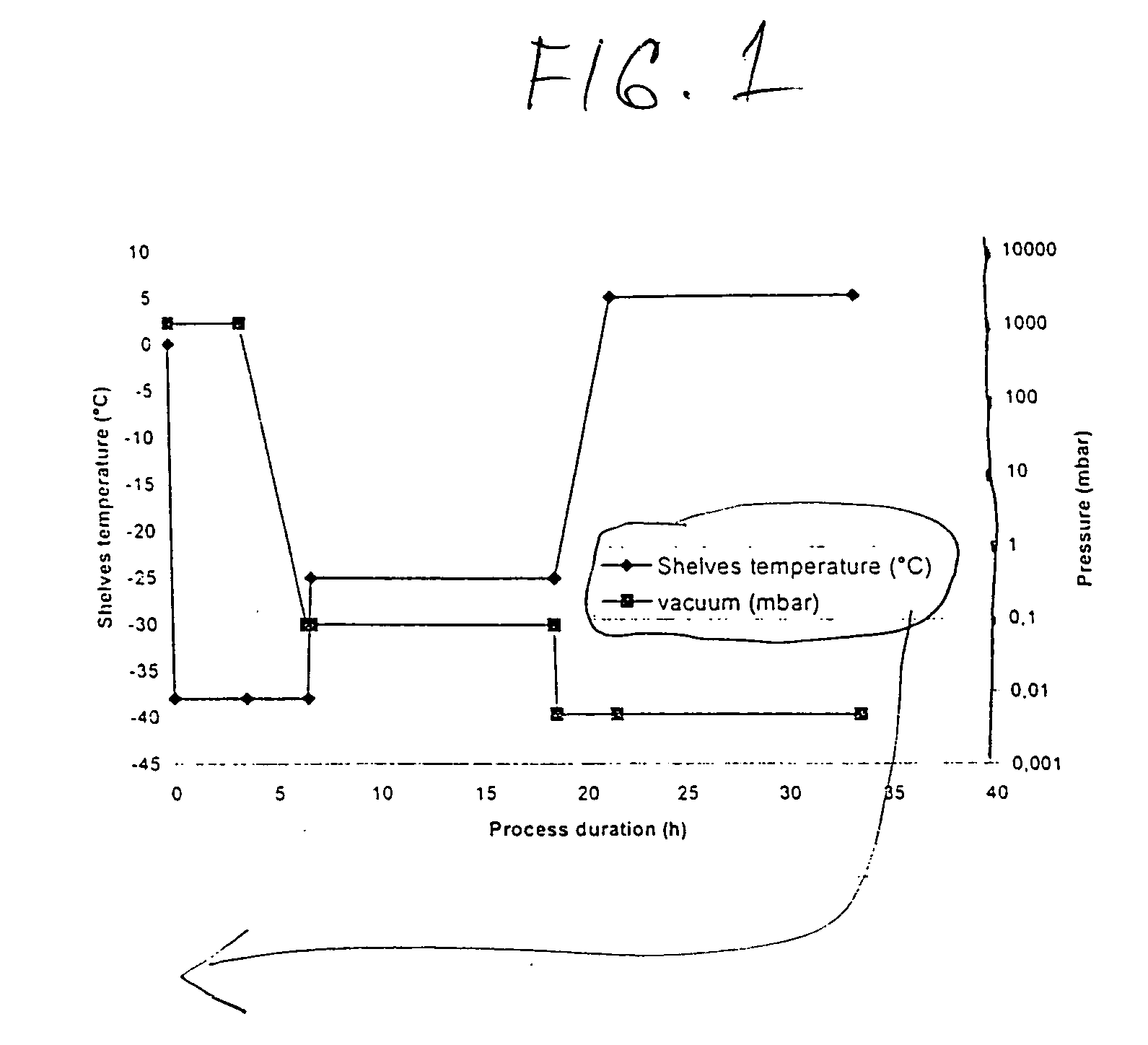
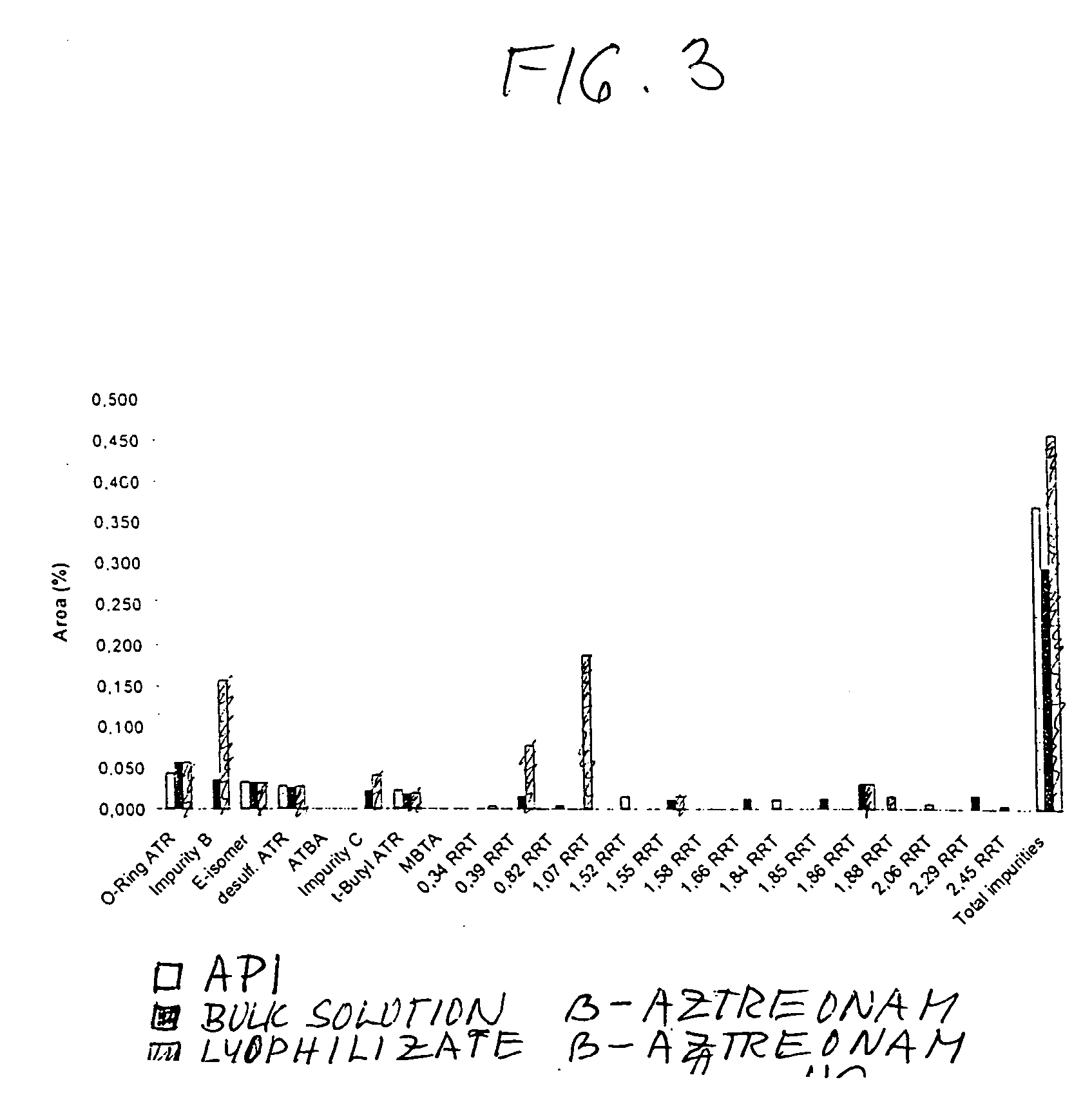
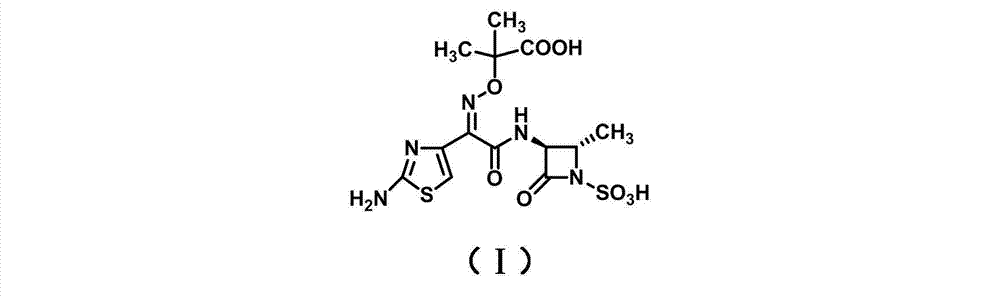
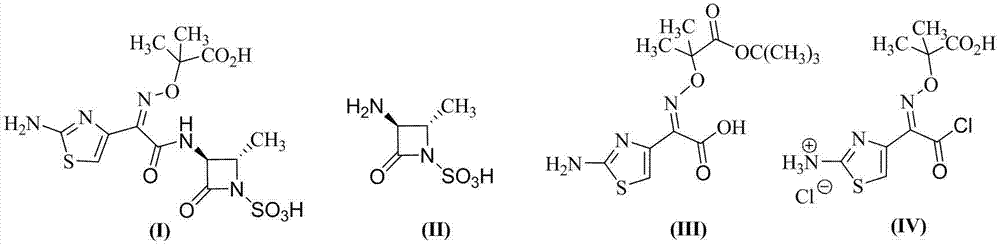
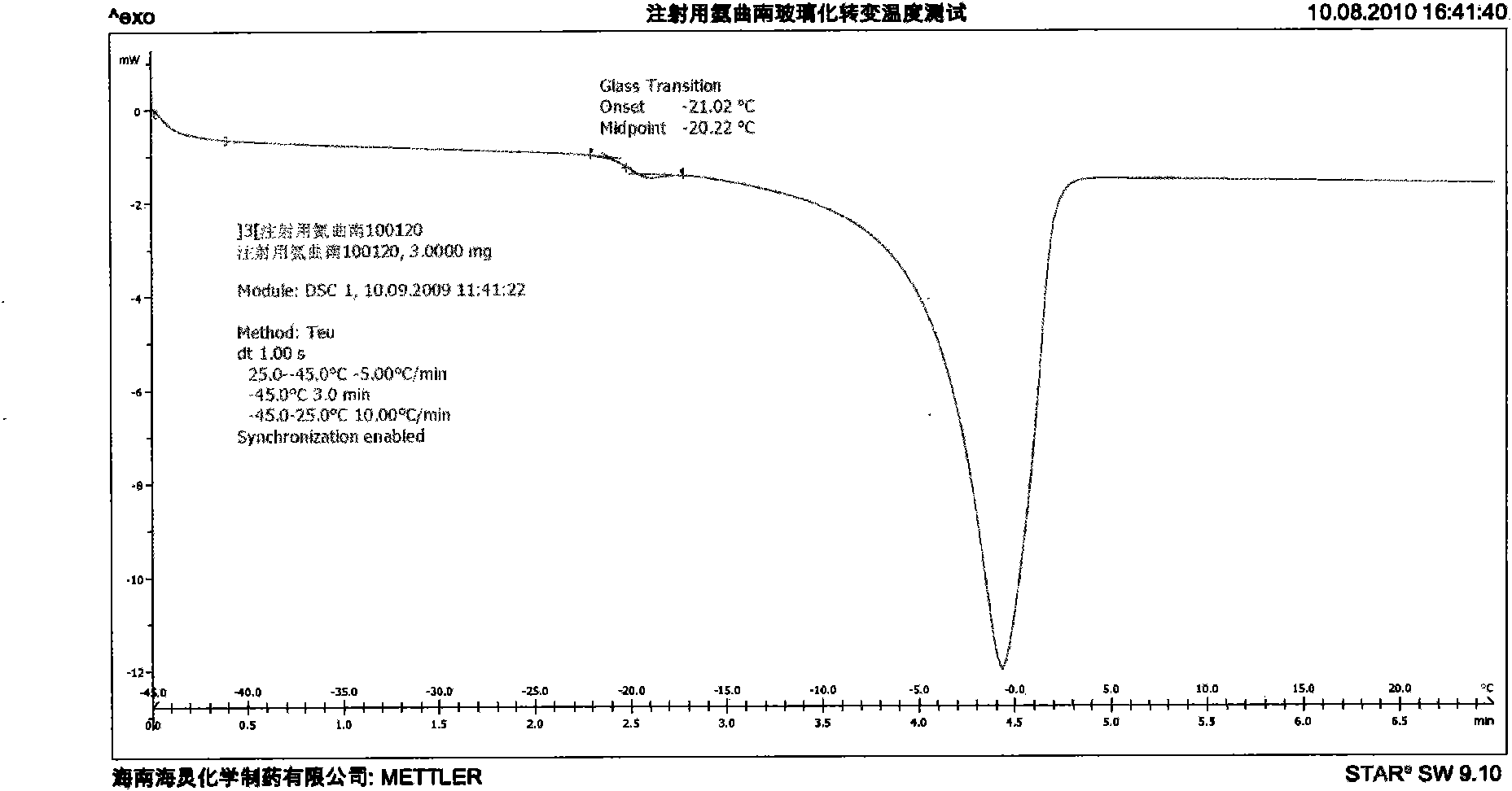
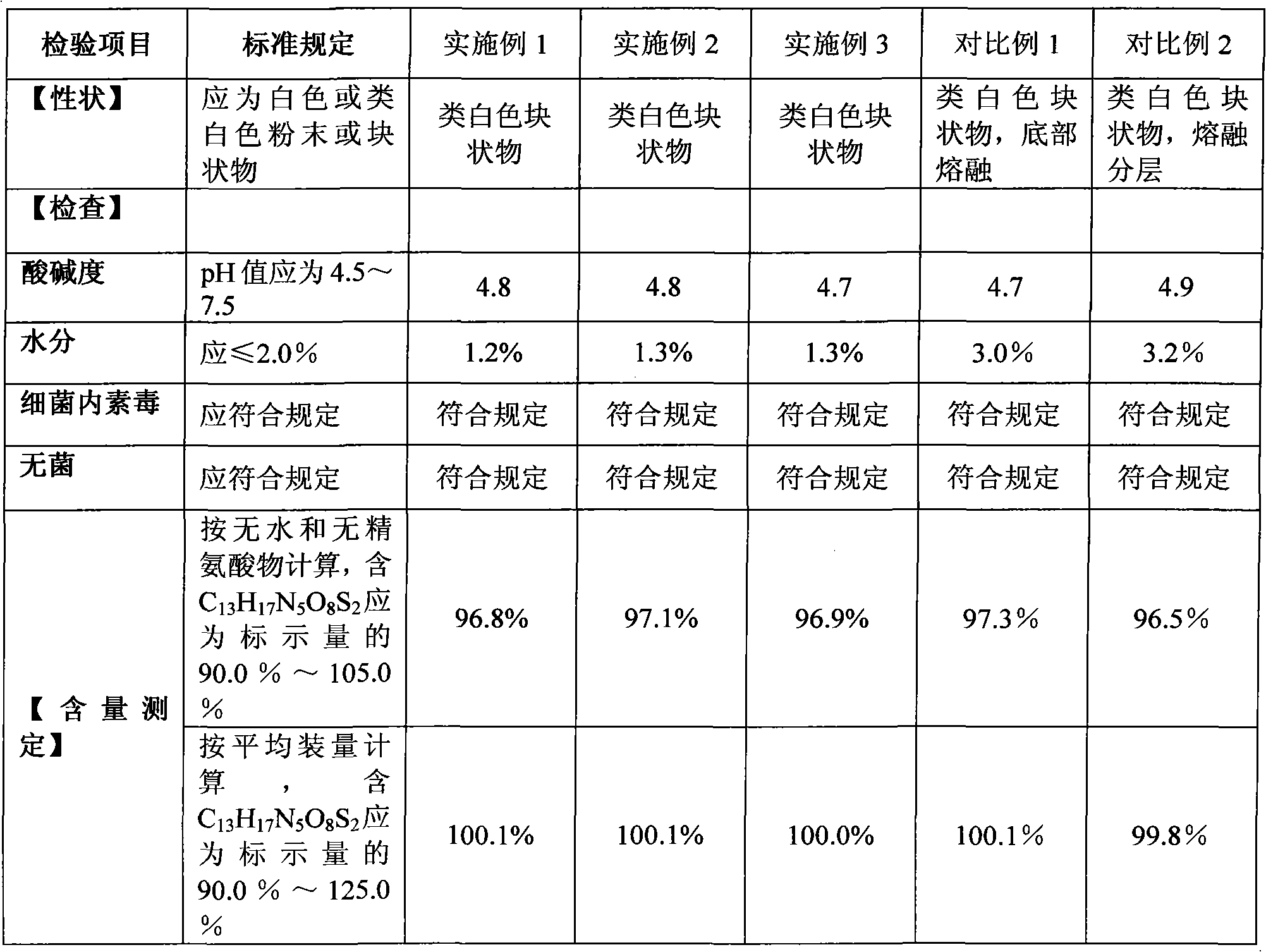
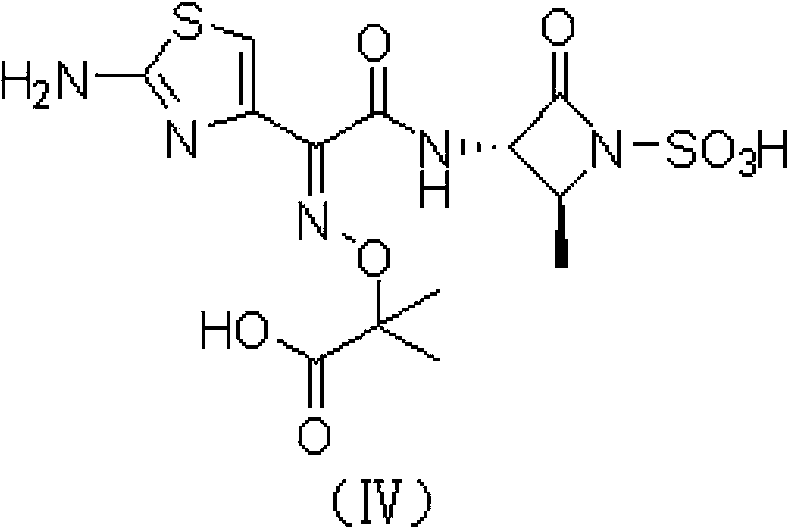
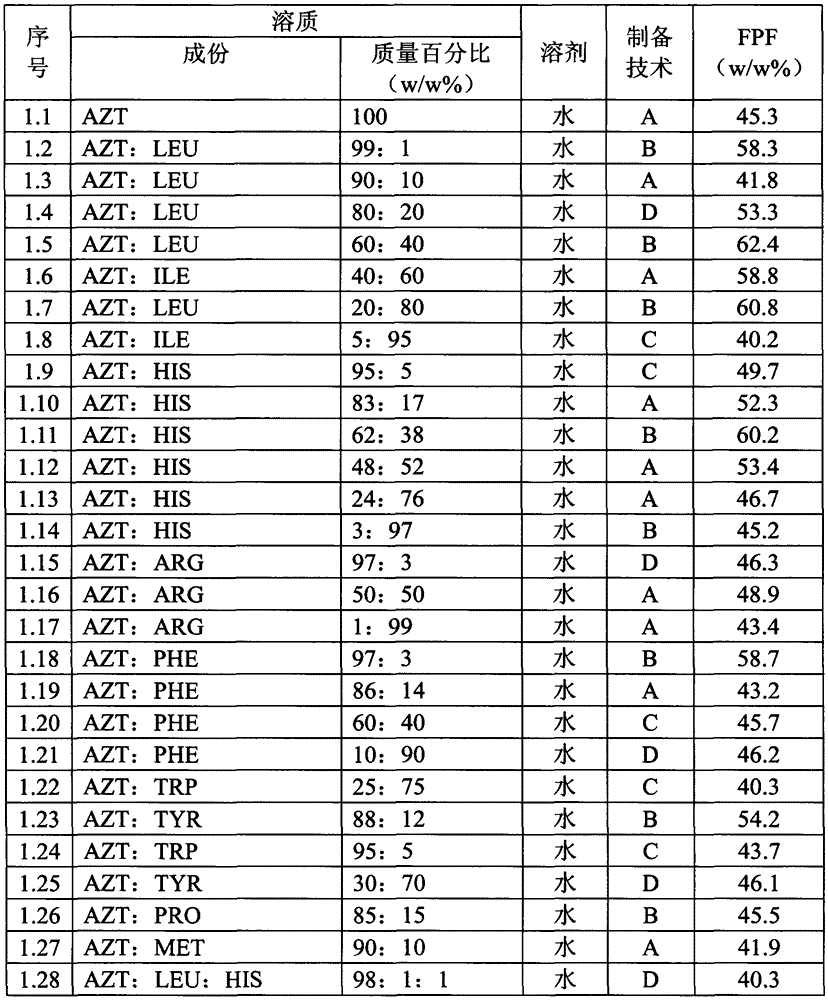
