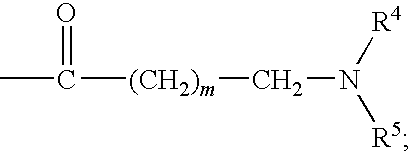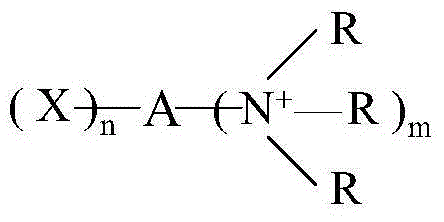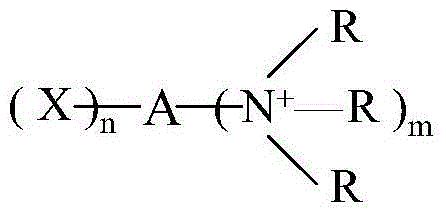Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
287 results about "Azetidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
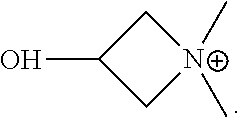
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
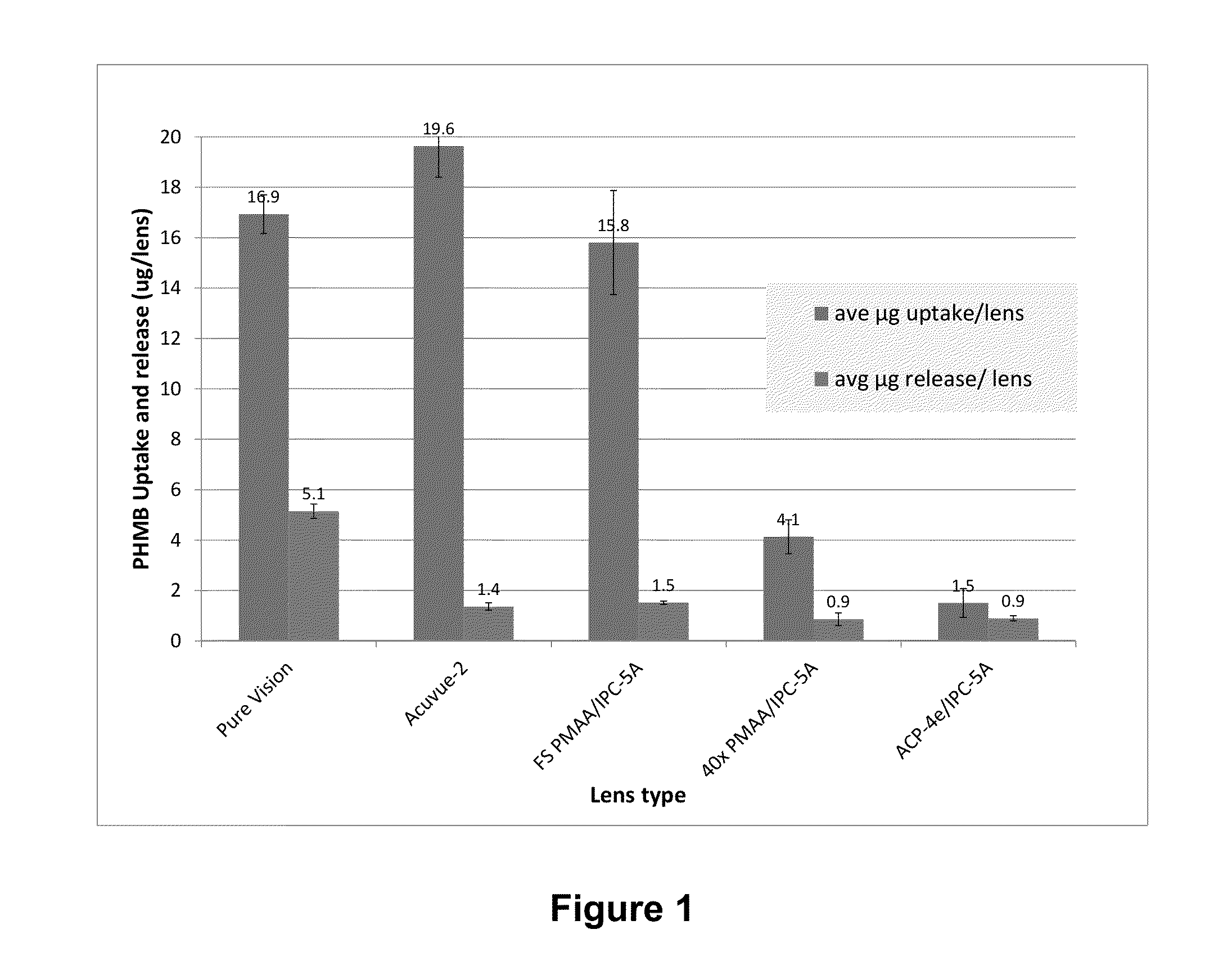
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20120026457A1Increased durabilityPicture framesSpecial ornamental structuresSilicone hydrogelWater soluble
Owner:ALCON INC
Azetidinium-containing copolymers and uses thereof
ActiveUS20130337160A1Good coating durabilityIncreased durabilityOrganic chemistryOptical articlesPolymer sciencePolymer chemistry
Owner:ALCON INC
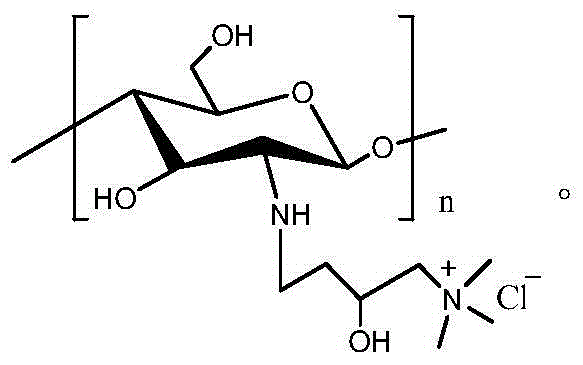
Azetidinium-functional polysaccharides and uses thereof
The present invention relates to polysaccharides that have been modified by providing azetidinium functionality thereto. Such functionality can be provided by crosslinking a polysaccharide with a resin having azetidinium functional groups. In one or more aspects, the polysaccharide can comprise one or more of starch, guar gum, alginate or derivatives thereof. Polysaccharides having azetidinium functionality according to the present invention are suitable for multiple uses. Such uses include, but are not limited to, removal of one or more solid materials from a liquid, beneficiation of an ore, removal of metallic ions from a liquid; providing oil from bitumen; and removal of mercury from synthetic gypsum. Other uses of the functionalized polysaccharides of the present invention include hydroseeding, dust control and corosion control.
Owner:GEORGIA PACIFIC CHEM LLC
Combinations of substituted azetidinones and CB1 antagonists
The present invention provides compositions, therapeutic combinations and methods including: (a) at least one selective CB1 antagonist; and (b) at least one substituted azetidinone or substituted β-lactam sterol absorption inhibitor which can be useful for treating vascular conditions, diabetes, obesity, metabolic syndrome and lowering plasma levels of sterols or 5α-stanols.
Owner:SCHERING CORP
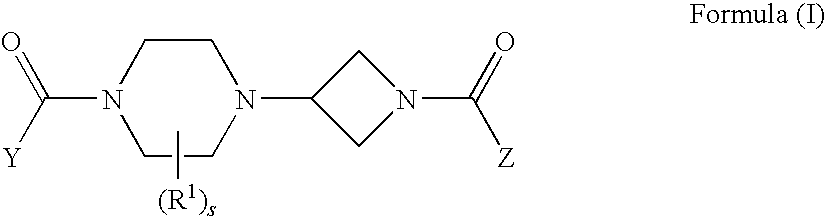
Azetidines as MEK inhibitors for the treatment of proliferative diseases
Disclosed are compounds of Formula (I) and pharmaceutically acceptable salts and solvates thereof. Such compounds are MEK inhibitors and are useful in the treatment of proliferative diseases, such as cancer. Also disclosed are pharmaceutical compositions containing such compounds as well as methods of using the compounds and compositions of the invention in the treatment of cancer.
Owner:EXELIXIS INC
Azetidinyl diamides as monoacylglycerol lipase inhibitors
Owner:JANSSEN PHARMA NV
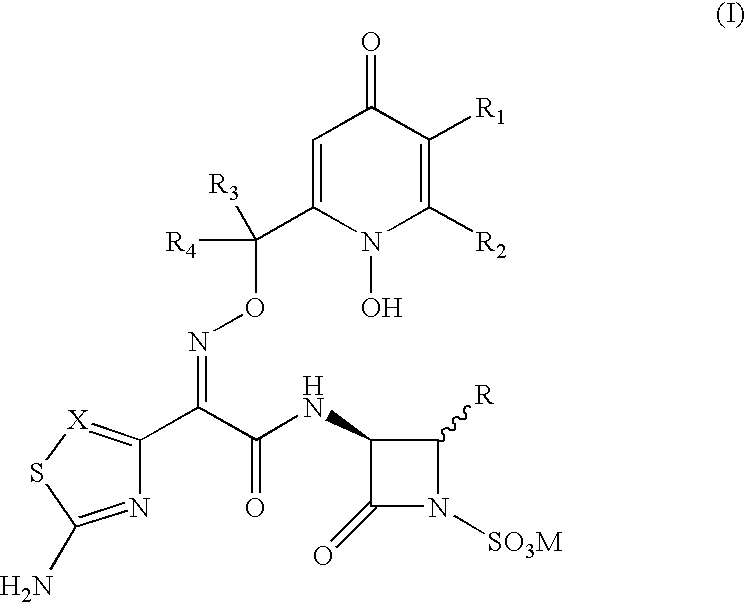
3-(Heteroaryl acetamido)-2-oxo-azetidine-1-sulfonic acids derivatives as antibacterial agents
The present invention relates to novel Syn isomers of racemates and optical isomers of 3-(heteroaryl acetamido)-2-oxo-azetidine-1-sulfonic acids of formula (I) and its use in treating the infections caused by gram-negative pathogenic bacteria.
Owner:REMPEX PHARM INC
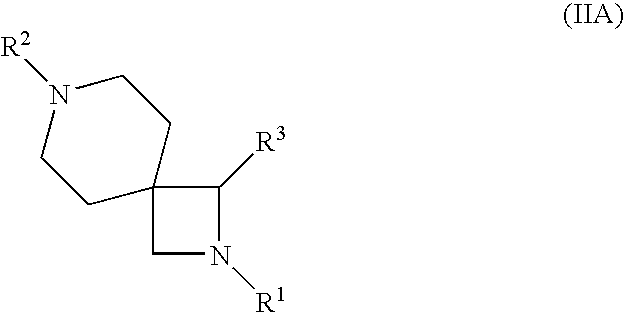
Azetidine derivatives useful in treating pain, diabetes and disorders of lipid metabolism
Disclosed are compounds of the formula: and compounds of the formula: wherein R1, R2, R3, R4, R5, u and v are as defined herein. Also disclosed are methods of treating pain (e.g., inflammatory pain, chronic pain, and neuropathic pain), methods of treating diabetes, and methods of inhibiting the absorption of cholesterol using compounds of formula I or IIA.
Owner:SCHERING CORP
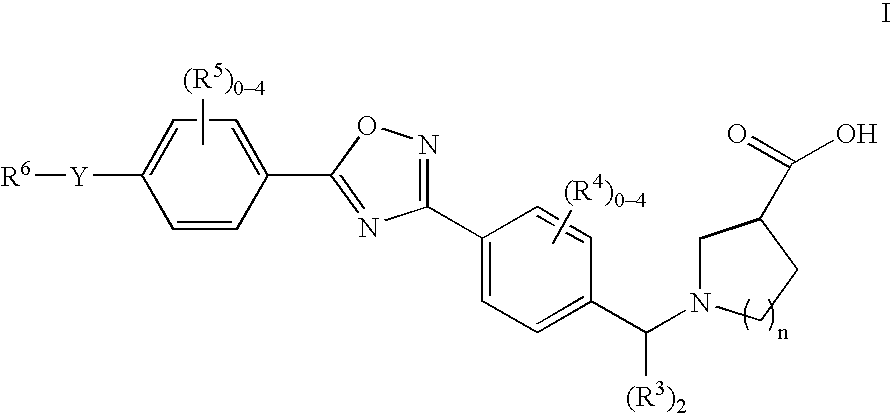
1-((5-aryl-1,2,4-oxadiazol-3-yl) benzyl)azetidine-3-carboxylates and 1-((5-aryl-1,2,4-oxadiazol-3-yl)benzyl) pyrrolidine-3-carboxylates as edg receptor agonists
The present invention encompasses compounds of Formula I: as well as the pharmaceutically acceptable salts and hydrates thereof. The compounds are useful for treating immune mediated diseases and conditions, such as bone marrow, organ and tissue transplant rejection. Pharmaceutical compositions and methods of use are included.
