum dot system containing Cd[x]Zn[1-x]S; and S4, addingcadmium carboxylate and a second sulfur precursor into the quantum dot system containing Cd[x]Zn[1-x]S to grow a shell layer, thereby obtaining a Cd[x]Zn[1-x]S / Cd[Y]Zn[1-Y]S core-shell quantum dot, wherein X is greater than 0 and smaller than 1, and Y is greater than 0 and smaller than 1. The Cd[x]Zn[1-x]S / Cd[Y]Zn[1-Y]S core-shell quantum dot obtained by the preparation method is relatively narrowin half-peak width in a blue-light area and is relatively high in fluorescence efficiency.
Owner:NANJING TECH CORP LTD
Crystallization method for preparing high-purity I-type clopidogrel hydrogen sulfate
The invention discloses a method for preparing high-purity I-type clopidogrel hydrogen sulfate, belonging to the technical field of finding and preparing of drug crystal forms. The method comprises the following four steps of: (A) transforming a clopidogrel salt into free alkali thereof at a lower temperature; (B) dropwise adding sulfuric acid into aqueous alkali at 20 to 25 DEG C, reacting and crystallizing; (C) growing crystals for 1 to 2 hours at the same temperature; and (D) washing an obtained solid by using ethyl acetate, and performing vacuum drying. An I-type clopidogrel hydrogen sulfate crystal prepared by using the method is determined to be the high crystal form purity I-type clopidogrel hydrogen sulfate after being subjected to X-ray powder diffraction, infrared spectrum and thermal analysis. An appropriate amount of seed crystal is added during crystallization, the crystallization speed is obviously increased, and the crystallization can be completed within about 5 hours.
Owner:JIANGNAN UNIV
Crystal-form ilaprazole sodium and preparation method thereof
The invention discloses a crystal-form ilaprazole sodium and a preparation method thereof. The novel ilaprazole sodium crystal form provided by the invention is easy to prepare, and has advantages of high purity and low impurity content. The preparation method provided by the invention needs low solvent amount, has advantages of low production cost, simple operation and mild reaction condition, is easy to control, and can produce the target product crystalline form doubtlessly and well-repeatedly.
Owner:LIVZON PHARM GRP INC
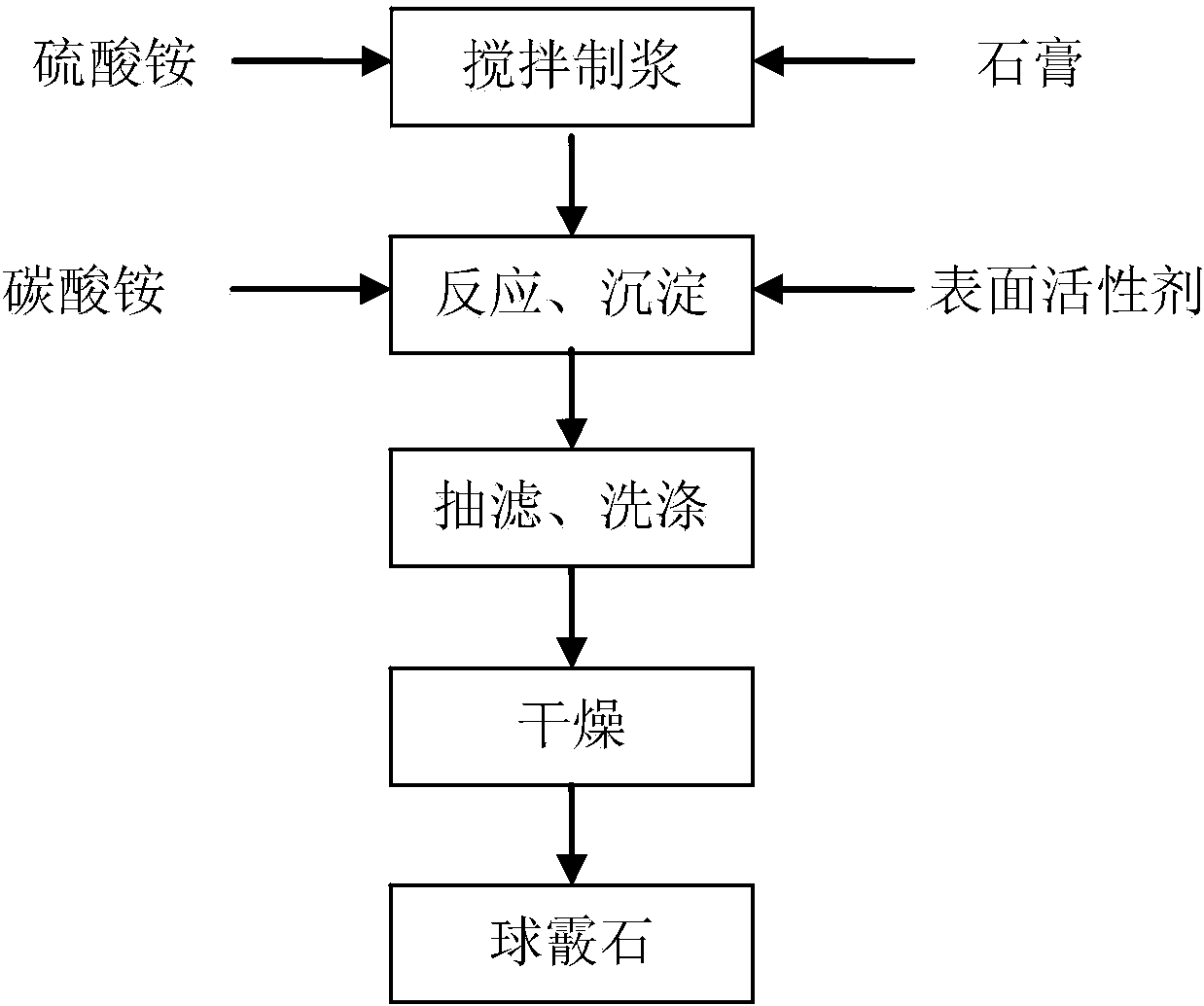
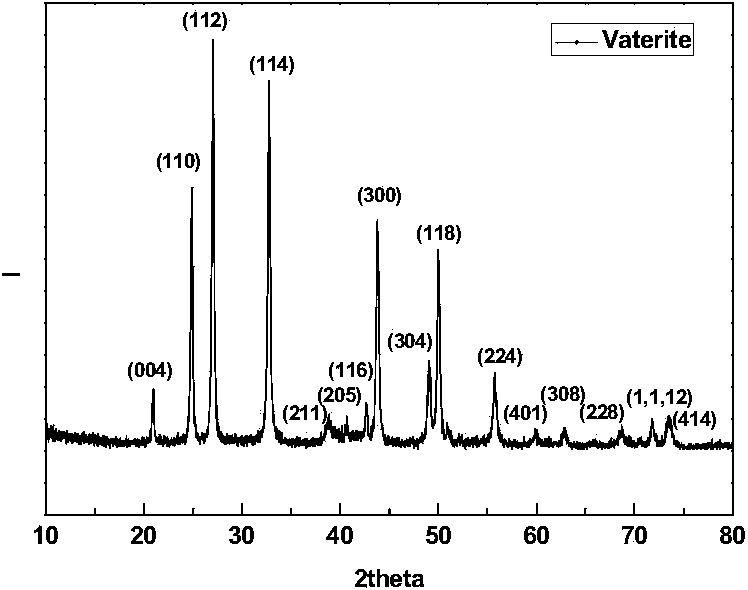
Method for preparing high-purity metastable vaterite calcium carbonate from gypsum
ActiveCN103922378ASolve the problem of low-quality utilizationEase of industrial productionCalcium/strontium/barium carbonatesHigh concentrationReaction temperature