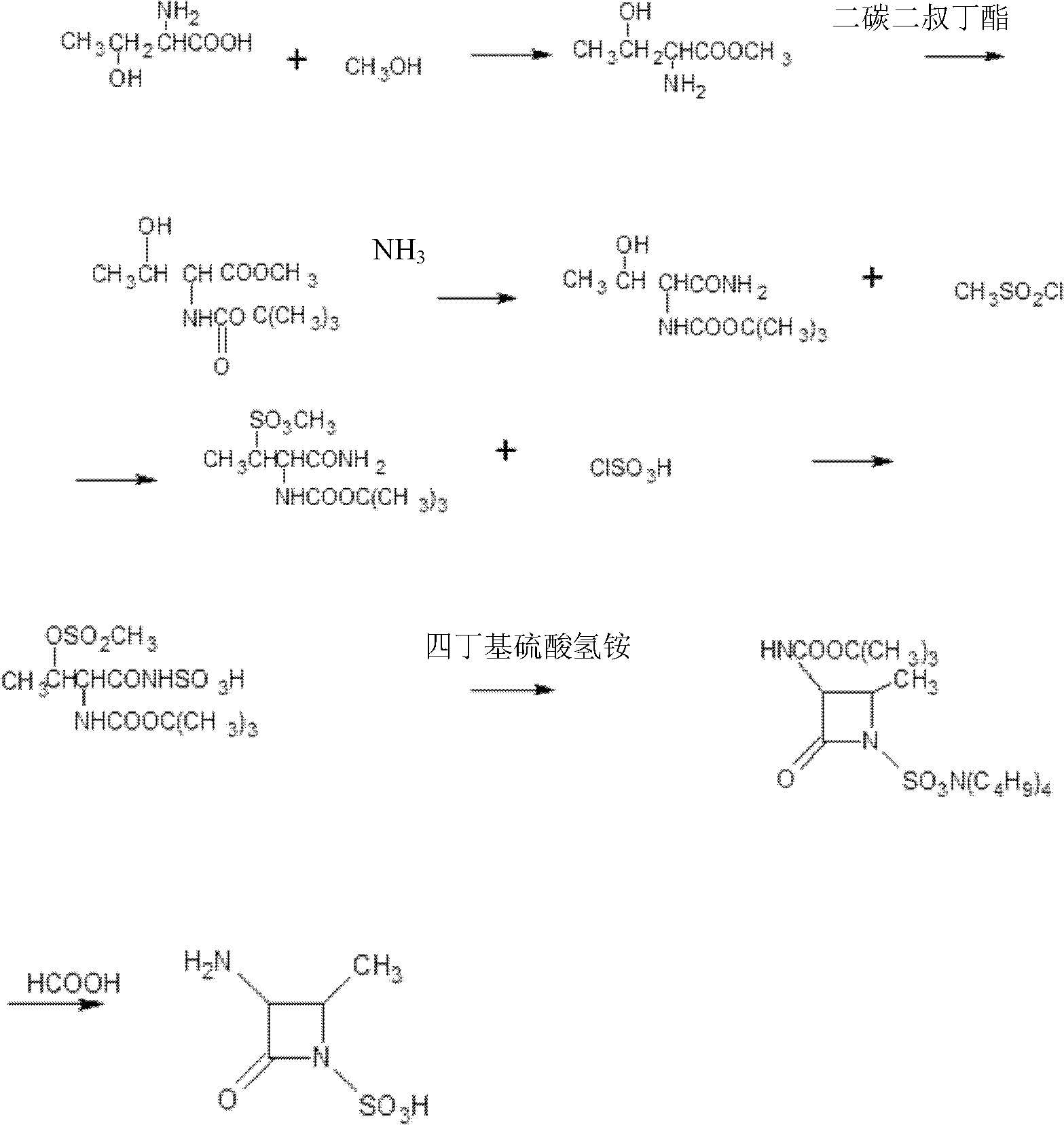Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
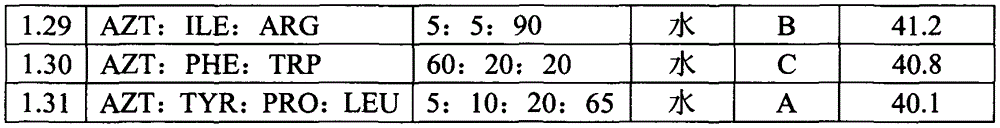
87 results about "Aztreonam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
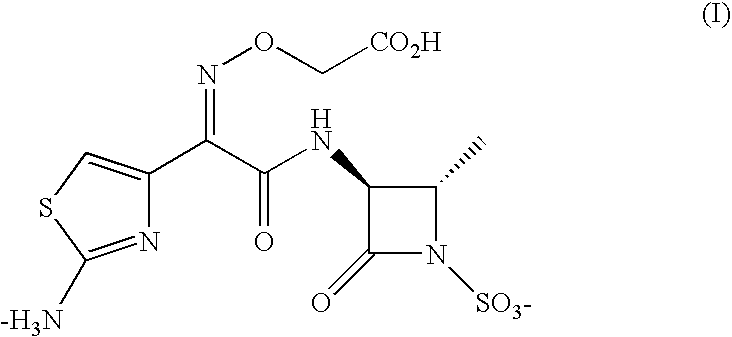
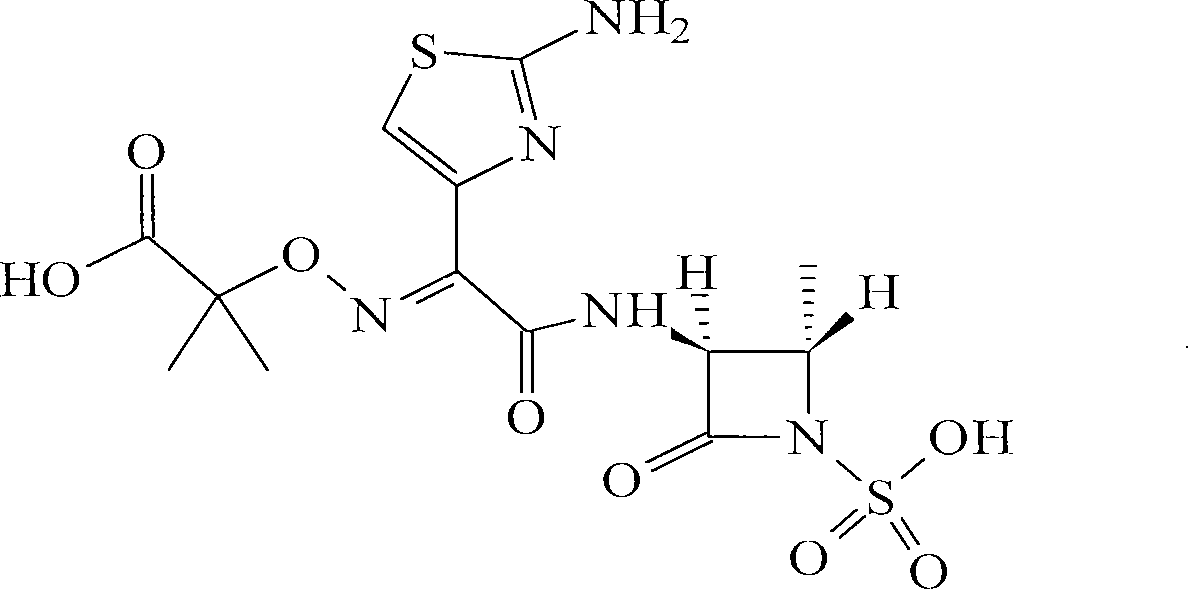
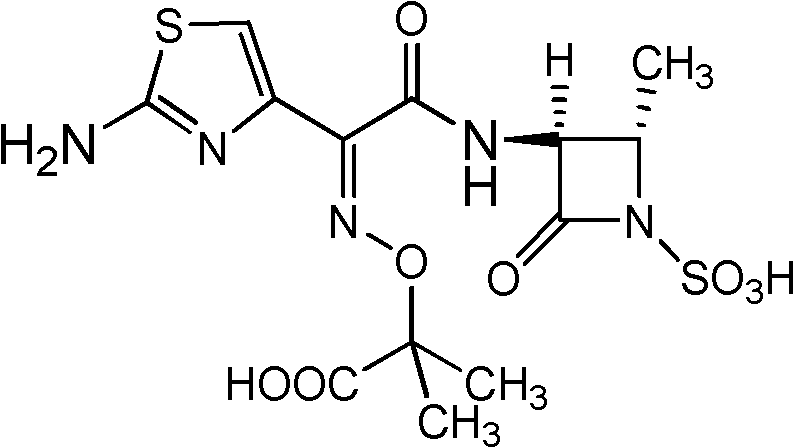
