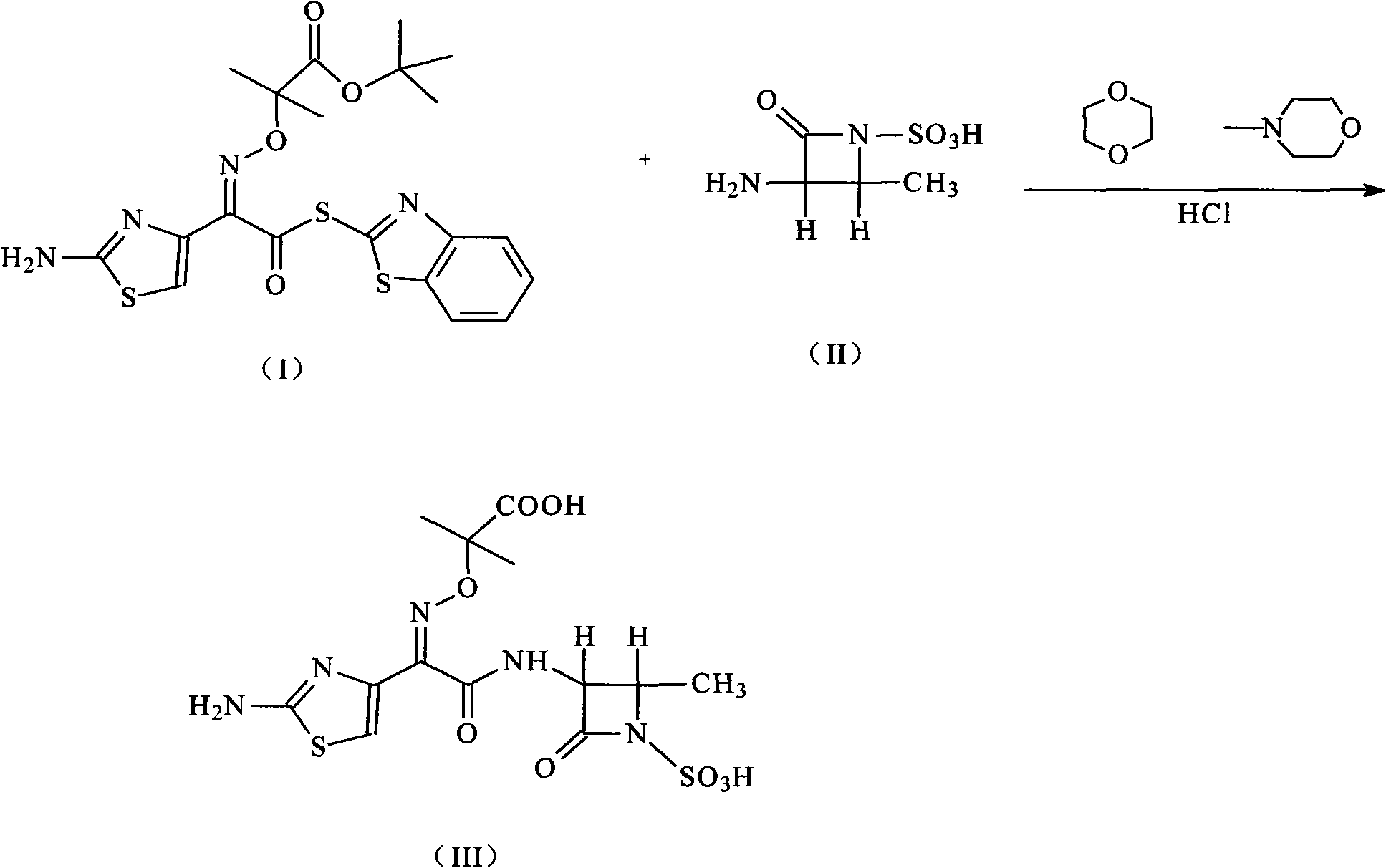
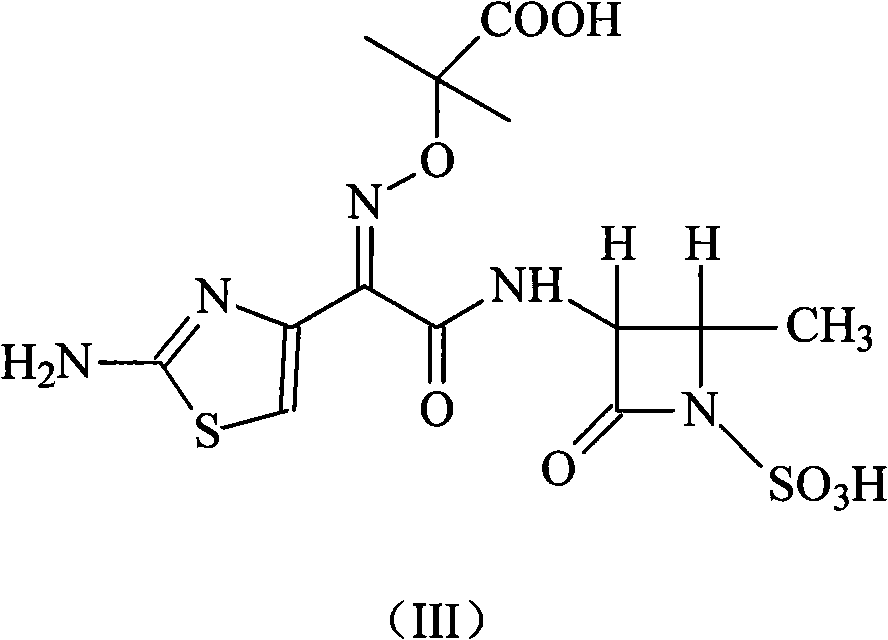
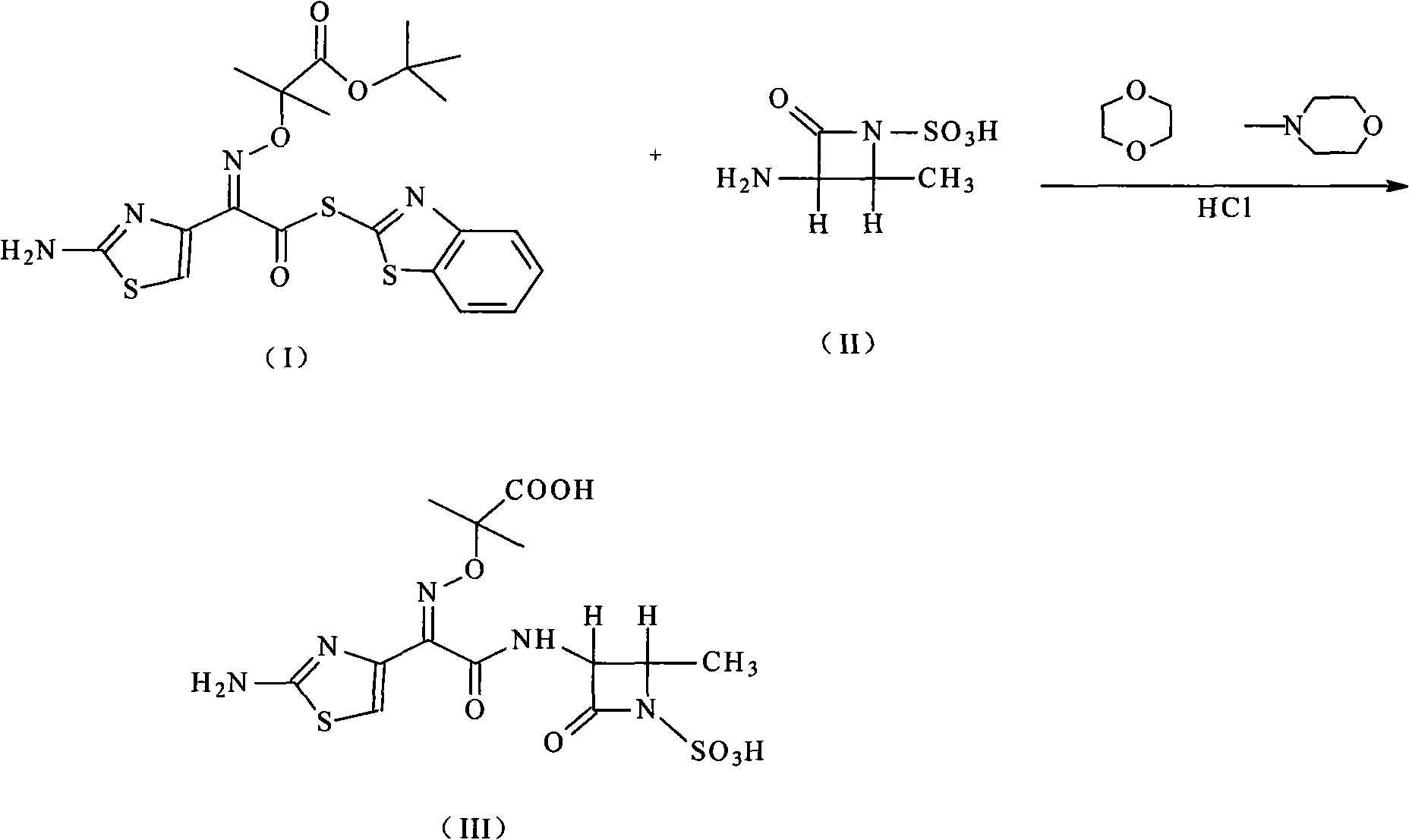
Compound of aztreonam and a synthetic method thereof
A synthesis method and technology of aztreonam, applied in the directions of organic chemistry, bulk chemical production, antibacterial drugs, etc., can solve the problems of low sulfuric acid yield, difficult treatment, environmental hazards, etc., and achieve improved reaction yield and product purity. Improve and simplify the effect of processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The synthesis of embodiment 1 aztreonam
[0022] Add 100 g (0.56 mol) of (3S-trans)-3-amino-4-methyl-2-oxo-1-azetidinesulfonic acid into the reaction flask, add 1,4-dioxane Ring 1000 ml and N-methylmorpholine 60 ml (0.6mol), the solution was