The invention relates to a method for preparing high-purity metastable vaterite calcium carbonate from gypsum. The method comprises the following concrete steps: (1) adding an ammonium sulfate solution into a reactor from the constant temperature to the reaction temperature, adding the gypsum into the reactor, and stirring for pulping for 25-35 minutes; (2) adding an ammonium carbonate solution into the reactor; (3) adding an anionic surfactant accounting for 0.1%-5% by mass of the gypsum into the reactor in the step (1); (4) adjusting the rotary speed of a stirring paddle and reacting at the constant temperature to obtain a reaction product; and (5) filtering the reaction product in the step (4) in a conventional way, washing, and drying to obtain the high-purity metastable vaterite calcium carbonate. The vaterite calcium carbonate produced by adopting the method adopts the gypsum as a raw material, a technical support can be provided for solving the problem of recycling of industrial solid wastes-gypsum, and wastes are turned into wealth; the vaterite calcium carbonate is high in crystal purity and easy to prepare, the yield of the vaterite calcium carbonate is increased due to high-concentration ammonium carbonate reaction, and the production cost of the vaterite calcium carbonate can be greatly reduced.
Owner:EAST CHINA UNIV OF SCI & TECH
Synthetic method of (ethoxymethylene)-malononitrile
ActiveCN102584626AReduce pollutionLow costCarboxylic acid nitrile preparationOrganic compound preparationEthyl esterEthyl fumarate
The invention relates to a synthetic method of (ethoxymethylene)-malononitril, which includes the following steps: (1) dropwise adding malononitrile into triethyl orthoformate in ice bath cooling condition, and adding catalyst anhydrous zinc chloride after dropwise adding for reaction for 2-4 hours, and (2) steaming and removing ethanol after the reaction is finished, cooling for dissolving out solids, and filtering and collecting the solids to obtain the (ethoxymethylene)-malononitrile. The synthetic method is easy to operate, high in yield, low in cost, environment-friendly, high in product crystallization purity, stable in quality and suitable for large-scale industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
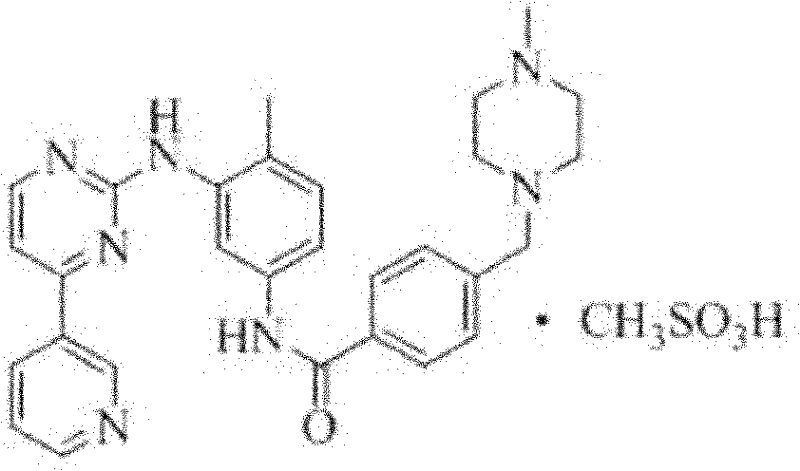
Method for preparing alpha-imatinib mesylate
ActiveCN102190649AHigh crystal purityReduce manufacturing costOrganic chemistryOrganic solventSolvent
The invention discloses a method for preparing alpha-imatinib mesylate, and belongs to the field of medicines. The method comprises the following steps of: suspending imatinib base in a mixed solvent of water and an organic solvent, stirring, and adjusting the temperature to be 20-30DEG C; dissolving methanesulfonic acid, and dripping into reaction liquid; after the dripping is finished, standingfor clarifying the liquid, heating to the temperature of between 50 and 60DEG C, and keeping the temperature to react for 0.5 to 1 hour; and reducing the temperature to 32-40DEG C, adding alpha-imatinib mesylate seed crystals, dripping the organic solvent, continuously reducing the temperature to 20-30DEG C, separating out crystals, filtering, and drying to obtain a product. The preparation method has the advantages of simple operation, short reaction time, high yield, high purity of alpha-imatinib mesylate, low production cost, and suitability for industrial production.