Owner:MERCK & CO INC
Ambient temperature rapid self-polymerization compositions of high cross-linked or linear type beta-amino-ester alternative co-polymers and their applications
InactiveUS20070299211A1Self-polymerization can be very rapidSelf-polymerization rate can be controlledCross-linkAmino esters
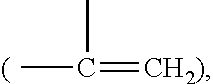
Self-polymerization of mono-aziridine (or azetidine) and multi-aziridine (or azetidine) containing compounds with vinyl group containing organic acid, such as acrylic acid (AA), 2-methylenesuccinic acid, 2,3-dimethylenesuccinic acid and etc, at ambient temperature results in the new type of cross-linked and linear type copolymers, respectively.The polymerization of multi-functional aziridine (or azetidine) containing compounds with vinyl group containing organic acid results in the formation of high cross-linked polymers. The self-polymerization takes place at ambient temperature and the resultants, cross-linked polymeric networked materials, are solvent insoluble and potential for adhesive, composite matrix and other applications. These insoluble materials are hydrolyzed in an acidic or basic condition to form the water soluble β-amino acids.A linear poly(β-aminoester) is obtained from the self-polymerization of vinyl group containing organic acid with mono-aziridine (or azetidine) containing compound at ambient temperature. poly(β-aminoester) is applicable for gene transfer, controlled drug release and other applications. This self-polymerization process offers a convenient route for preparing poly(β-aminoesters).
Owner:TAMKANG UNIVERSITY
Azetidinium modified polymers and fabric treatment composition
InactiveUS20050060812A1Organic detergent compounding agentsPhysical treatmentPolymer chemistryAcrylate
The invention relates to a fabric treatment composition which comprises a self-crosslinking polymer possessing pendant azetidinium groups. A first aspect of the invention comprises an azetidinium functionalised polymer containing primary or secondary amine groups. Cross-linking reactions between the azetidinium group and primary or secondary amine groups does not form quaternary groups and consequently does not result in a charged, cross-linked polymer. This lack of charge is believed to overcome the problems of stain fixing and dye adsorption. A second aspect of the present invention subsists in a azetidinium functionalized polymer of which the monomers comprise: an amino-acrylate and / or amino-alkacrylate monomer, and, optionally, further non-amino acrylate and / or alkacrylate monomer. A third aspect of the present invention provides a textile treatment composition which comprises a azetidinium functionalised polymer in accordance with the first or second aspect of the invention and a textile compatible carrier.
Owner:HENKEL IP & HOLDING GMBH
Azetidine 2-Carboxamide Derivatives Which Modulate The CB2 Receptor
Compounds of the formula (I), (II) and (III) which modulate the CB2 receptor are disclosed. Compounds according to the invention bind to and are agonists of the CB2 receptor, and are useful for treating inflammation. Those compounds which are agonists are additionally useful for treating pain.
Owner:BOEHRINGER INGELHEIM INT GMBH
Piperidine And Azetidine Derivatives As Glyt1 Inhibitors
The present invention provides compounds of formula (I), wherein both p's are one or two, R1 is generally heteroaryl or cycloalkyl, R2 is C3-6cycloalkyl or phenyl and R3 is heteroaryl, and pharmaceutically acceptable salts thereof, as GlyT1 inhibitors for treating schizophrenia, pharmaceutical compositions comprising the same and methods for their preparation.
Owner:MERCK SHARP & DOHME (UK) LTD
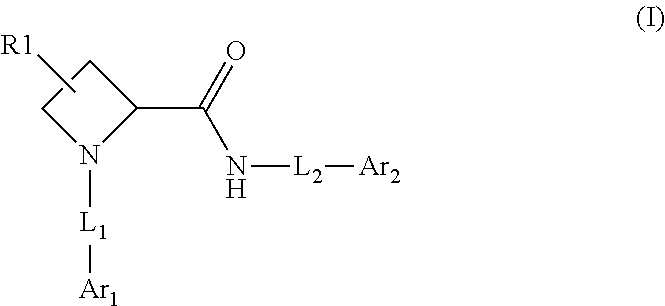
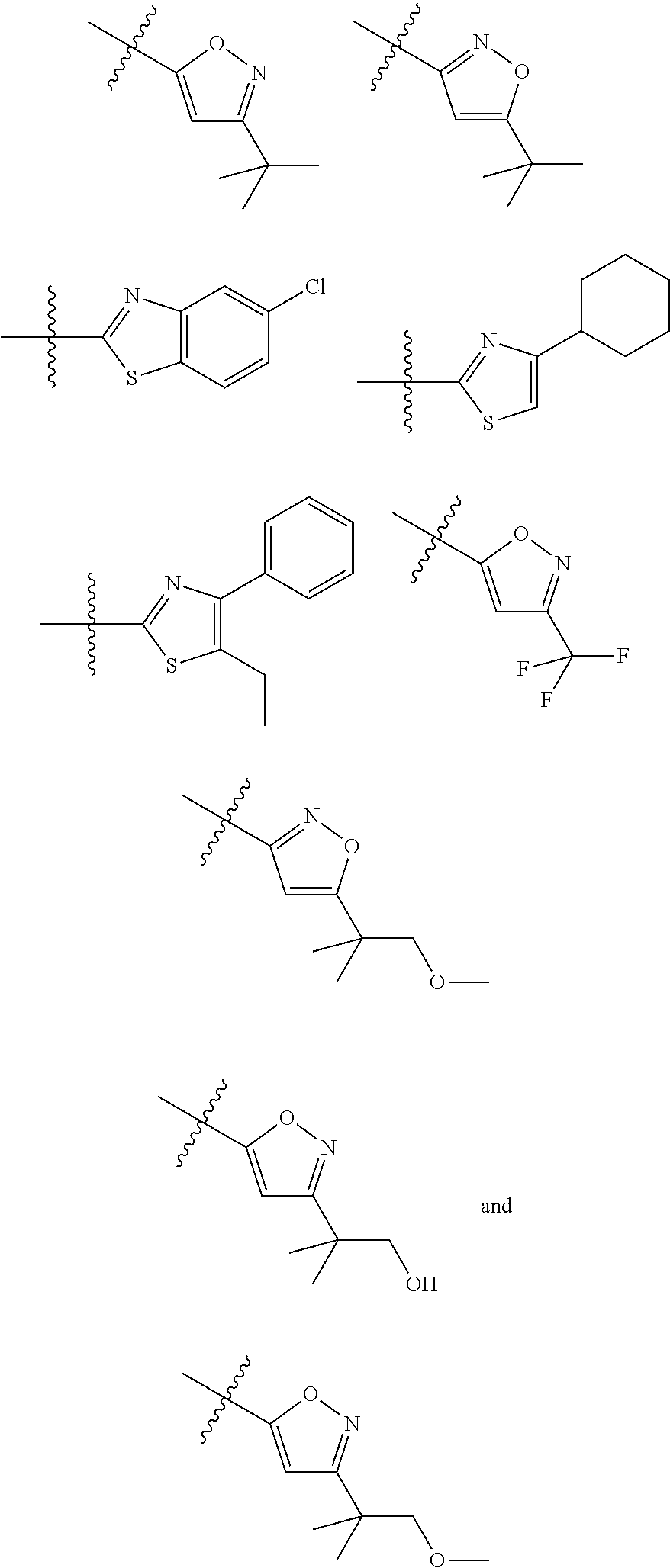
1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1 yl)prop-2-en-1-one derivatives and similar compounds as kras g12c modulators for treating cancer
ActiveUS20190382377A1Prevent proliferationOrganic chemistryPharmaceutical delivery mechanismDiseaseBenzoxazole
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, isotopic form or stereoisomer thereof, wherein A is a five-membered heteroaryl comprising 1 or 2 non-adjacent heteroatoms, inclusive of X and Y; W, X, Y, Z, L, L1, E, R1, R2bR2c and the dotted circle are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and compounds for use in methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided. Preferred compounds are e.g. 1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1yl)prop-2-en-1-one derivatives and related compounds such as e.g. the corresponding derivatives with e.g. a benzoimidazole, indole, benzooxazole, imidazopyridine or imidazole core structure, substituted on ring A by e.g. azetidine, pyrrolidine, azepane or bicyclopentane-amine (L1) each substituted by e.g. propenone (E), and the core structure substituted on the six-membered ring with e.g. 3-hydroxynaphthalene or indazole or hydroxy-, alkoxy- and / or fluoro-substituted phenyl (R1).
Owner:ARAXES PHARMA LLC
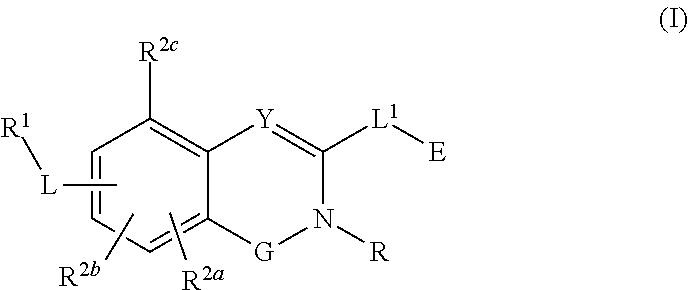
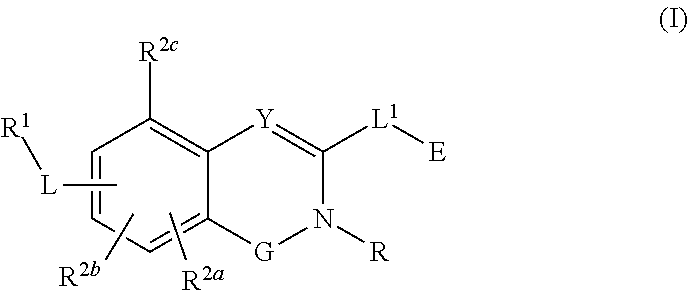
Substituted quinazoline and quinazolinone compounds and methods of use thereof
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): (Formula (I)) or a pharmaceutically acceptable salt, stereoisomer thereof, wherein G, Y, R, R1, R2a, R2b, R2c, L, L1 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided. Preferred compounds are quinazoline and quinazolinone derivatives and in particular 1-(6-(3-hydroxynaphthalen-1-yl)quinazolin-2-yl)azetidin-1-yl)prop-2-en-1-one derivatives and similar compounds.
Owner:ARAXES PHARMA LLC
Method for producing phenoxypyridine derivative
InactiveCN101454286AHas HGFR inhibitory effectHas anti-tumor effectOrganic active ingredientsOrganic chemistryHydrogen atomHydrogen
A process for preparing a compound represented by the formula (I): comprising reacting a compound represented by the formula (II) or salt thereof: with a compound represented by the formula (III): in the presence of a condensation reagent, wherein R 1 represents 1) optionally substituted azetidin-1-yl, 2) optionally substituted pyrrolidin-1-yl, 3) optionally substituted piperidin-1-yl, etc, R2, R3, R4 and R5 may be the same or different and each represents hydrogen or fluorine; and R6 represents hydrogen or fluorine.