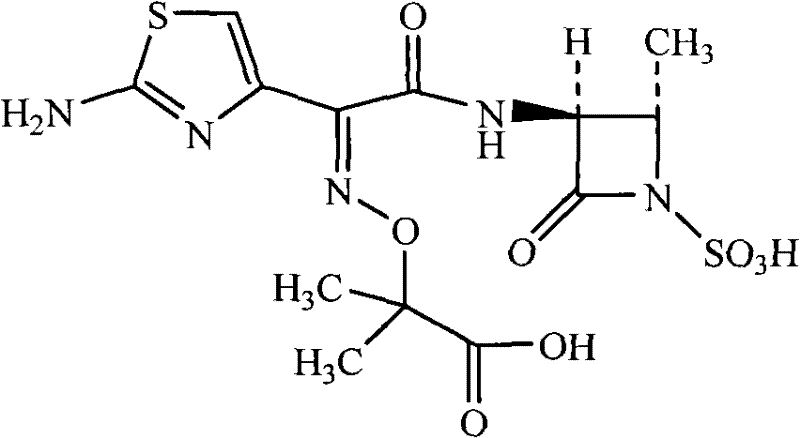
Aztreonam is used to treat a wide variety of bacterial infections.
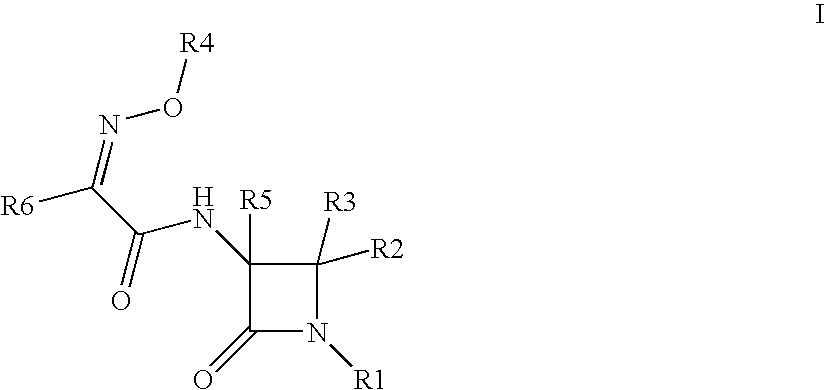
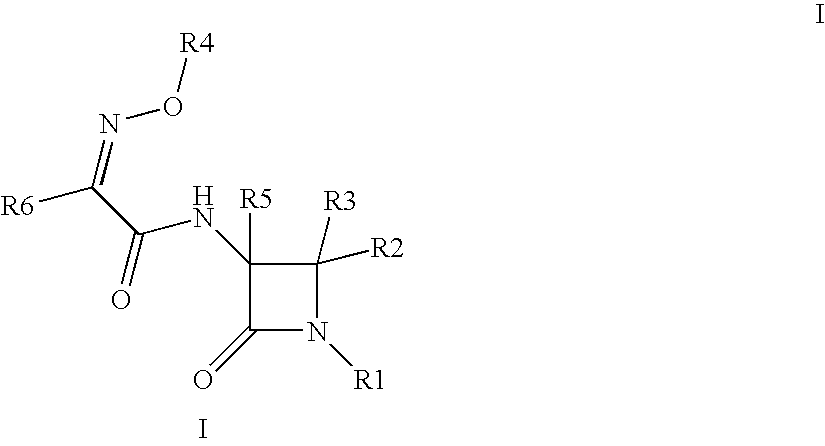
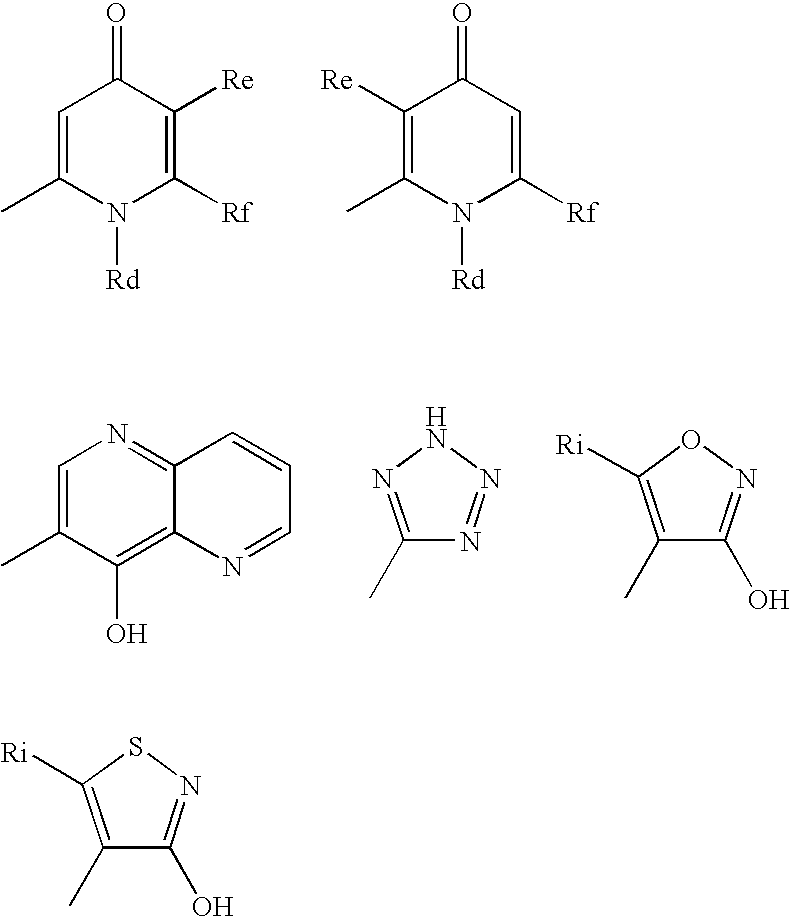
Useful Combinations of Monobactam Antibiotics With Beta-Lactamase Inhibitors