Owner:山东安信制药有限公司
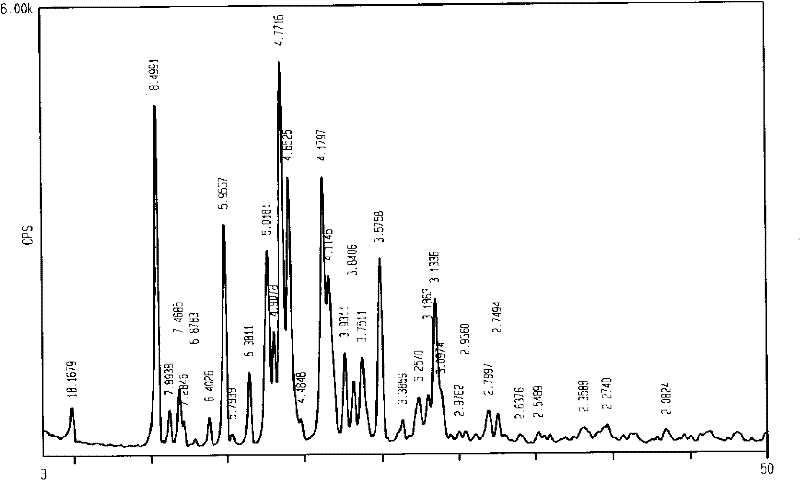
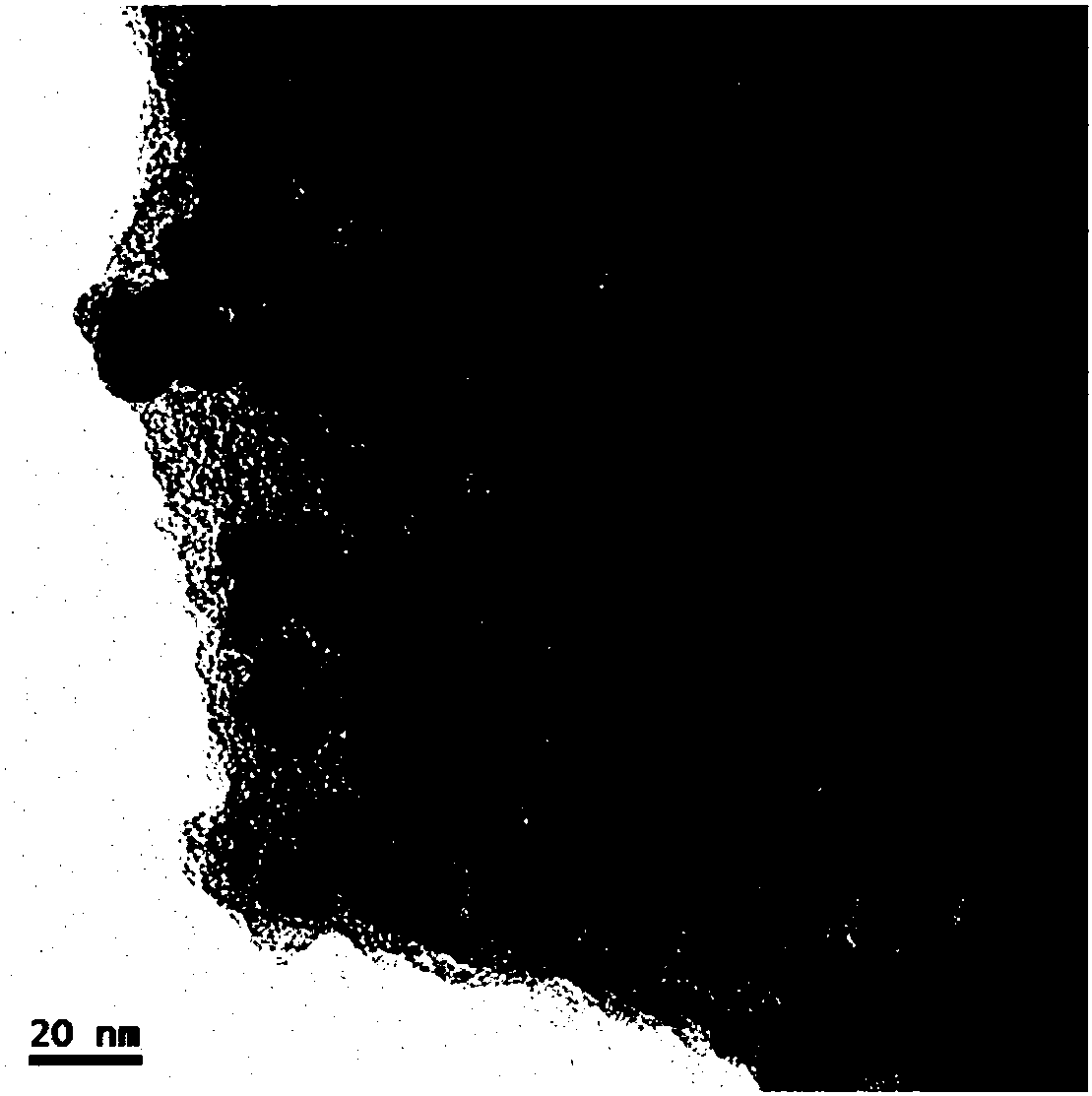
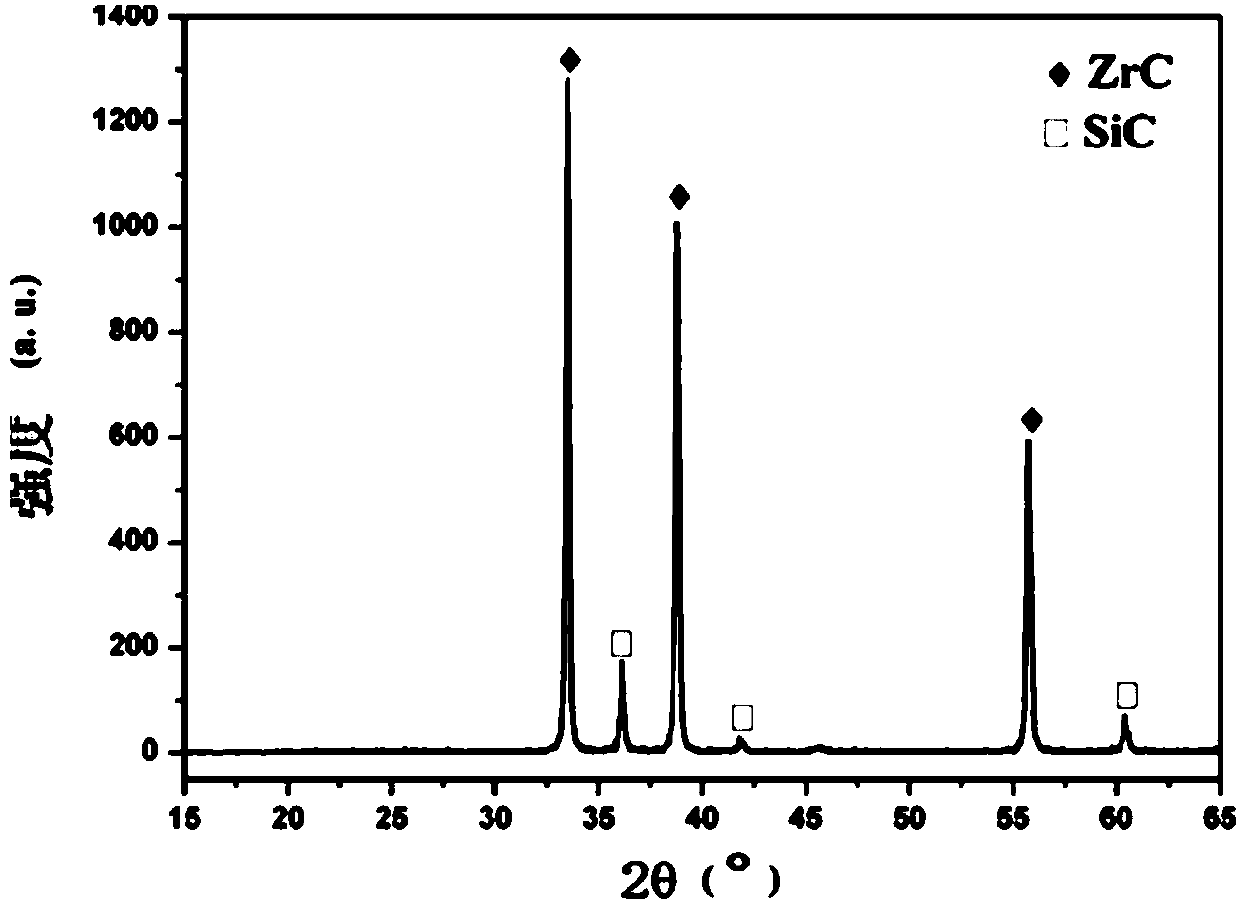
Zirconium carbide-silicon carbide composite powder material and preparation method thereof
The application discloses a zirconium carbide-silicon carbide composite powder material, wherein a molar ratio of components in the zirconium carbide-silicon carbide composite powder material satisfies ZrC:SiC=1:0.1-1:10; a preparation process is easy, the cost is low, and the process is easy to control; and the obtained zirconium carbide-silicon carbide composite powder material has the advantages of submicron level, uniform composition and high purity.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI +1
A kind of preparation method of fluidized bed catalyst
ActiveCN104549528BAdequate responseQuick buildCatalyst carriersCatalyst activation/preparationVapor liquidAluminate
The invention discloses a preparation method of an ebullated bed catalyst. The method includes the following contents: (1) adding the reaction solution at the bottom of the impinging flow reactor and heating it, and starting the stirring paddle at the bottom; (2) injecting the alkali metal aluminate aqueous solution and the CO2 gas flow through the acceleration tube on the upper part of the impinging flow reactor respectively , after the alkali metal aluminate aqueous solution is atomized, it undergoes a gas-liquid impact flow reaction with the CO2 gas flow to generate aluminum hydroxide crystal nuclei and enters the sedimentation zone; III At the same time, add acidic aluminum salt aqueous solution and alkali metal aluminate aqueous solution or alkaline precipitant continuously to adjust the pH value and neutralize the reaction; (4) aging, filtering, washing and drying to obtain alumina dry glue; (5) adding Alumina dry glue, small-hole SB powder and safflower powder are mixed evenly, then an adhesive is added to form a plastic body, extruded into a strip, dried and roasted to obtain an alumina carrier, impregnated with active metals, dried and roasted to obtain a fluidized bed catalyst. In the method of the invention, an impingement flow reactor is adopted to strengthen the transfer characteristics between the gas-liquid two-phase and the liquid-liquid two-phase, and the prepared ebullated bed catalyst has high strength and low wear, and the pore volume and pore diameter of the catalyst are relatively large.