Owner:EISIA R&D MANAGEMENT CO LTD
Oxopiperazine-azetidine amides and oxodiazepine-azetidine amides as monoacylglycerol lipase inhibitors
Owner:JANSSEN PHARMA NV
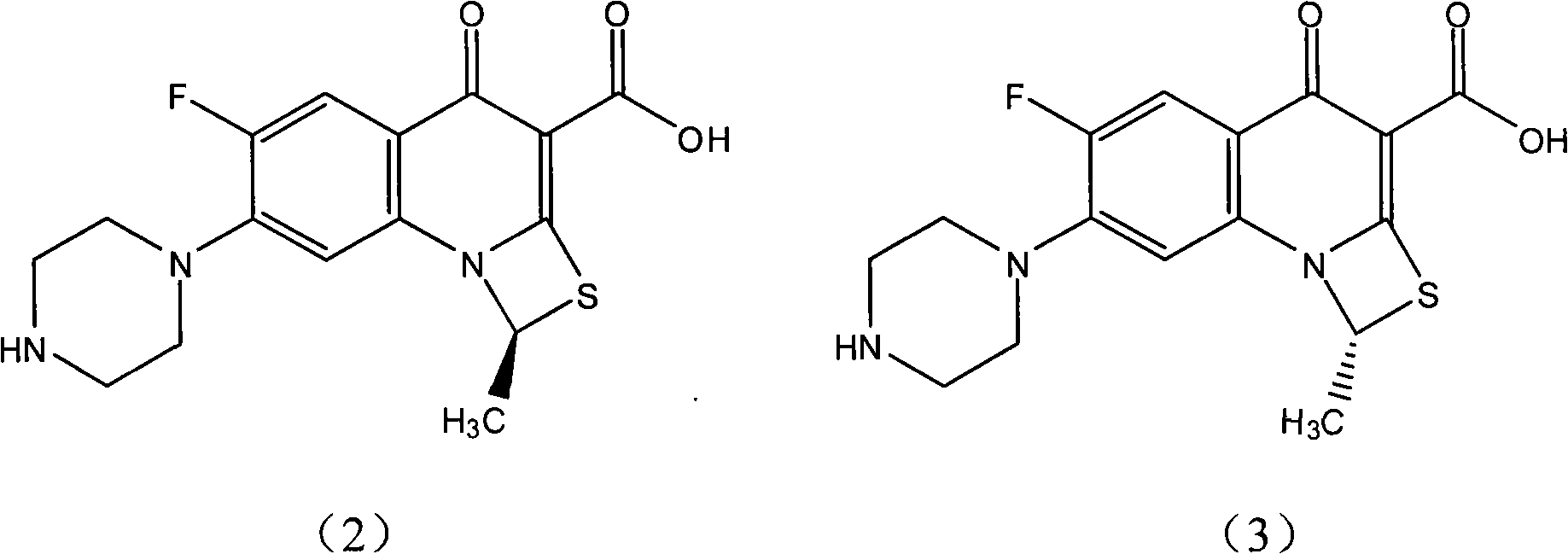
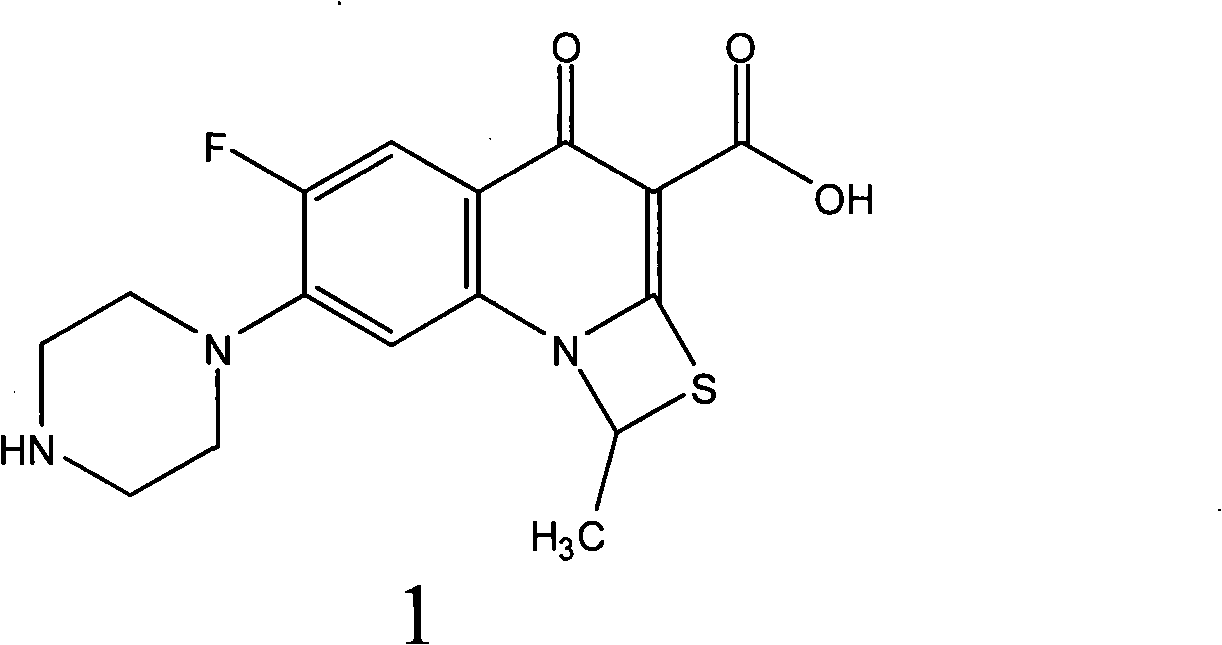
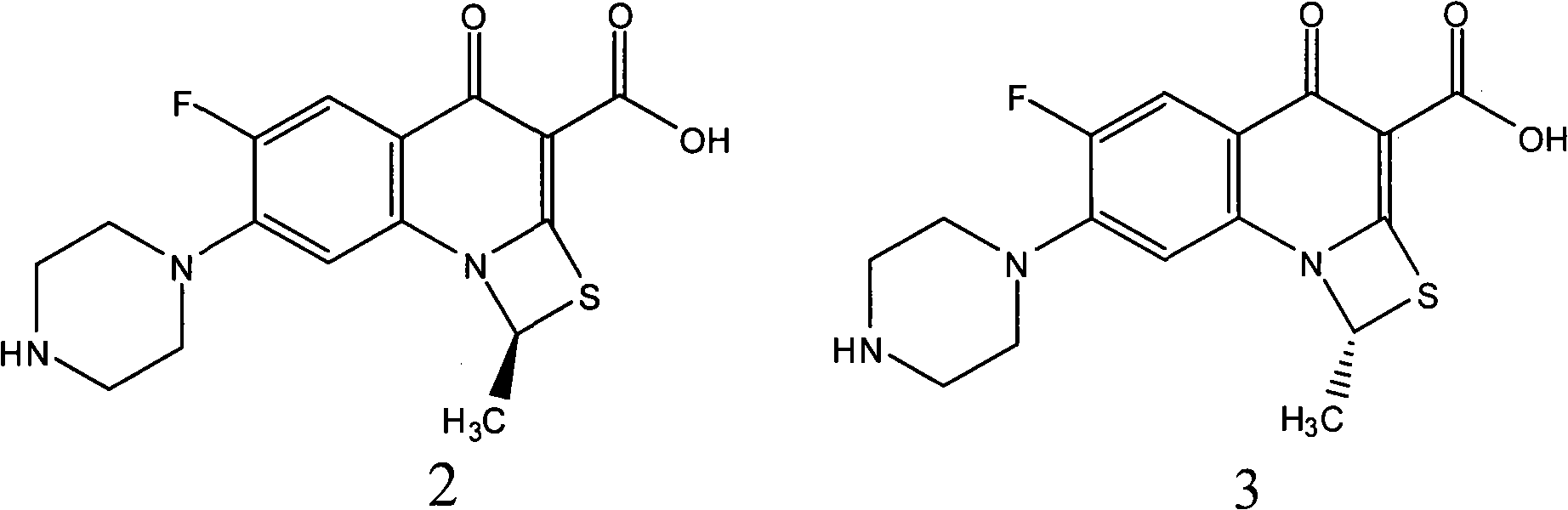
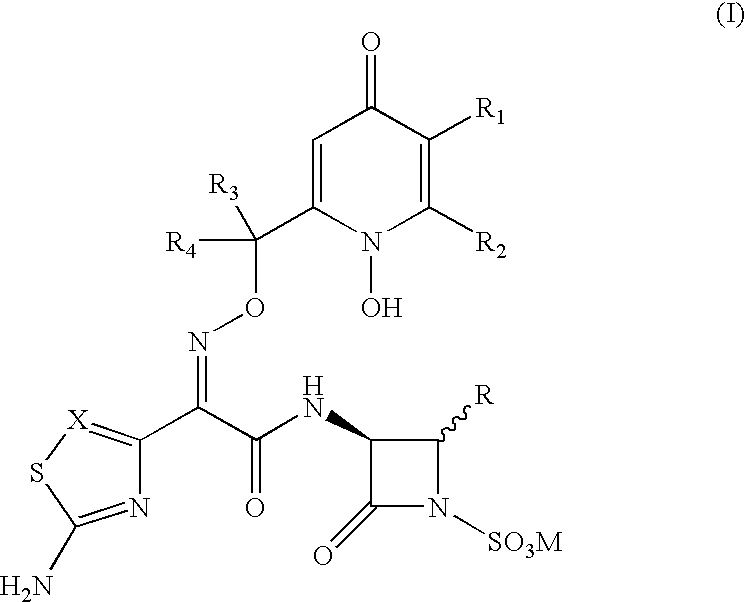
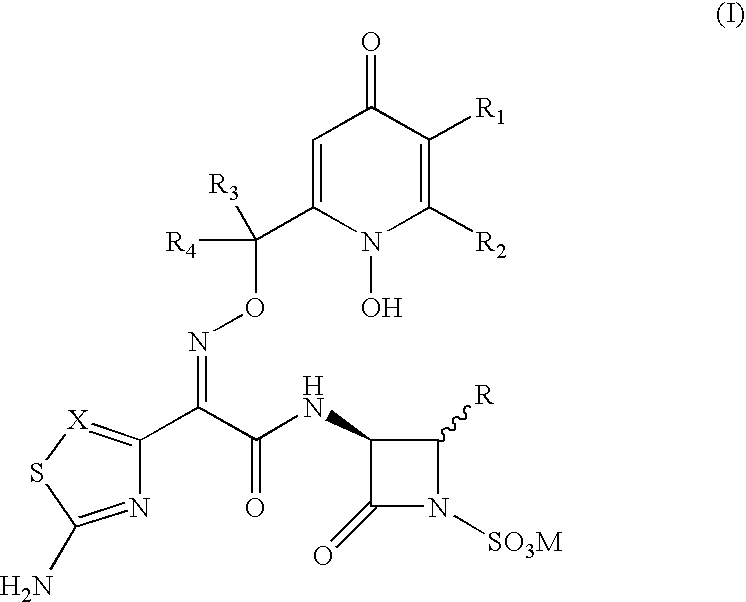
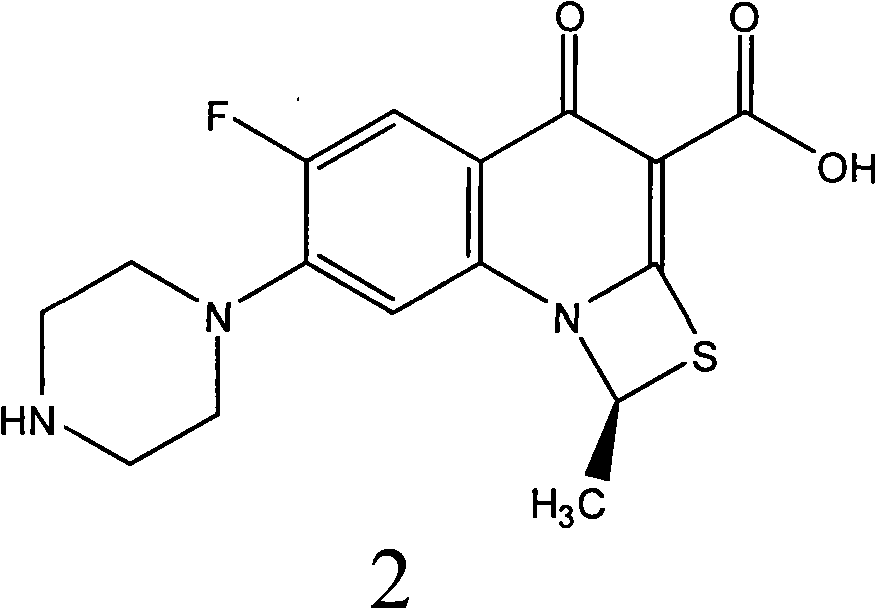
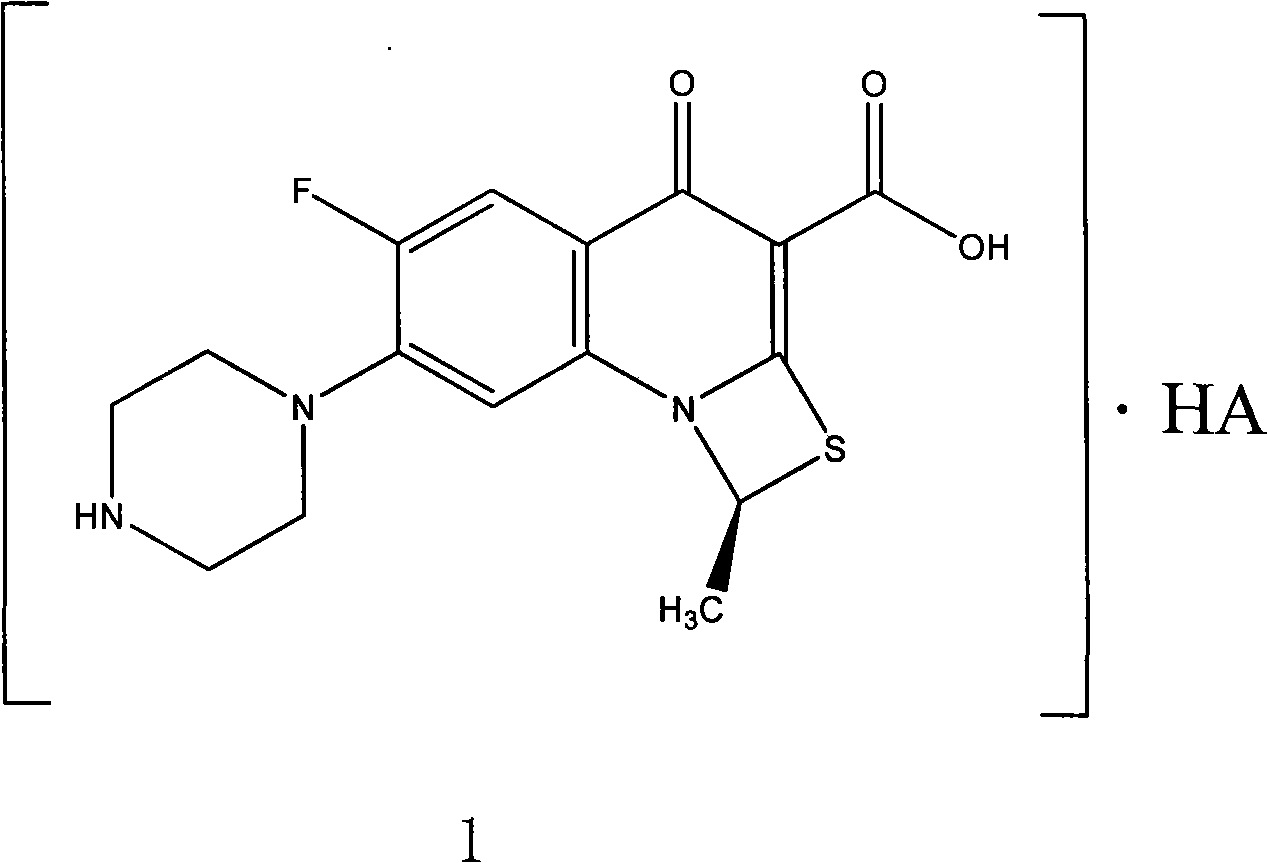
Fluorine-containing optically active composition for anti-infection
ActiveCN101550153AStable in natureLower pHOrganic active ingredientsOrganic chemistrySolubilitySide effect
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
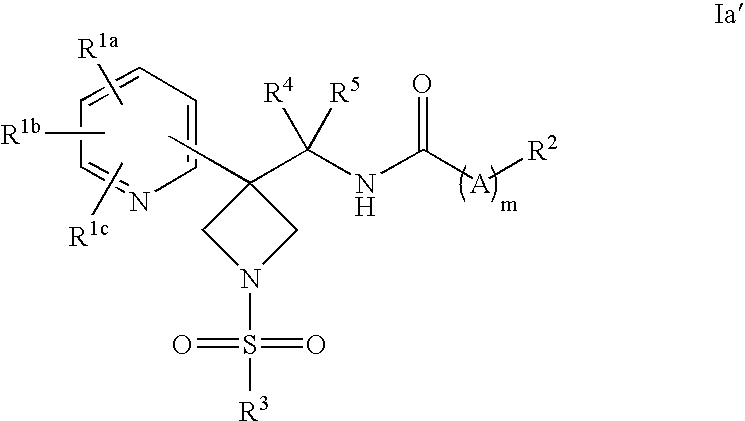