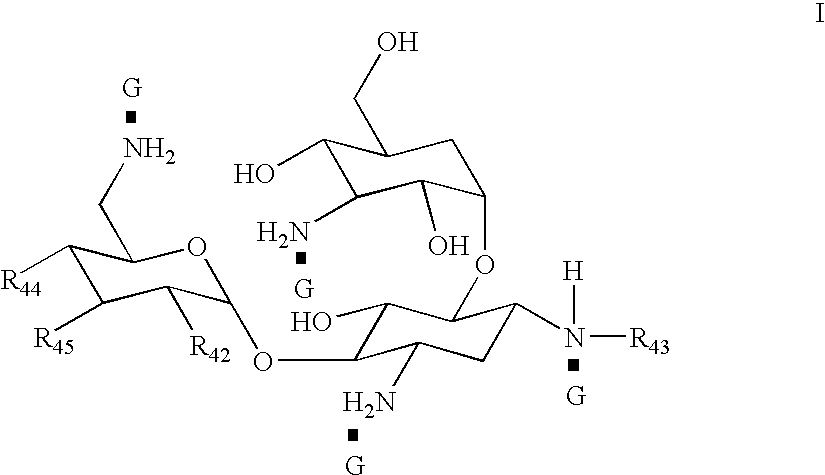
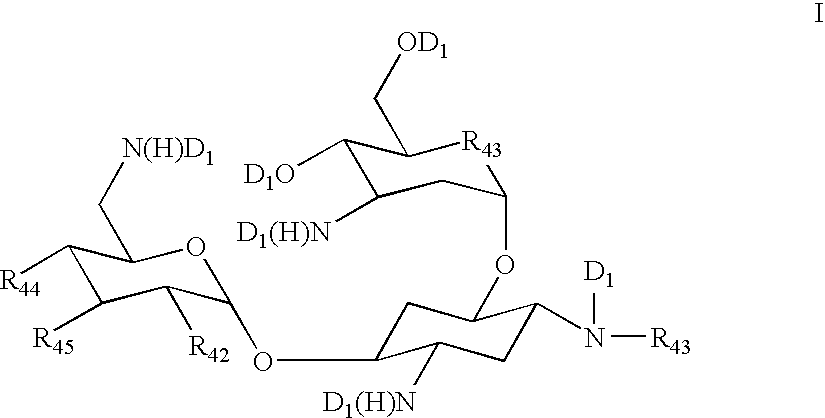
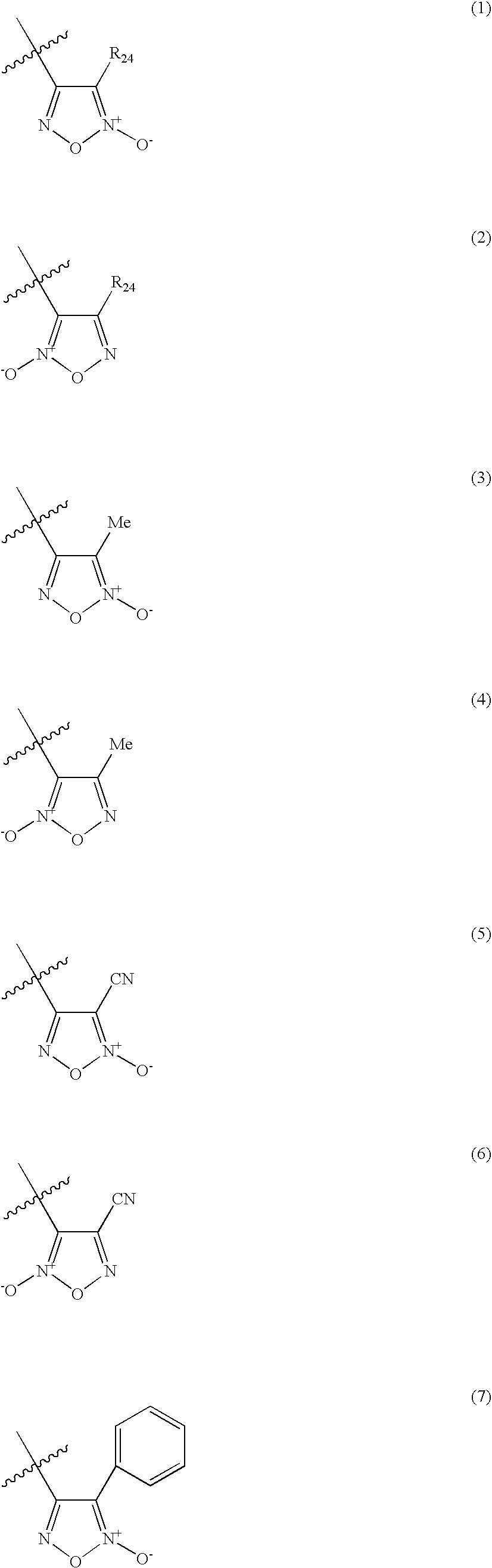
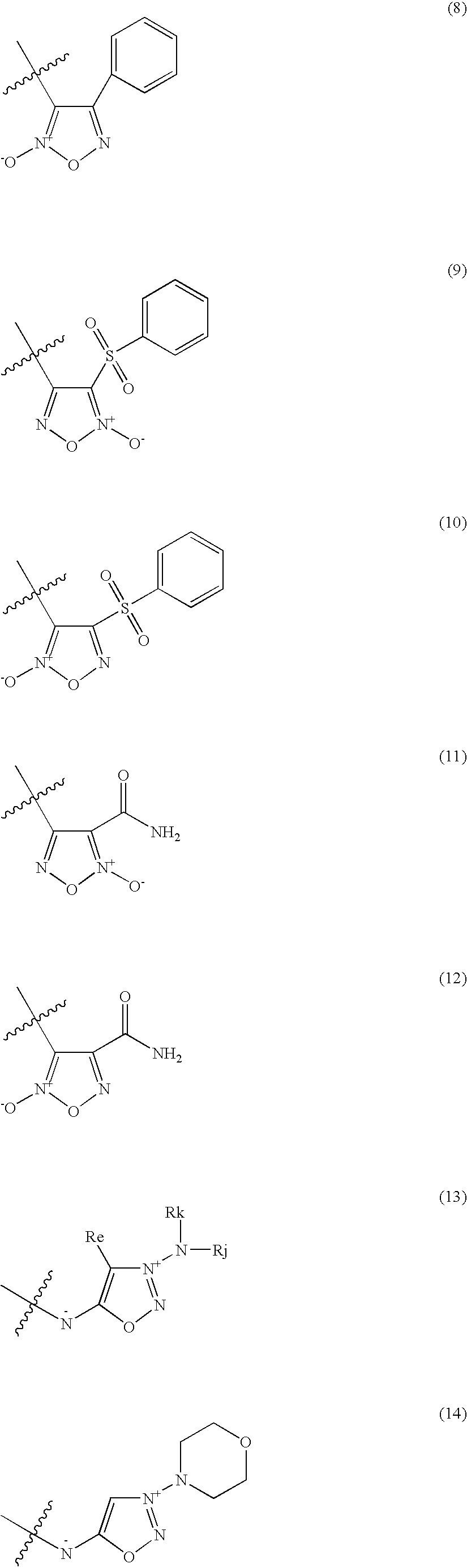
A pharmaceutically composition, comprising a combination of an antibiotically active compound of the formula (I): with a β-lactamase inhibitor of one of the formulae (II) to (XIII) are active against Gram-negative bacteria, in particular such bacteria which have become resistant against antibiotics such as aztreonam, carumonam and tigemonam. Optionally the compositions may comprise another β-lactamase inhibitor of one of the formulae (II) to (XIII), particularly of formula (V) or formula (VI).