Owner:CHINA PETROLEUM & CHEM CORP +1
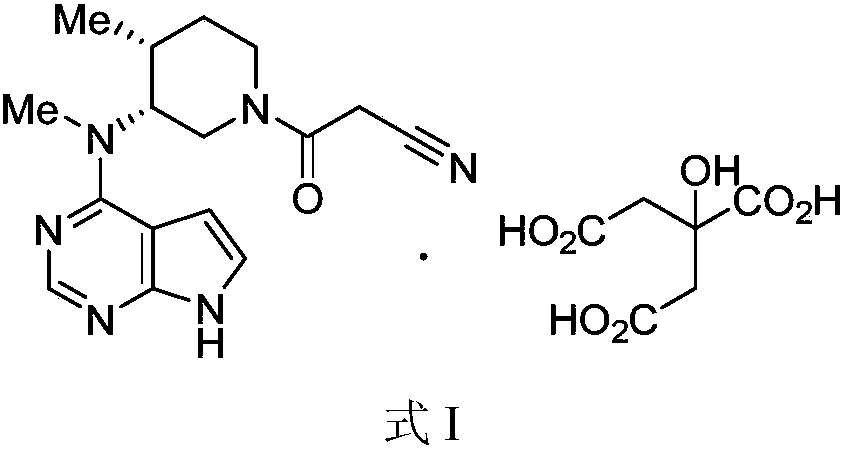
New method for preparing citric acid tofacitinib medicinal crystal form
InactiveCN107814802AImprove the purification effectEasy to operateOrganic chemistryTofacitinibCitric acid
The invention belongs to the field of medicine and particularly relates to a new method for preparing a citric acid tofacitinib medicinal crystal form. The method has the advantages of being simple inoperation, good in reproducibility, high in yield, good in purifying effect for API related substances, high in crystal form purity, suitable for industrialized mass production and the like, and overcomes the problems of complicated operation, low reproducibility, low yield, poor purifying effect for API related substances, low crystal form purify, unsuitability for industrialized production andthe like.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Preparation method of sodium citrate crystal through solvent-out crystallization
ActiveCN104262139AHigh yieldUniform grainOrganic compound preparationOrganic chemistry methodsReaction temperatureCrystallization temperature
The invention relates to a preparation method of sodium citrate crystal through solvent-out crystallization. The preparation method comprises the following steps: (A) carrying out salt-forming reactions: preparing critic acid and a salt forming agent according to a certain mole ratio, dissolving the salt forming agent into water at first, then slowly adding critic acid into the water, controlling the reaction temperature at a range of 20 to 30 DEG C, wherein the pH value of the solution is in a range of 7.5 to 9.0 when the reactions finish, and filtering the solution after the reactions so as to obtain a mixed solution, wherein the mixed solution concentration calculated by waterless sodium citrate is 285 g / L to 570 g / L; (B) carrying out solvent-out crystallization: adding a solvent-out agent into the mixed solution obtained in the step (A) according to a certain volume ratio to crystallize the sodium citrate crystals, and controlling the crystallization temperature at 20 to 75 DEG C; (C) filtering and drying so as to obtain the sodium citrate product; wherein the sodium citrate product comprises waterless sodium citrate, dihydrate sodium citrate, and pentahydrate sodium citrate. The provided preparation method is simple and convenient, the yield is high, the crystal is large, the purity is high, and the sodium citrate quality is largely improved.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
Preparation method of I type clopidogrel hydrogen sulfate
InactiveCN101805354AHigh crystal purityGood repeatabilityOrganic chemistryReaction temperatureNitrogen
The invention discloses a preparation method of I type clopidogrel hydrogen sulfate. The method comprises the following steps: (1) dissolving clopidogrel free base in tetrahydrofuran, and adding concentrated sulfuric acid at the temperature of minus 14-0 DEG C; (2) leading the mixture to react for 1-2 hours, then slowly increasing the reaction temperature to 20-40 DEG C, continuing the reaction for 10-12 hours, and fully precipitating crystals; and (3) filtering after obtaining the crystals, washing a filter cake with the tetrahydrofuran, draining, vacuum-drying and obtaining the I type clopidogrel hydrogen sulfate. The I type clopidogrel hydrogen sulfate prepared through the method is not doped with II type clopidogrel hydrogen sulfate and has high crystal phase purity, and the preparation process does not need the steps of nitrogen production and the like, thereby reducing the production cost, leading the operation method to be simple and easy, realizing high repeatability and being easy to realize mass production.
Owner:SUN YAT SEN UNIV
Method for preparing arctin from burdock
InactiveCN101759735ALarge adsorption capacitySolution to short lifeSugar derivativesSugar derivatives preparationMicrofiltrationDiethyl ether
The invention relates to a method for preparing arctin from burdock, which adopts burdock medicinal material as the raw material and comprises the following steps: grinding the burdock medicinal material, reflux extracting for 2-3 times by ethanol, de-coloring by active carbon, concentrating to be low-concentration ethanol after microfiltration and ultrafiltration, adjusting pH, adsorbing by macro-porous absorption resin, eluting by water and ethanol, collecting burdock fraction, recovering a reagent, placing for crystallization, washing by petroleum ether and re-crystallizing by acetone and diethyl ether. The process is easy to enlarge production.