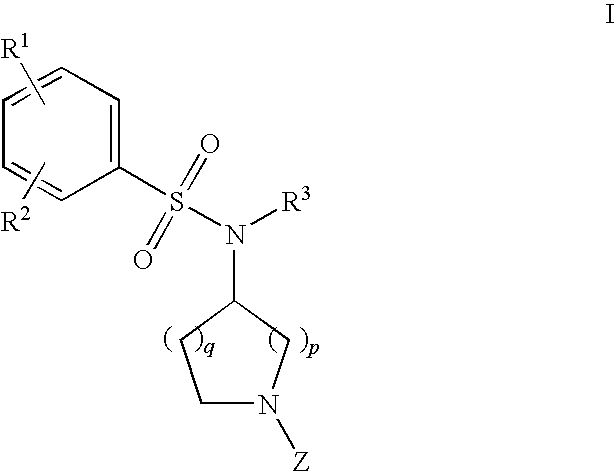
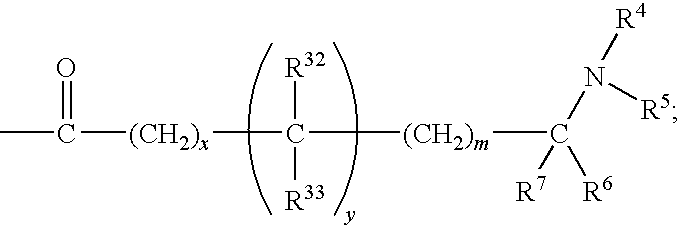
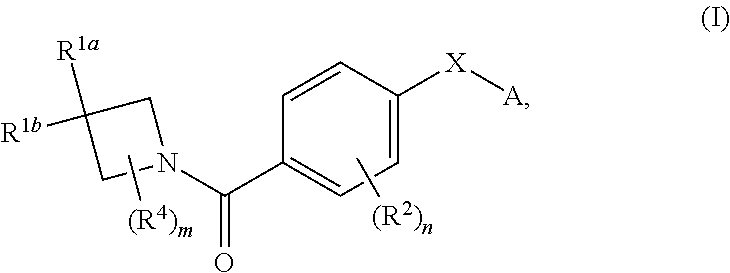
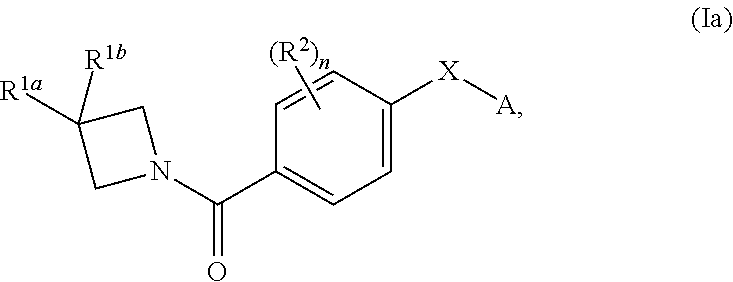
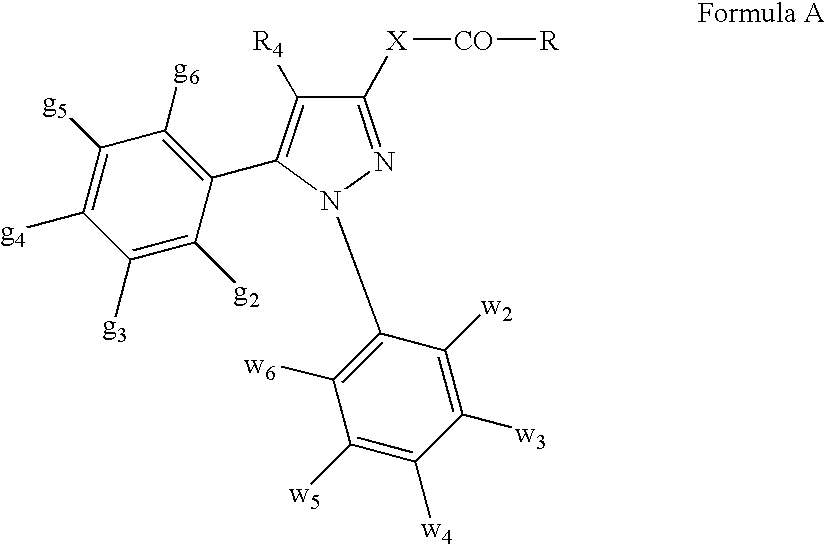
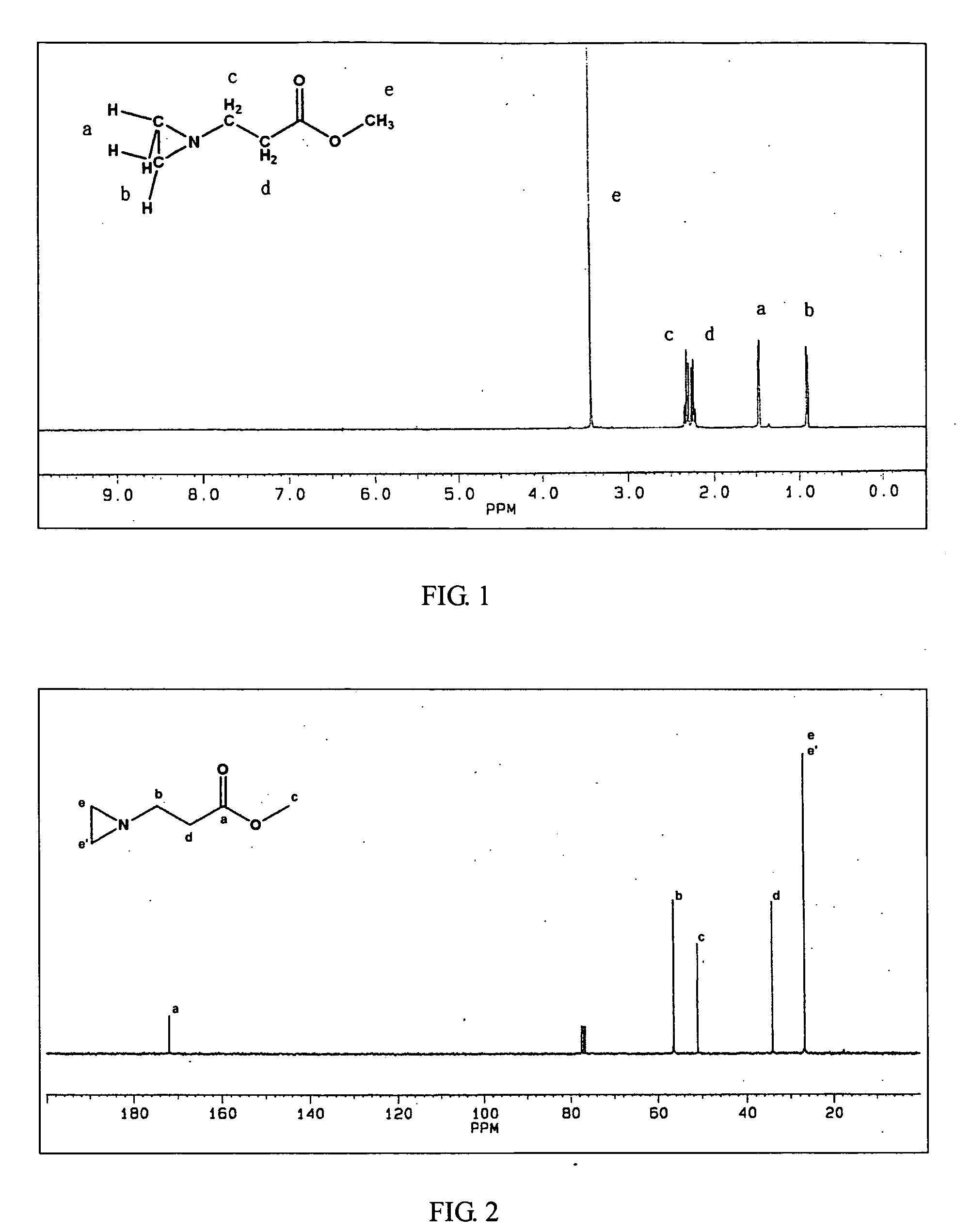
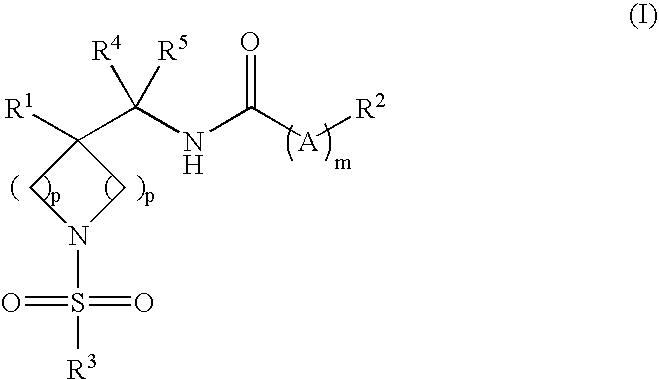
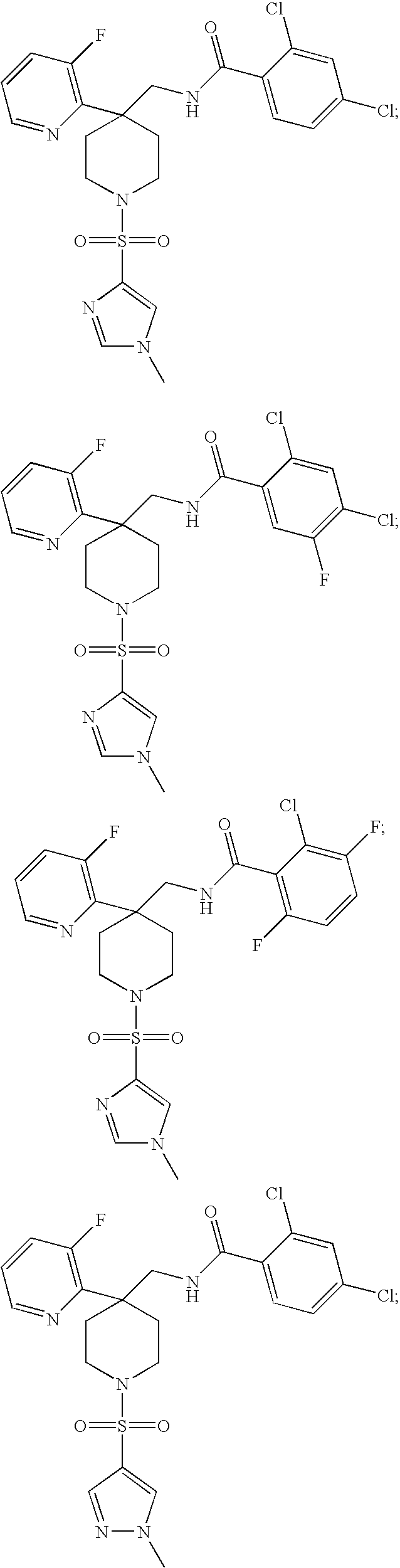
DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES
ActiveUS20060229287A1Enhanced inhibitory effectAntibacterial agentsBiocideCarboxylic acidInhibitory effect
Derivatives of 6,7-dihydro-5H-imidazo[1,2-a]imidazole-3-sulfonyl]-azetidine-carboxylic acids, esters and amides which exhibit good inhibitory effect upon the interaction of CAMs and Leukointegrins and are thus useful in the treatment of inflammatory disease.