Owner:BASILEA PHARMACEUTICA AG
Inhalable aztreonam aerosol for treatment and prevention of pulmonary bacterial infections
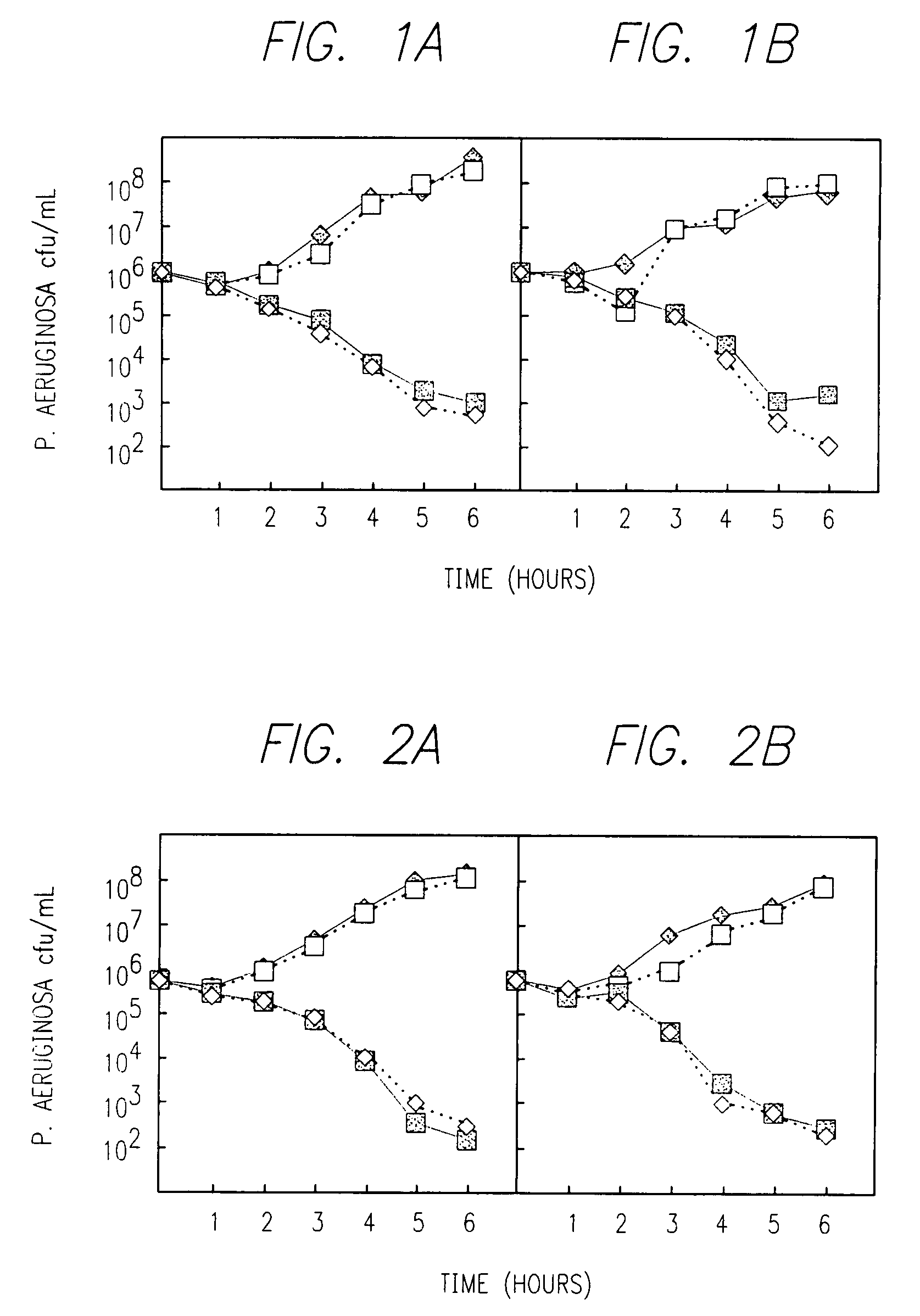
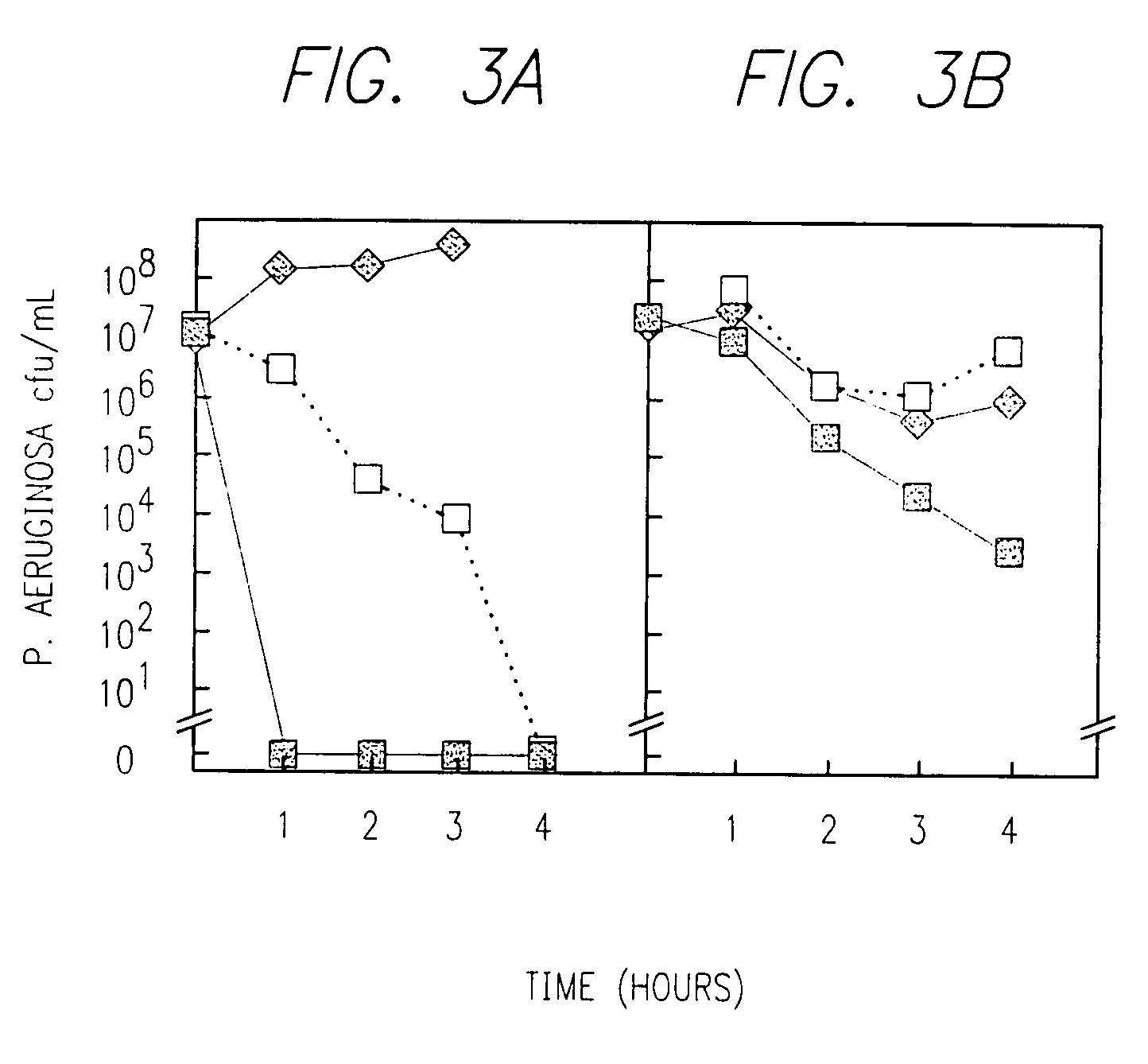
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam, or a pharmaceutically acceptable salt thereof, delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Beta-lactamase detecting reagent composition, detection kit and detection method
InactiveUS20060014230A1Quick checkOrganic chemistryMicrobiological testing/measurementΒ lactamasesNitrostyrol
The present invention provides a reagent composition for detecting β-lactamase including as a β-lactamase detection substrate 3-[2,4-dinitrostyryl]-7-(2-thienylacetamido]-3-cephem-4-carboxylic acid, or 7-[2-(2-aminothiazol-4-yl)-2-(1-carboxy-1-methylethoxy-imino)acetamido]-3-(2,4-dinitrostyryl)-3-cephem-4-carboxylic acid, and at least one β-lactamase inhibitor selected from the group consisting of clavulanic acid, aztreonam, ethylenediaminetetraacetic acid, and cloxacillin, which composition can detect β-lactamases rapidly and easily with high sensitivity. The present invention also provides a detection kit including the detecting reagent composition. Further, the present invention provides a β-lactamase detection method where a liquid specimen containing a target substance to be analyzed is brought into contact with the composition.