Owner:NANJING ZELANG MEDICAL TECH
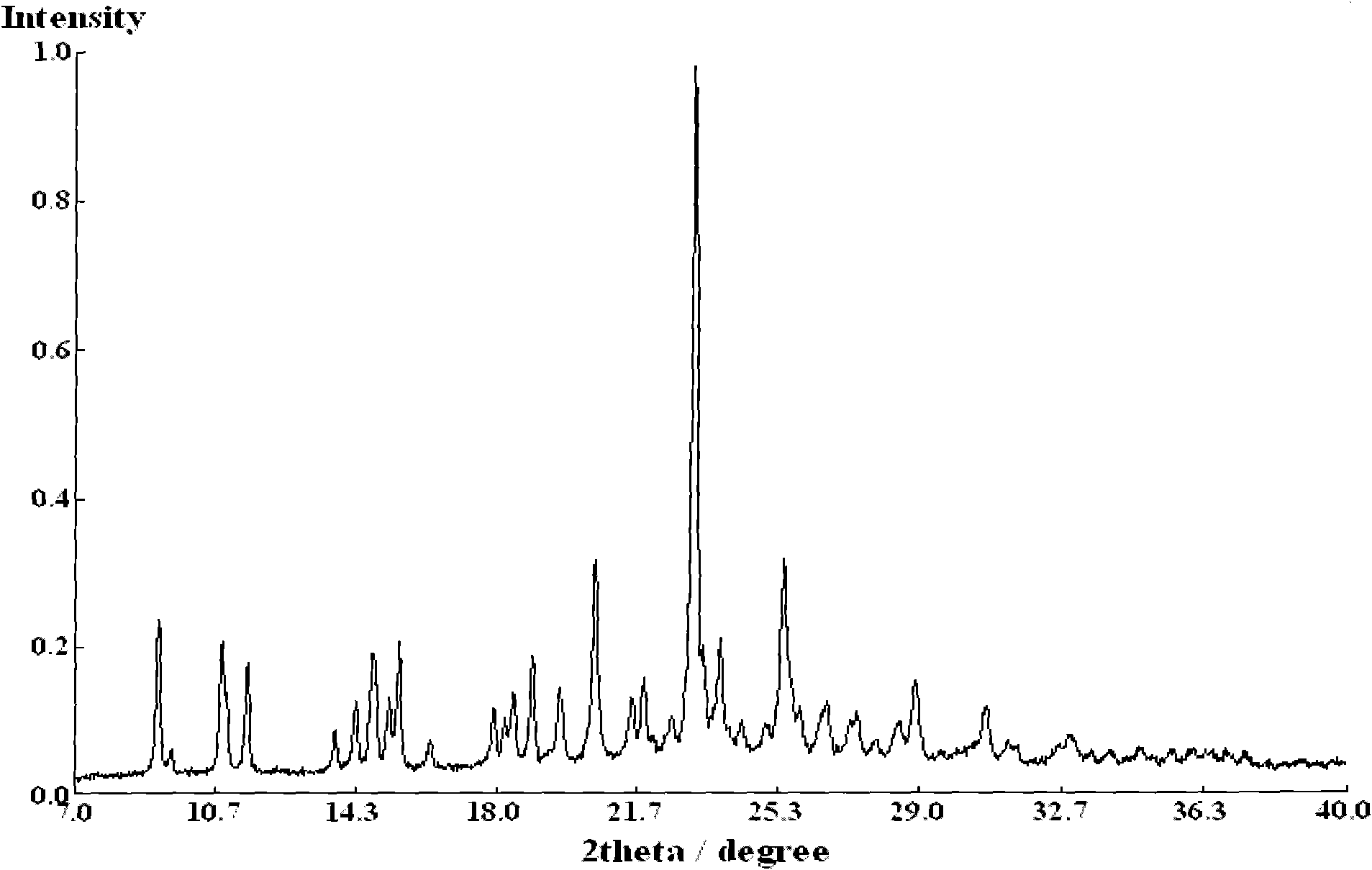
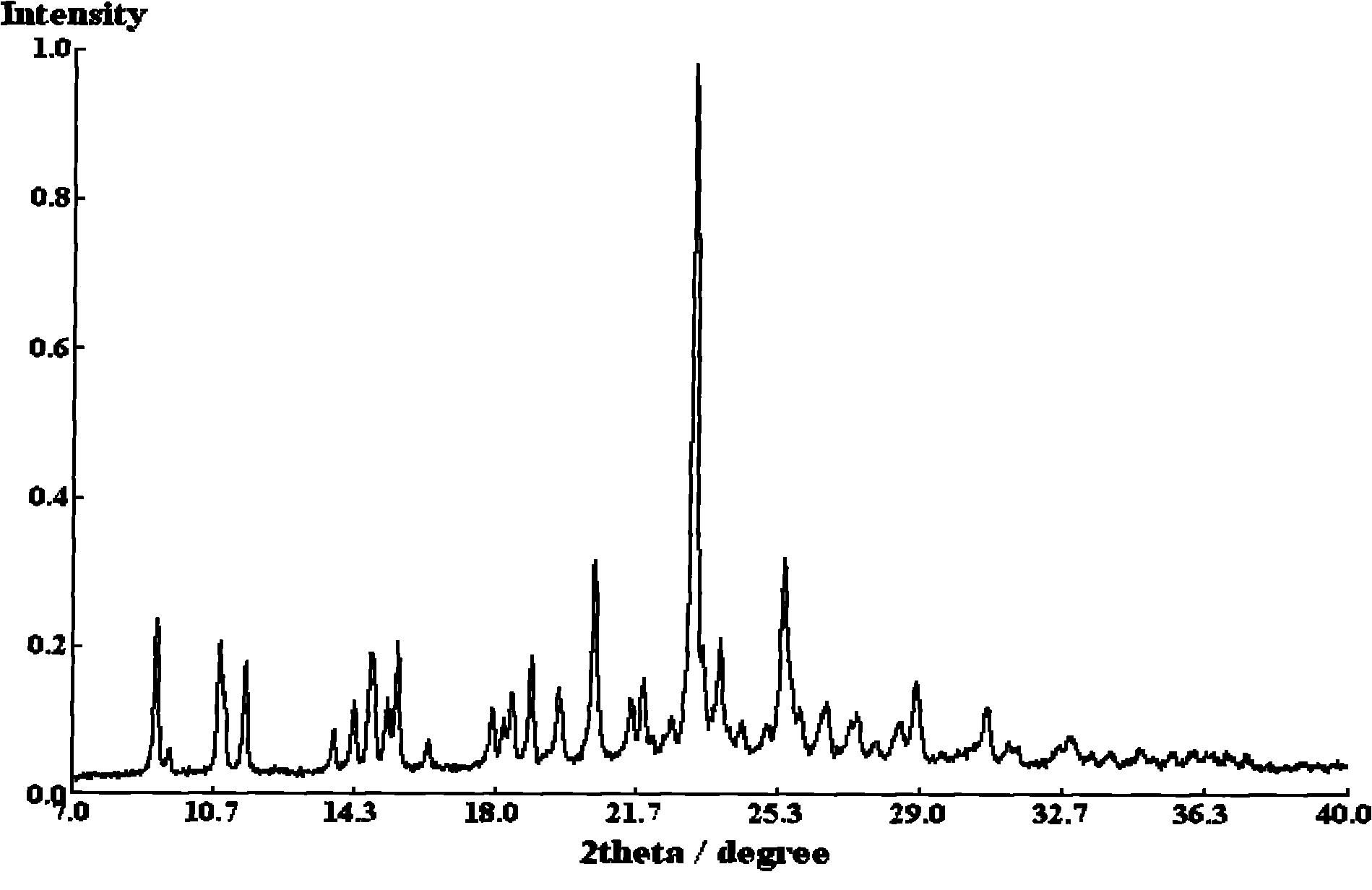
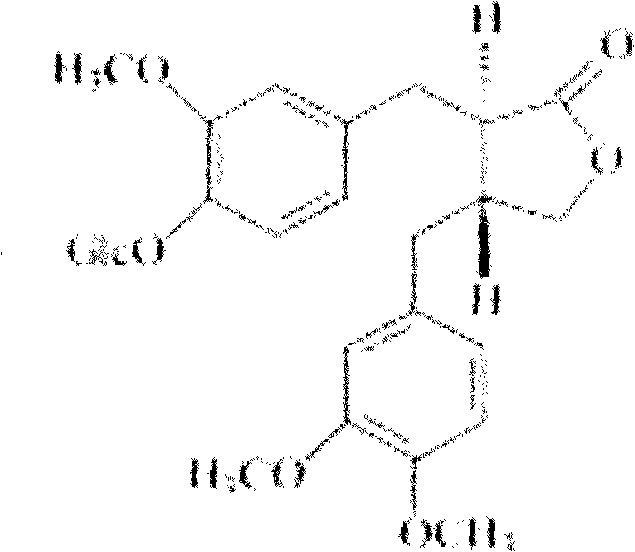
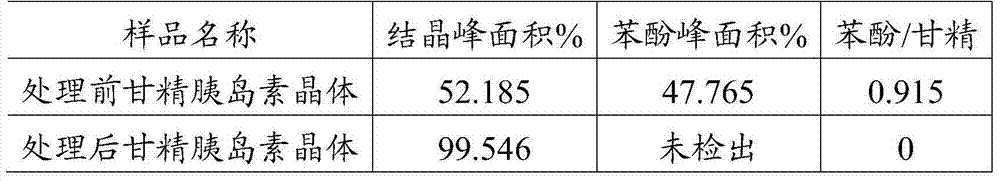
Purification method for insulin crystal or insulin analogue crystal
ActiveCN103709244AEasy to operateHigh crystal purityPeptide preparation methodsInsulinsDispersityPurification methods
The invention belongs to the field of pharmaceutical chemistry, and discloses a purification method for a crystal of insulin or an analogue thereof. The purification method comprises: adopting an easy-volatilizing and low-toxicity organic solvent to rinse and leach an insulin crystal or an insulin analogue crystal in a short time so as to completely remove the trace poor-volatility and high-toxicity organic solvent remaining in the crystal, and drying to completely remove the good-volatility organic