Owner:BOEHRINGER INGELHEIM INT GMBH
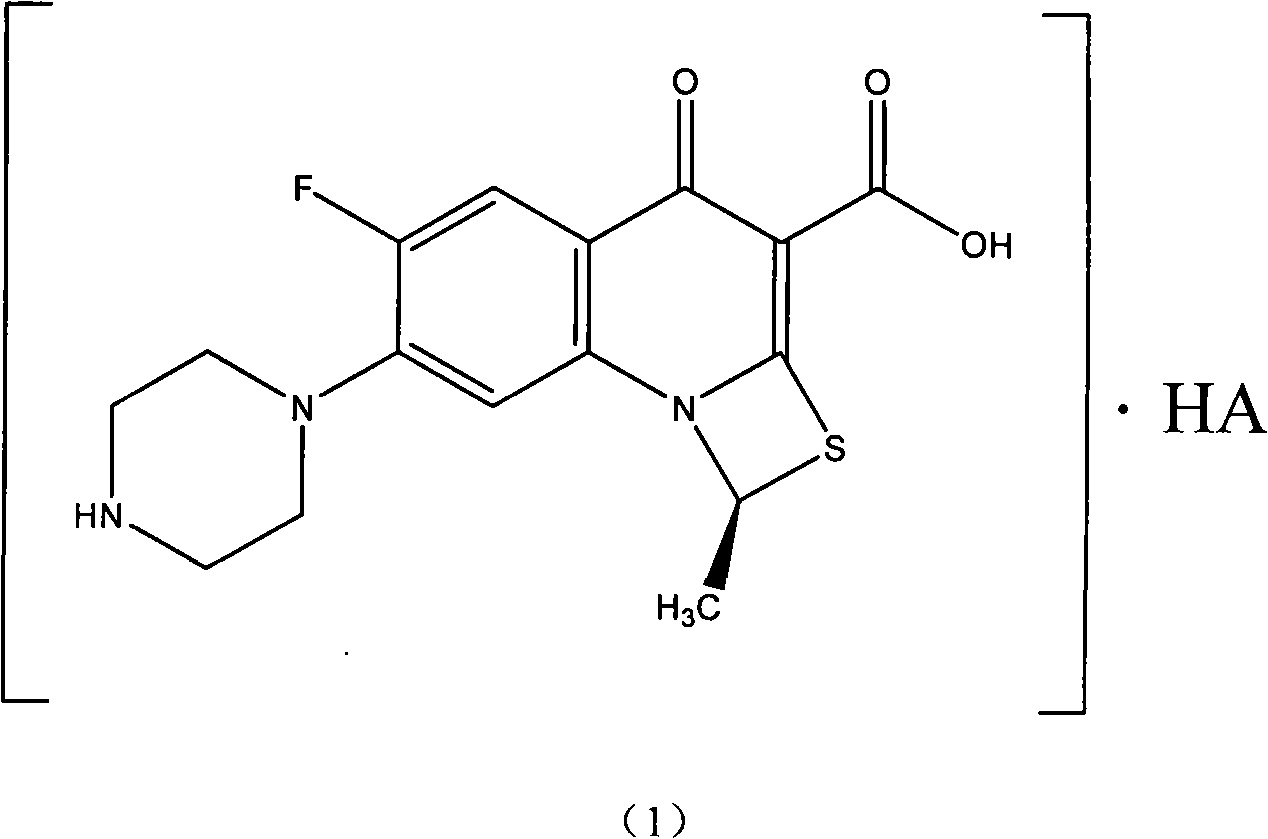
Preparation method of ulifloxacin optical isomer
The invention relates to a preparation method of a ulifloxacin optical isomer, in particular to a preparation method of a 6-fluorine-1-methyl-4-oxo-(1-piperazinyl)-1H and 4H-(1, 3) sulfur azetidine combined with (3, 2-a) quinoline-3-carboxylic acid optical isomer, which comprises the following steps: (+ / -) ulifloxacin is taken to be dissolved in dimethyl sulfoxide, a dimethyl sulfoxide solution of D-tartaric acid is dropped into the dimethyl sulfoxide to obtain (S)-ulifloxacin-D-tartrate precipitation, the pH value of the (S)-ulifloxacin-D-tartrate is regulated by a NaOH solution in water after the (S)-ulifloxacin-D-tartrate is recrystallized so as to obtain precipitates, the precipitates are filtered and dried to obtain (S)-ulifloxacin, and the optical purity e.e. of the (S)-ulifloxacin is more than 95 percent. The preparation method of the ulifloxacin optical isomer is simple and convenient without special equipment requests and is beneficial to industrialized batch production, and an obtained optically active body has high purity.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Azetidine Glycine Transporter Inhibitors
The present invention is directed to azetidine compounds that inhibit the glycine transporter GlyT1 and which are useful in the treatment of neurological and psychiatric disorders associated with glycinergic or glutamatergic neurotransmission dysfunction and diseases in which the glycine transporter GlyT1 is involved.
Owner:MERCK SHARP & DOHME LTD +1
Benzenesulfonamide Compounds and the Use Thereof
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula (I): and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3, Z, p and q are defined as set forth in the specification. The invention is also directed to the use compounds of Formula (I) to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
Benzenesulfonamide Compounds and Their Use
The invention relates to azetidinyl, pyrrolidinyl, piperidinyl, and hexahydroazepinyl compounds of Formula I and pharmaceutically acceptable salts, prodrugs, or solvates thereof, wherein R1-R3 and Z are defined as set forth in the specification. The invention is also directed to the use compounds of Formula I to treat, prevent or ameliorate a disorder responsive to the blockade of calcium channels, and particularly N-type calcium channels. Compounds of the present invention are especially useful for treating pain.
Owner:PURDUE PHARMA LP
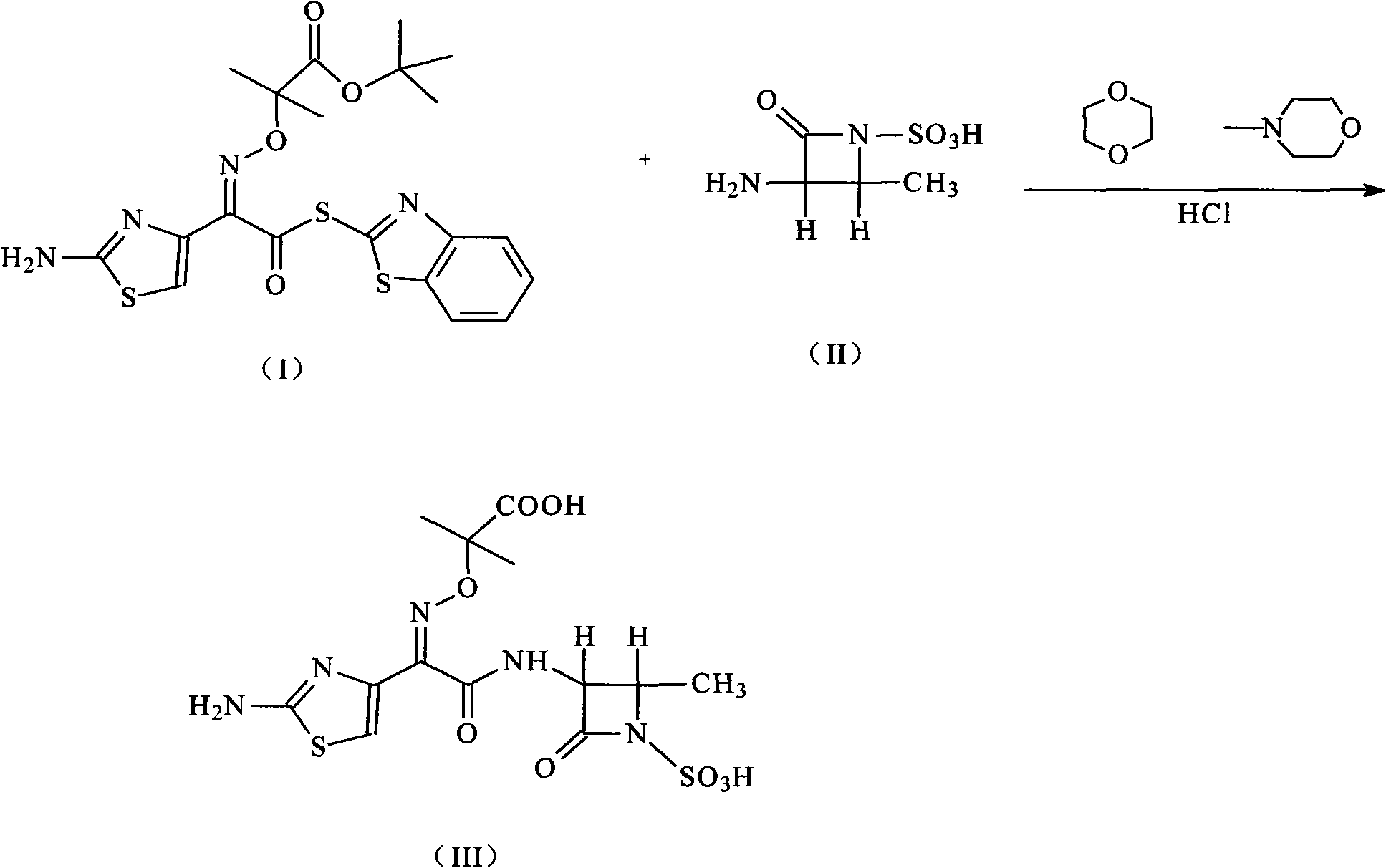
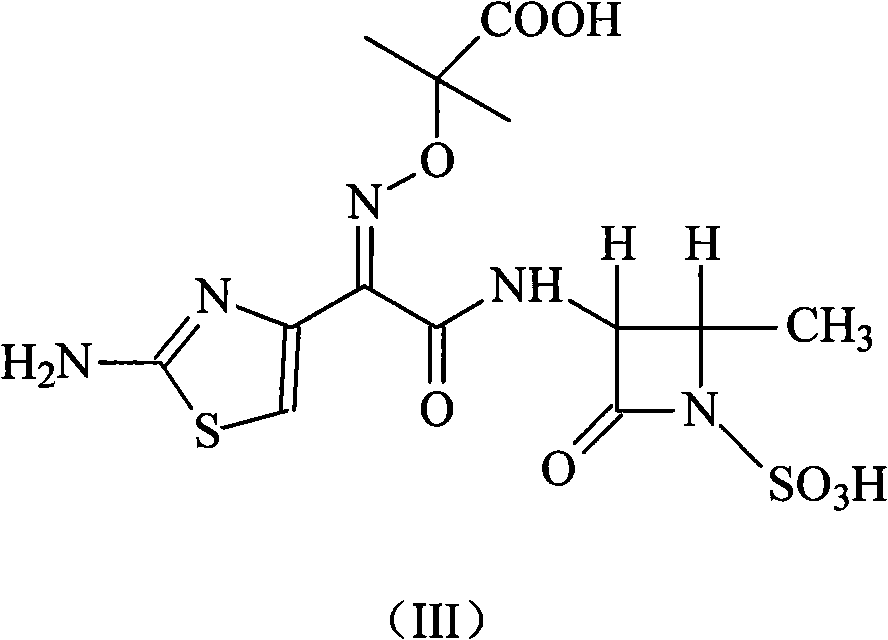
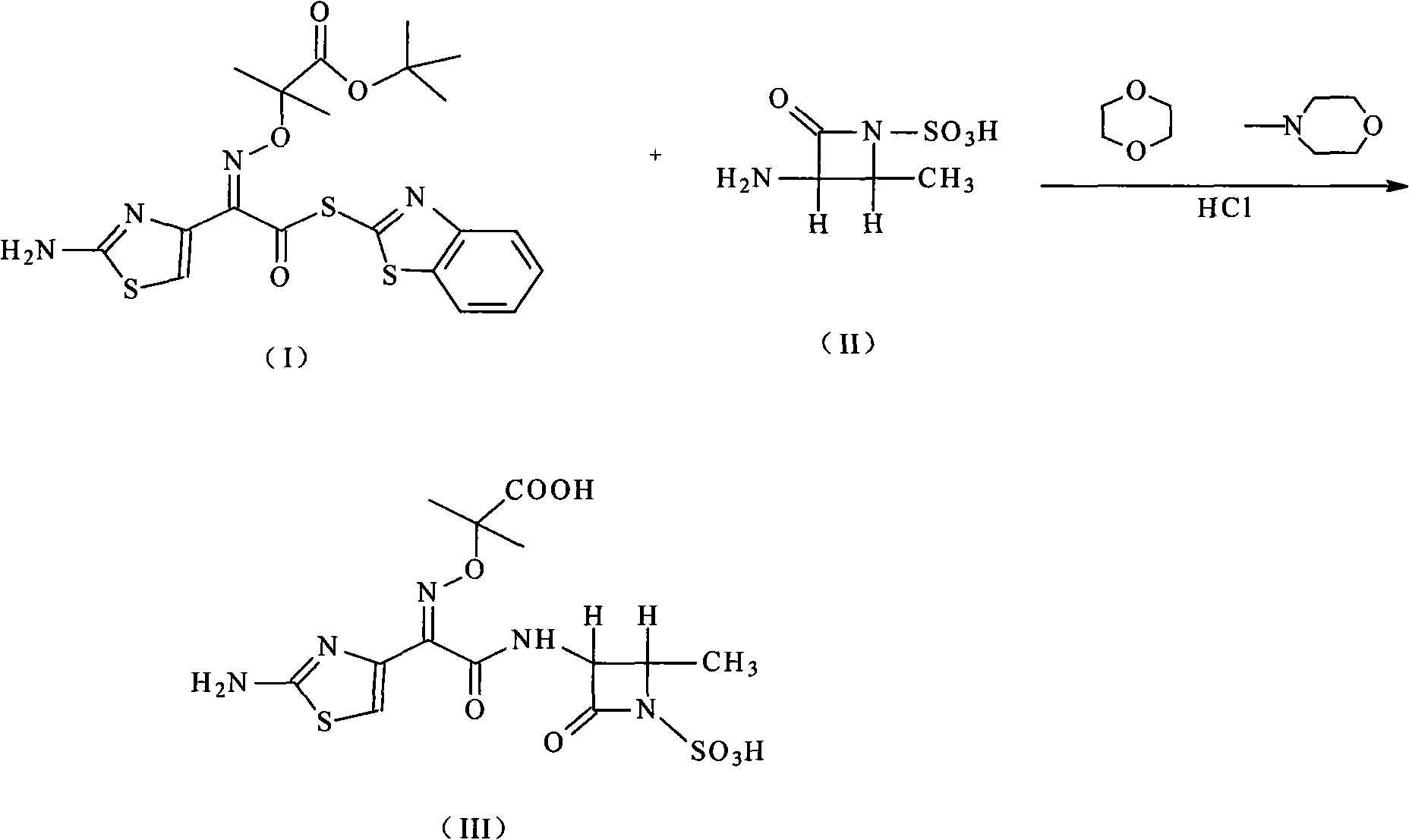
Compound of aztreonam and a synthetic method thereof
InactiveCN101514200AEasy to handleImprove responseAntibacterial agentsOrganic chemistryAcetic acidIminodiacetic acid