Owner:SHOWA YAKUHIN KAKO +1
Inhalable aztreonam lysinate formulation for treatment and prevention of pulmonary bacterial infections
InactiveUS7214364B2Improve stabilityHigh purityAntibacterial agentsPowder deliveryK pneumoniaeKlebsiella oxytoca
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam lysinate delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Organic nitric oxide donor salts of antimicrobial compounds, compositions and methods of use
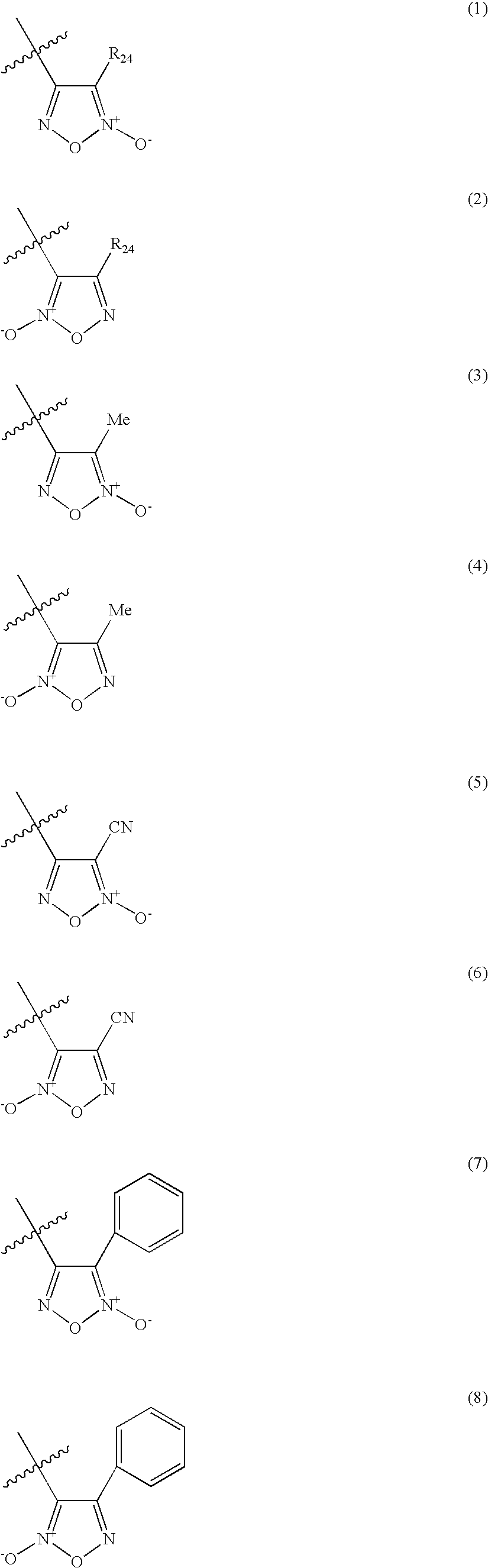
The invention describes novel organic nitric oxide donor salts of a antimicrobial compounds, and novel compositions and kits comprising at least one organic nitric oxide donor salt of an antimicrobial compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating bacterial infections; (b) treating viral infections; (c) treating fungal infections; and (d) treating lesions. In one embodiment the antimicrobial compounds of the invention are aztreonam, ciprofloxacin, doripenam, duramycin and tobramycin. The organic nitric oxide donors that form salts are preferably organic nitrates, organic nitrites, nitrosothiols, thionitrites and heterocyclic nitric oxide donors. The heterocyclic nitric oxide donors are preferably furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines. The methods of the invention are preferably for the treatment of bacterial infections associated with pulmonary diseases such as cystic fibrosis and for treating Bacillus anthracis infections.
Owner:NICOX SA
Aztreonam liposomes freeze-dry preparations and method of preparing the same
InactiveCN101249074AImprove stabilitySolve the problem of quality stabilityAntibacterial agentsOrganic active ingredientsFreeze-dryingAztreonam
The invention discloses a freeze-dried preparation of aztreonam liposomes, wherein aztreonam is encapsulated in antioxidant-containing liposomes made of neutral phospholipids, negative charge phospholipids and cholesterol. The freeze-dried preparation has stable quality. Additionally, the aztreonam is encapsulated to minimize toxicity and adverse effects without affecting drug effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
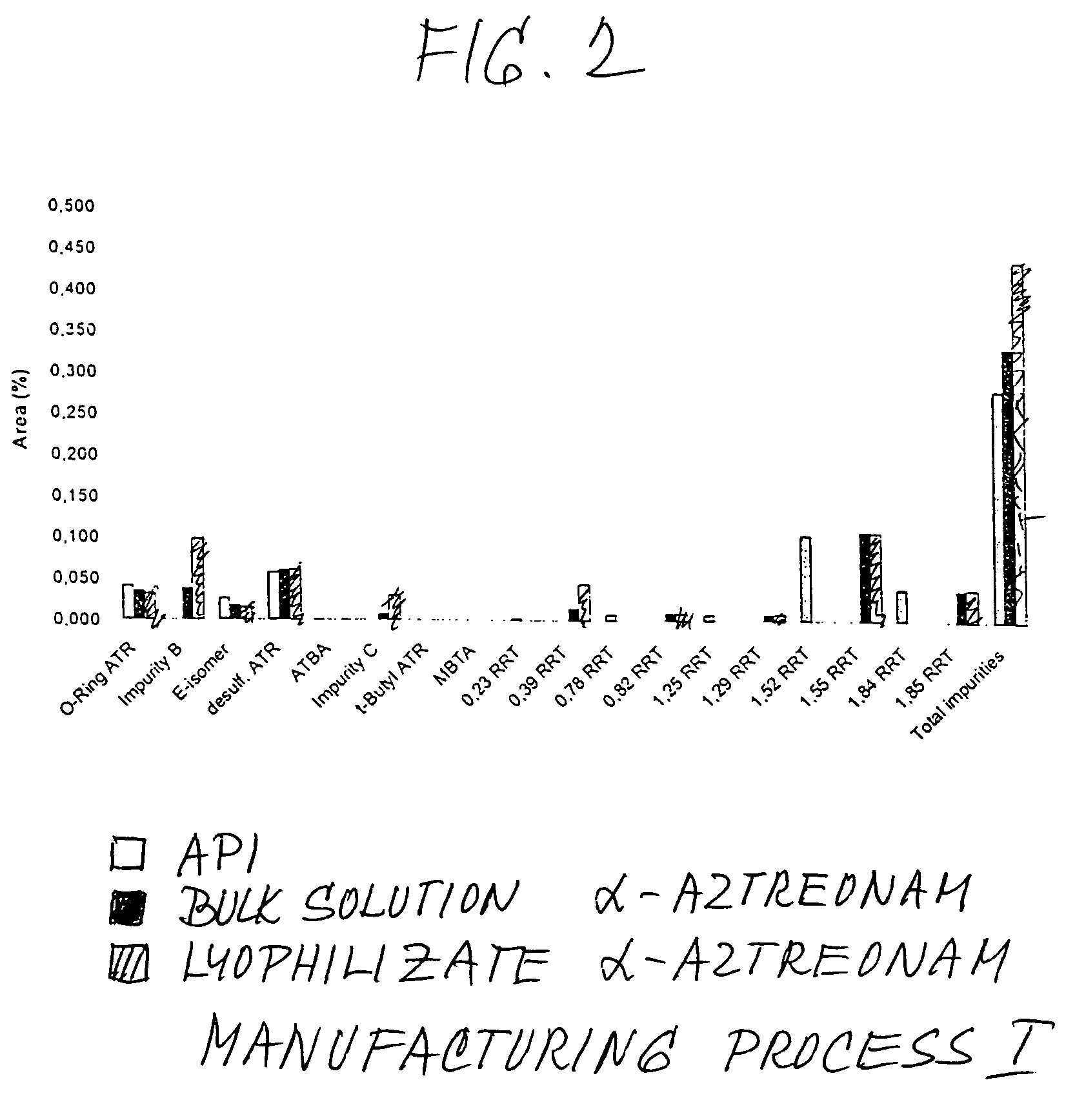
Process for manufacturing bulk solutions and a lyophilized pure α-aztreonam lysinate
A process for manufacturing bulk solutions and lyophilized pure α-aztreonam lysinate for large scale production of an inhalable aztreonam is disclosed, as is a pure α-aztreonam lysinate for inhalation. A dry powder or lyophilized pure α-aztreonam lysinate composition for inhalation is also disclosed.