solvent introduced during rinsing and leaching after completing leaching so as to solve the problem of residue of the difficultly-volatilized organic solvent in the crystal. According to the purification method, the operation is simple and quick, the purified insulin crystal or insulin analogue crystal finished product has characteristics of high crystal purity, low solvent residue, uniform particle, good dispersity, and easy storage and sub-package compared with the untreated crystal.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
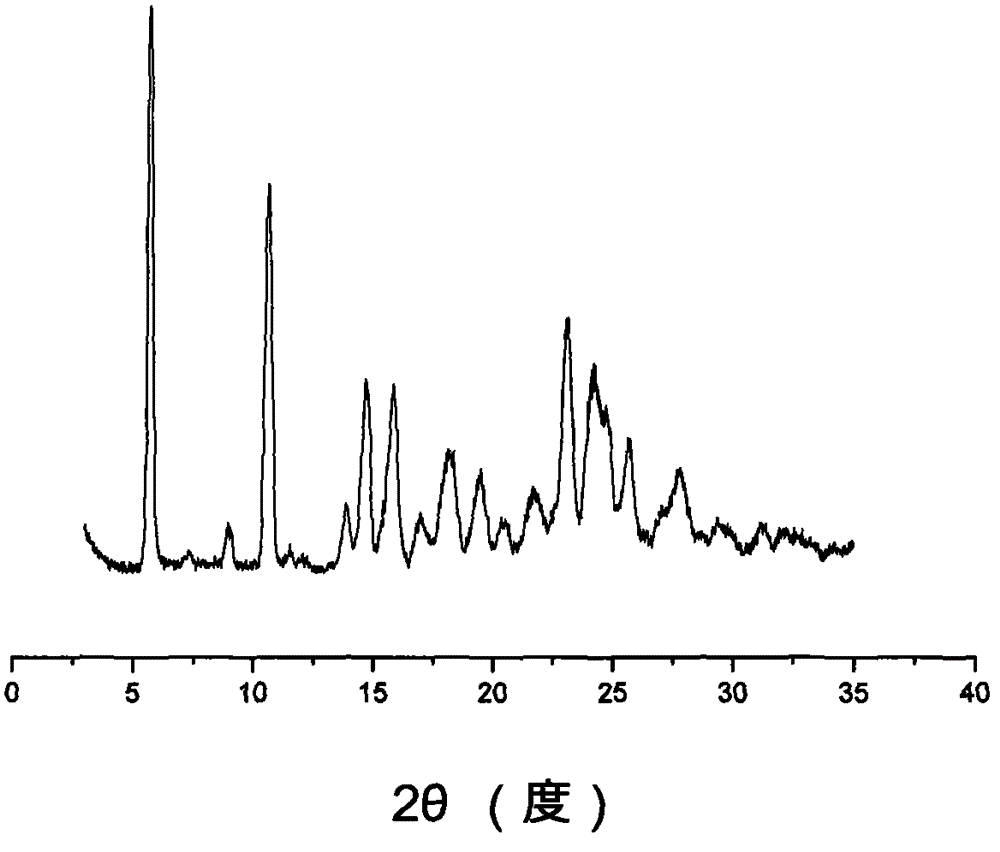
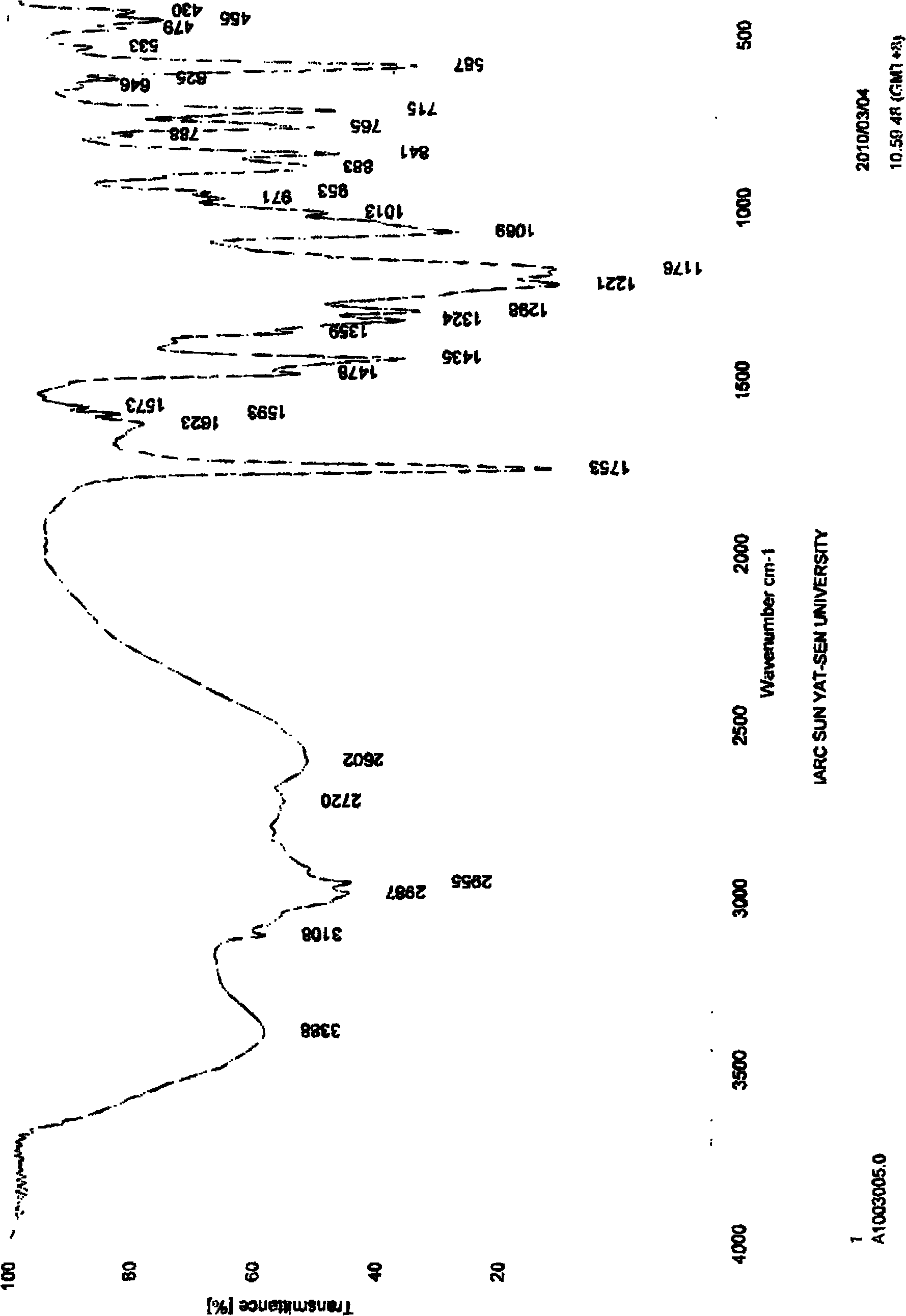
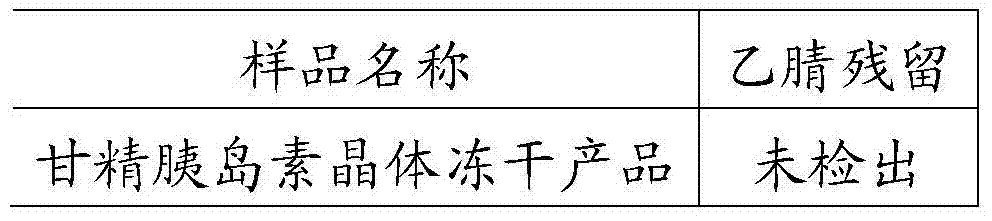
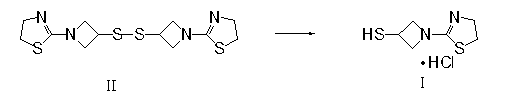
3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride crystal and preparation method thereof
The invention provides a 3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride crystal. X-ray powder diffraction detection indicates that a characteristic absorption peak exists at a diffraction angle (2 theta), as shown in Figure 1. The invention also provides a preparation method of the 3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride crystal, which comprises the following steps: adding grease containing 3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride into one or two mixed solvents, and crystallizing at 0-40 DEG C; and filtering to obtain the crystalline 3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride. The 3-sulfhydryl-1-(1,3-thiazoline-2-yl)-azetidine hydrochloride crystal provided by the invention has a unique crystal shape; and the crystal has the characteristics of high purity and stability and the like, and is beneficial to preservation.
Owner:天津市医药集团技术发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com