The invention provides a compound of aztreonam and a synthetic method thereof. In the method, general solvents are used, suitable amines are chosen, (2-aminothiazole-4-group)-2-(tert-butoxycarbonyl)-iminodiacetic acid isopropoxide acid-2-ester mercaptobenzothiazole and (3-S-trans form)-3-amino-4-methyl-2-oxo-1-sulfonic acid azetidine are used as intermediates; therefore, the method simplifies reaction and has good effect on depriving protecting groups by the mixed aqueous solution of acetic acid and hydrochloric acid.
Owner:HAINAN LINGKANG PHARMA CO LTD
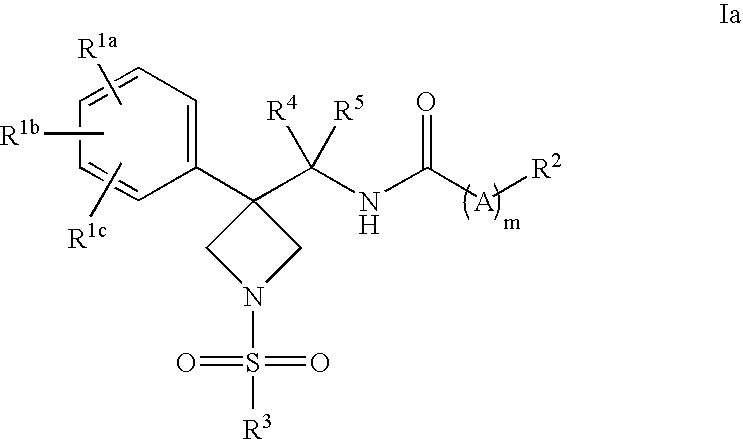
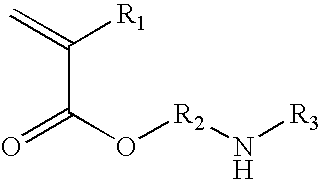
Injectable or orally deliverable formulations of azetidine derivatives
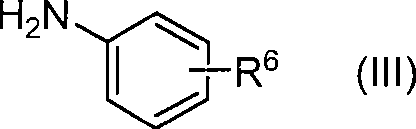
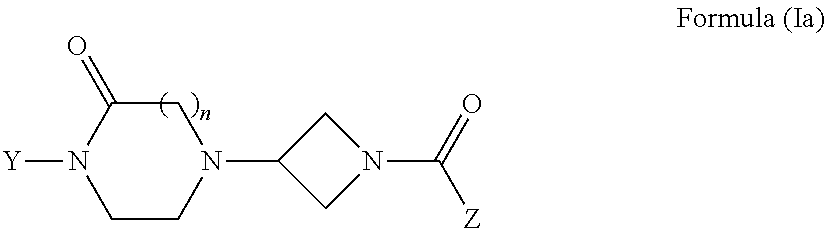
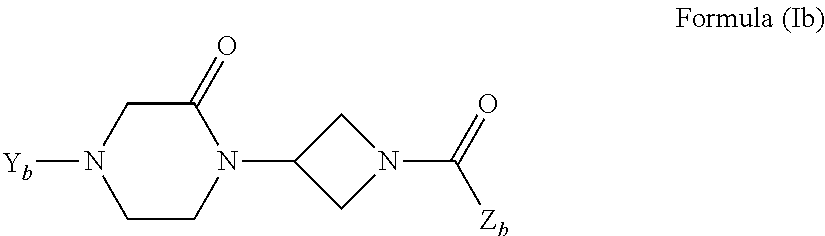
The invention concerns injectable or orally deliverable binary or ternary formulations of azetidine derivatives. The azetidine derivatives used in the inventive pharmaceutical compositions can be represented by the general formulae (Ia) or (Ib), wherein Ar is an aromatic or heteroaromatic group optionally substituted by one or more among (C1-C4)alkyl, halogen, NO2, CN, (C1-C4) alkoxy or OH.
Owner:AVENTIS PHARMA SA (US)
N-(4-(azetidine-1-carbonyl) phenyl)-(hetero-) arylsulfonamide derivatives as pyruvate kinase M2 (PMK2) modulators
Compounds of general Formula (I), and compositions comprising compounds of general formula I that modulate pyruvate kinase M2 (PKM2) are described herein. Also described herein are methods of using the compounds that modulate PKM2 in the treatment of cancer.
Owner:AGIOS PHARM INC
Preparation method of regenerated cellulose fiber subjected to cation grafting modification and salt-free dyeing
InactiveCN104532408AEvenly distributedEliminate electrostatic repulsionCellulose/protein filament chemical after-treatmentSpinningPolyactive
The invention discloses a preparation method of regenerated cellulose fiber subjected to cation grafting modification and salt-free dyeing, which adopts cellulose pulp as a main raw material, and orderly comprises the following procedures of dipping, squeezing, crushing, aging, yellowing, dissolving, filtering, defoaming, spinning, post-treatment, and drying; a cellulose cation modifier is added in the dissolving procedure or the spinning procedure; the cellulose cation modifier is one or a mixture of more than one of an amination reagent, a quaternization reagent, a azetidine cation compound, and a modified natural cation reagent. Therefore, the method of the invention prevents the disadvantages of dye waste, uneven dyeing, and the like caused by the adoption of dyes with multiple active groups, and also prevents the problem of process flow increasing caused by the adoption of a pretreatment mode.