cooled to 5 ° C, stirred until clear, added 800 ml of (2-aminothiazol-4-yl)-2-(tert-butoxycarbonyl )-isopropoxyiminoacetic acid-2-mercaptobenzothiazolyl ester 268 grams (0.56mol) of the solution of 1,4-dioxane, stirred and reacted for 3 hours, then added 3000 milliliters of acetic acid aqueous solution of pH=5 and 1000 ml of ethyl acetate, stirred for 1 hour, separated into layers, removed the ethyl acetate layer, the ethyl acetate can be recovered and used mechanically, the aqueous phase was added with 10% hydrochloric acid to adjust the pH=1, the mixture was heated to 40°C, and kept for 2 hours, A solid was formed, cooled to room temperature, stirred, the solid was precipitated, left overnight, filtered, washed with water, and dr...
Embodiment 2
[0027] The refining of embodiment 2 aztreonam
[0028] Heat 3000 ml of absolute ethanol to 55°C, add 50 g of aztreonam crude product, stir until dissolved, add 6.04 g of activated carbon, stir and adsorb for 30 minutes, filter while hot, and slowly cool the filtrate to 0°C, precipitate solid, continue stirring for 4 hours, filtered, washed with 50 ml of water, and vacuum-dried at 50°C to obtain 46.2 g of white β-form aztreonam refined product, with a yield of 92.4%, a purity of 99.8%, and mp: 239-241°C.
[0029] Elemental analysis Molecular formula: C 13 h 19 N 5 o 8 S 2
[0030] Theoretical value C: 35.7%, H: 4.4%, N: 16.0%, O: 29.3%, S: 14.6%;
[0031]Experimental values C: 35.5%, H: 4.4%, N: 16.1%, O: 29.4%, S: 14.7%.
[0032] H-NMR (D 2 O): δ6.95 (s, 1H), 4.67 (d, 1H, J=2Hz), 4.82 (qd, 1H, J=7, 2.5Hz), 1.58 (d, 1H, J=7.0Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com