Owner:JIANGSU GOLDSUN TEXTILE SCI & TECH
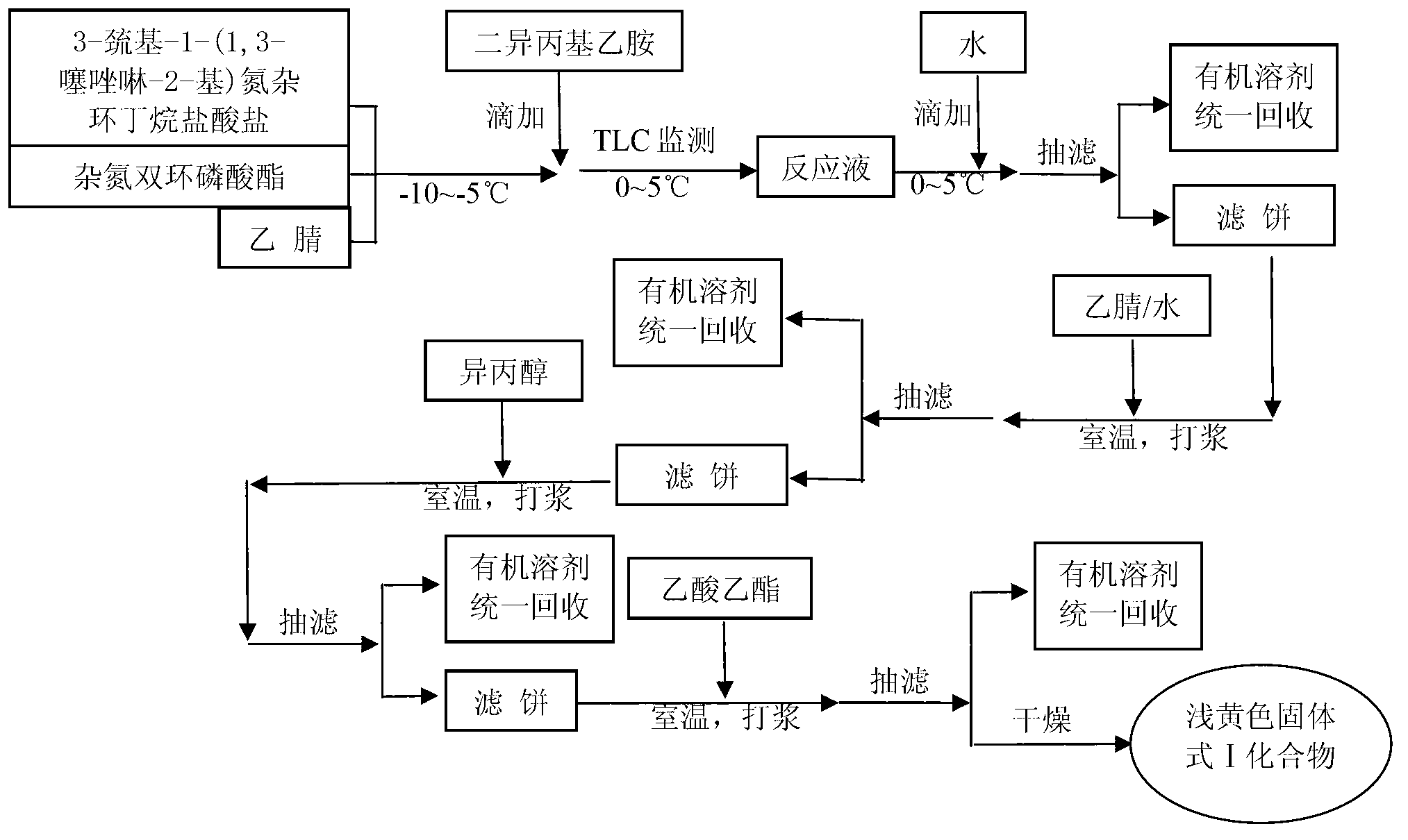
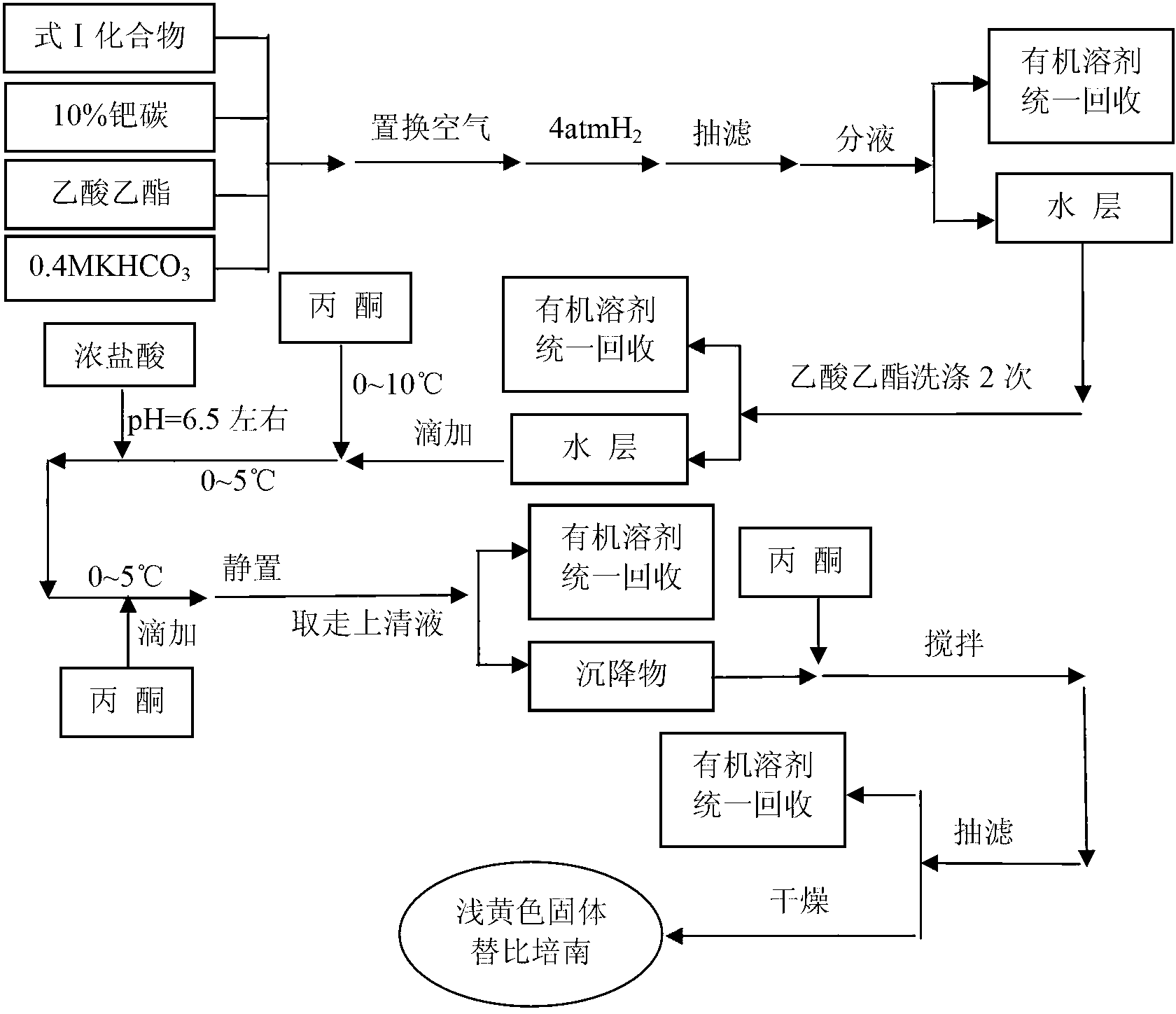
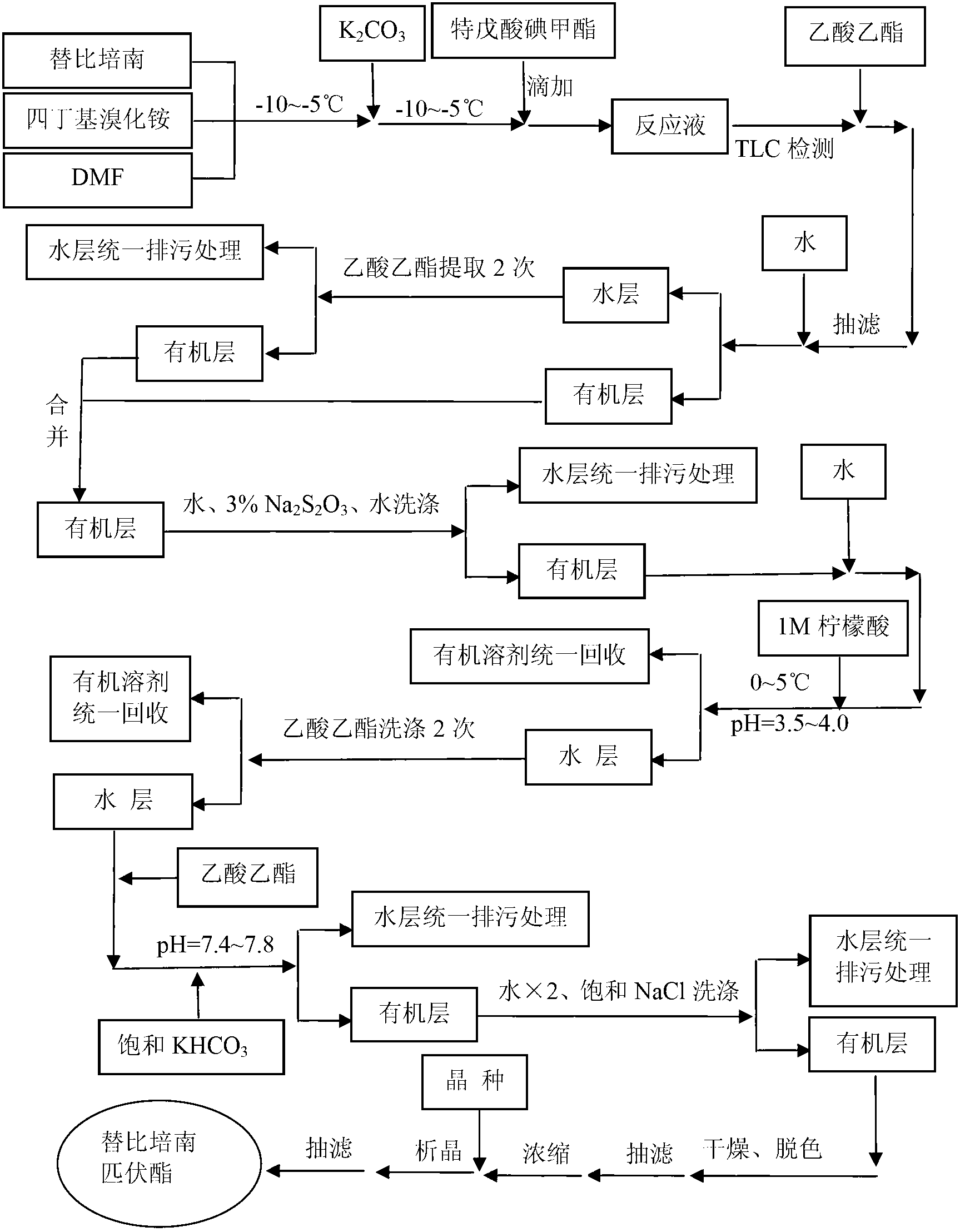
Preparation method of tebipenem pivoxil
ActiveCN103059028AHigh yieldSuitable for industrial productionOrganic chemistryChemical synthesisPhosphate
The invention relates to the field of chemical synthesis, and discloses a preparation method of tebipenem pivoxil. The preparation method comprises the following steps of: performing condensation reaction on azabicyclo phosphate and 3-mercapto-1-(1,3-thiazoline-2-yl) azetidine hydrochloride by taking acetonitrile as a solvent in the presence of diisopropylethylamine; in a mixed solvent consisting of acetic ether and a potassium bicarbonate aqueous solution, performing hydrogenization on the compound shown by formula I to remove p-nitrobenzyl to obtain tebipenem; and under the catalysis of a phase transfer catalyst, adding anhydrous potassium carbonate into the tebipenem and iodomethyl pivalate to perform condensation reaction to obtain the tebipenem pivoxil. By adopting the preparation method, according to the defects of related solvents and operations of purification and the like in the conventional method for preparing the tebipenem pivoxil, co-adapted solvents and purification operations are selected during preparation according to a reaction mechanism, so that the yield of the tebipenem pivoxil is improved, and the preparation method is suitable for industrial production. The formula I is shown in the description.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
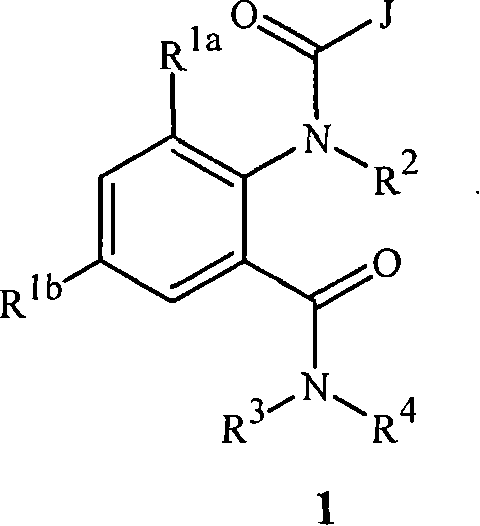
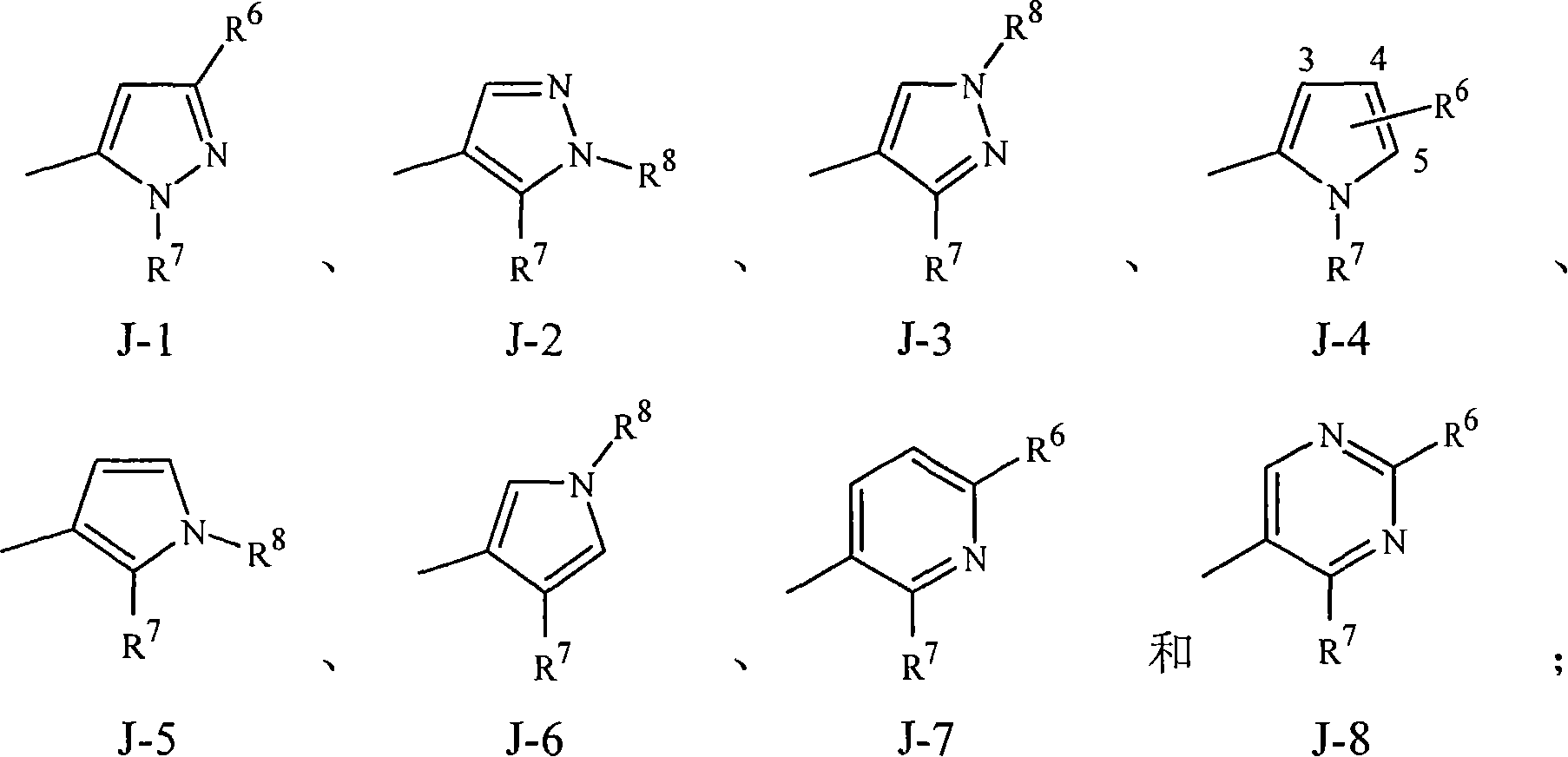
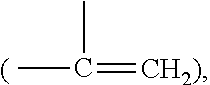
Anthranilamide insecticides
Disclosed are compounds of Formula 1, including all geometric and stereoisomers, N-oxides, and salts thereof, wherein J is a phenyl optionally substituted with one to four substituents independently selected from R5; or J is a heterocyclic ring selected from the group consisting of J-1 to J-8; R4 is C4-C12 alkylcycloalkyl, C5-C12 alkenylcycloalkyl, C5-C12 alkynylcycloalkyl, C4-C12 cycloalkylalkyl, C5-C12 cycloalkylalkenyl, C5-C12 cycloalkylalkynyl, C4-C12 cycloalkenylalkyl or C4-C12 alkylcycloalkenyl; each optionally substituted with one to six substituents selected from CH3 and halogen; or R4 is C3-C5 oxiranylalkyl, C3-C5 thiiranylalkyl, C4-C6 oxetanylalkyl, C4-C6 thietanylalkyl, 3-oxetanyl or 3-thietanyl, each optionally substituted with one to five substituents independently selected from C1-C3 alkyl, C1-C3 haloalkyl, halogen, CN, C2-C4 alkoxycarbonyl and C2-C4 haloalkoxycarbonyl; or R4 is C3-C5 aziridinylalkyl, C4-C6 azetidinylalkyl or 3-azetidinyl, each with R10 attached to the nitrogen atom, and optionally substituted on carbon atoms with one to five substituents independently selected from Cl-C3 alkyl, C1-C3 haloalkyl, halogen, CN, C2-C4 alkoxycarbonyl and C2-C4 haloalkoxycarbonyl; and Rla. R1b, R2, R3 and R5 are as defined in the disclosure. Also disclosed are compositions containing the compounds of Formula 1 and methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a compound or a composition of the invention.
Owner:FMC AGRO SINGAPORE PTE LTD +1
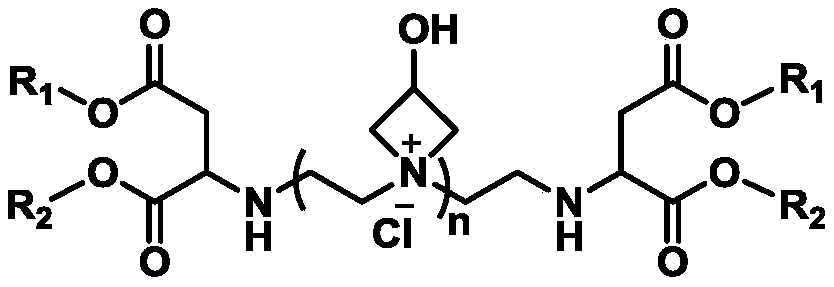
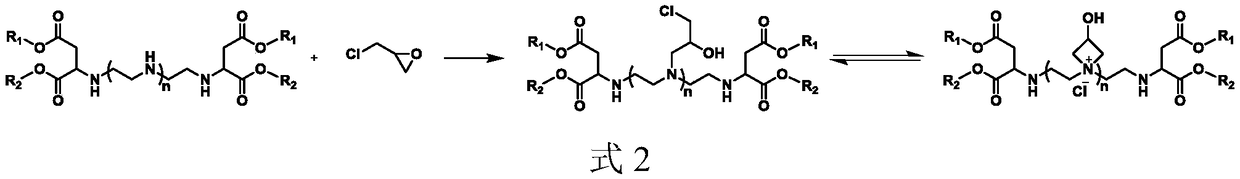
Water-soluble cationic polyaspartic ester, preparation method and application thereof
ActiveCN109020859AImprove stabilityHigh mechanical strengthOrganic chemistryPolyurea/polyurethane coatingsSynthesis methodsPolyaspartic
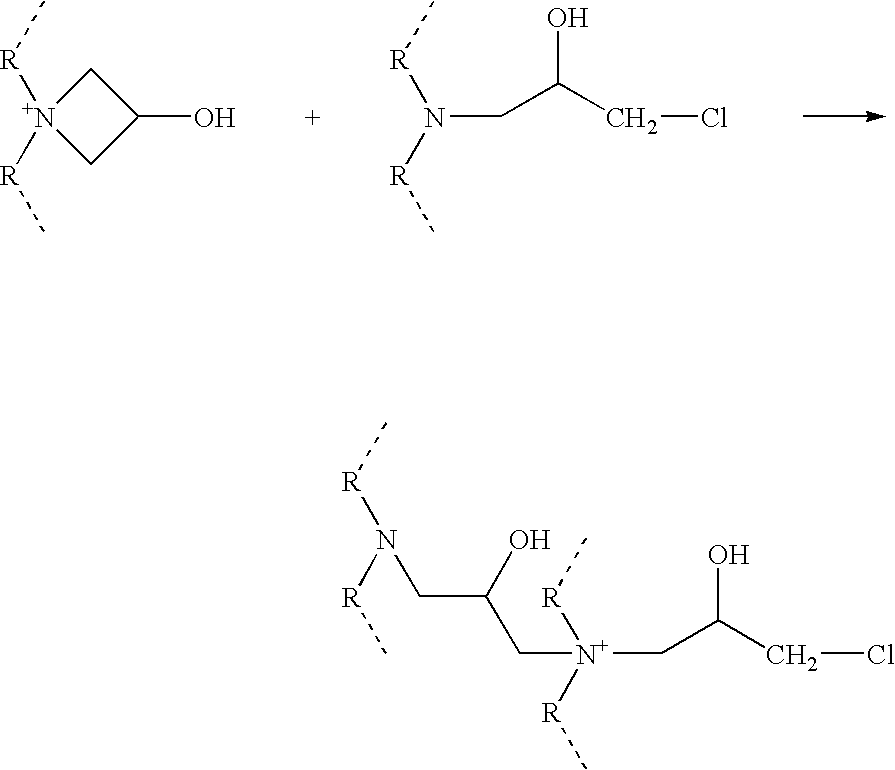
The invention provides a water-soluble cationic polyaspartic ester and a synthesis method thereof. The substance has a 3-hydroxyazetidine structure. During synthesis, firstly butene diacid diester reacts with polyethylene polyamine to obtain polyaspartic ester polyamine with unreacted secondary amine group, and then adding epoxy chloropropane for reaction with the secondary amine group so as to obtain water-soluble polyaspartic ester with azetidine cationic group. The cationic polyaspartic ester monomer can be compounded with isocyanate to develop water-soluble polyurea or polyurea / polyurethane coating resin.
Owner:WANHUA CHEM GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
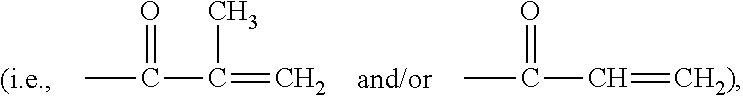
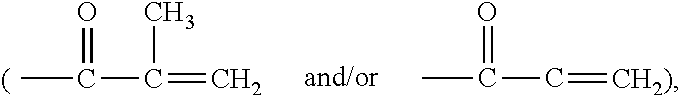


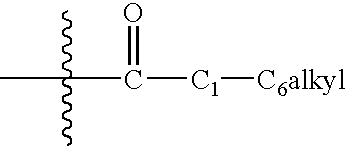




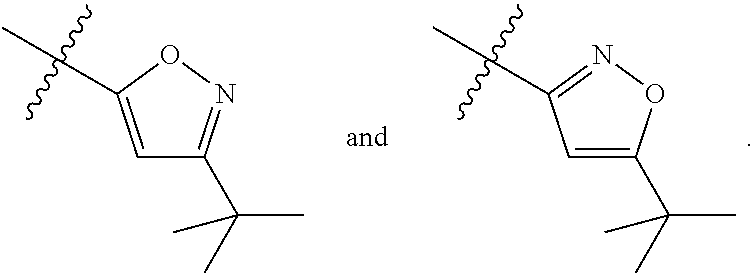

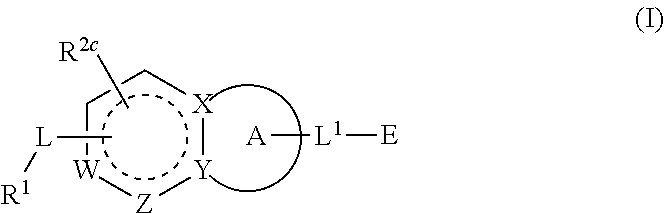
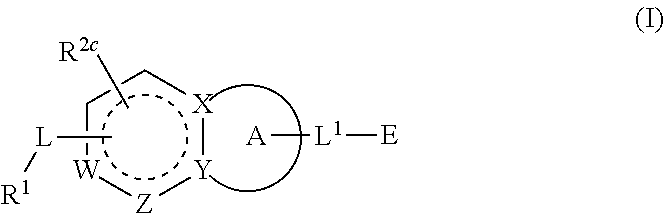



![DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/70df3e34-455d-4435-83f5-1411de8a7527/US20060229287A1-20061012-C00001.png)
![DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/70df3e34-455d-4435-83f5-1411de8a7527/US20060229287A1-20061012-C00002.png)
![DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES DERIVATIVES OF [6,7-DIHYDRO-5H-IMIDAZO[1,2-a]IMIDAZOLE-3-SULFONYL]-AZETIDINE-CARBOXYLIC ACIDS, ESTERS AND AMIDES](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/70df3e34-455d-4435-83f5-1411de8a7527/US20060229287A1-20061012-C00003.png)