Owner:GILEAD SCI INC
Nitric Oxide Enhancing Antimicrobial Compounds, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one nitric oxide enhancing group antimicrobial compound, or pharmaceutically acceptable salts thereof, and novel compositions comprising at least one nitric oxide enhancing antimicrobial compound, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating bacterial infections; (b) treating viral infections; (c) treating fungal infections; and (d) treating lesions. The antimicrobial compounds of the invention are preferably tobramycin, aztreonam, ciprofloxacin and doripenam. The nitric oxide enhancing antimicrobial compounds are substituted with at least one heterocyclic nitric oxide donor group and / or at least one nitroxide group. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors. The heterocyclic nitric oxide donors are furoxans, sydnonimines, oxatriazole-5-ones and / or oxatriazole-5-imines. In one embodiment the methods of the invention are for the treatment of bacterial infections associated with pulmonary diseases such as cystic fibrosis and for treating Bacillus anthracis infections.
Owner:NICOX SA
Aztreonam for injection and production method thereof
ActiveCN101579336ALess impuritiesExact ingredient ratioAntibacterial agentsOrganic active ingredientsGranularityAdditive ingredient
The invention discloses a new aztreonam for injection and a production method thereof. The aztreonam for injection consists of beta crystal form aztreonam and L-arginine, and the weight ratio of the beta crystal form aztreonam to the L-arginine is 1:0.74-1:0.86, wherein the components of aztreonam and L-arginine both need to be ground with the granularity respectively capable of passing a medicinal sieve ranging from 80 meshes to 120 meshes, preferentially a medicinal sieve of 100 meshes. The aztreonam for injection has the advantage of ensuring the crystal form of the aztreonam for injection same as that of raw materials, as well as the advantages of accurate rate of two ingredients and subpackage dosage, small subpackage difference among every bottle, uniform mixing, no delamination of two ingredients in production, transportation and storage, and the like. Simultaneously, the invention reduces the impurities of ring-opening aztreonam to some extent.
Owner:福安药业集团庆余堂制药有限公司
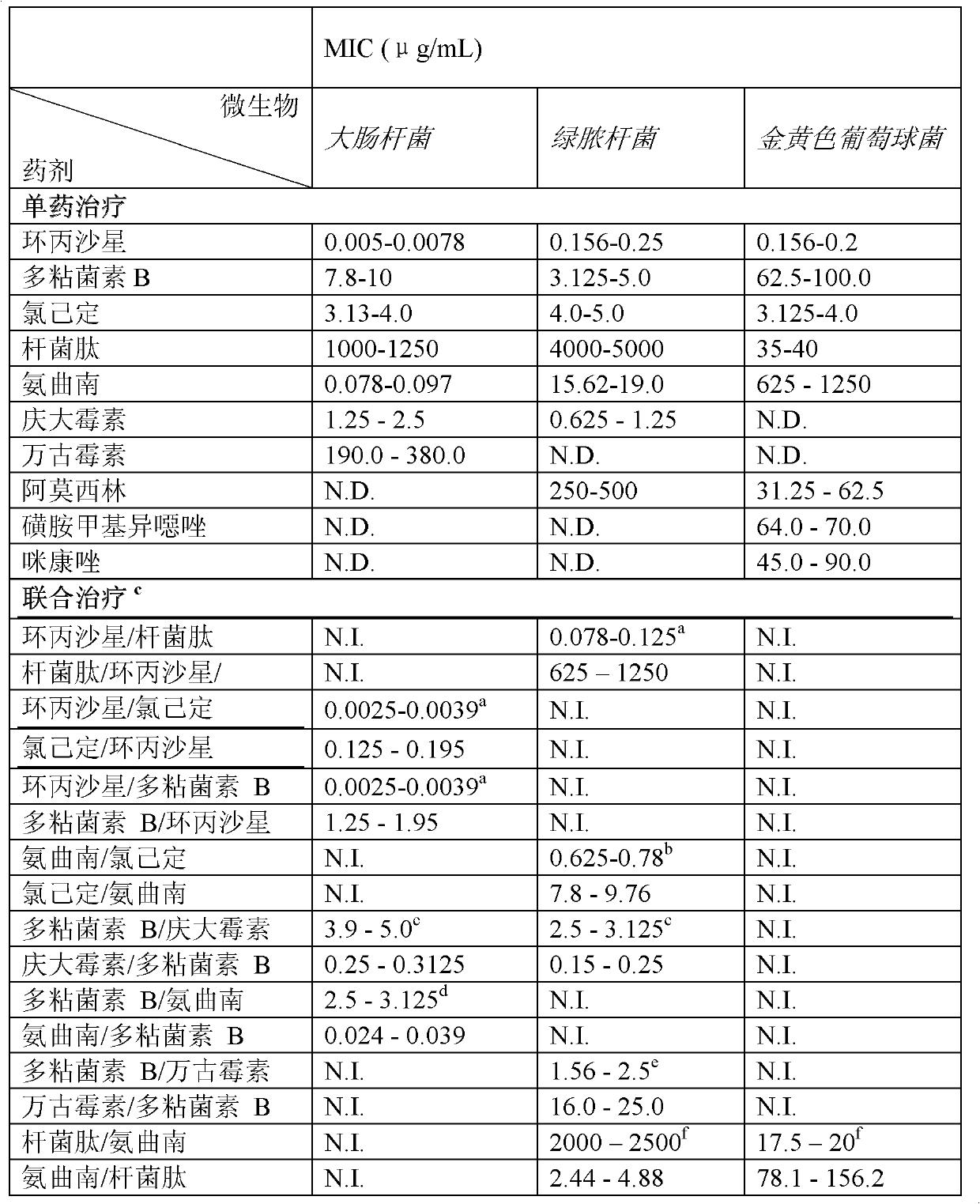
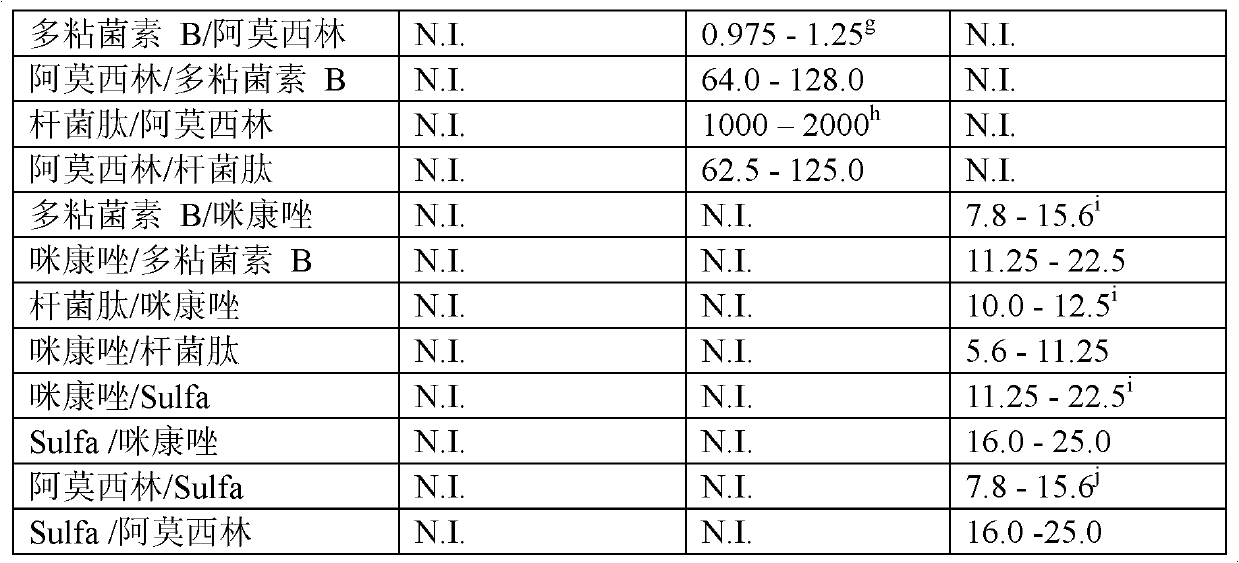
Combination therapy for the treatment of bacterial infections
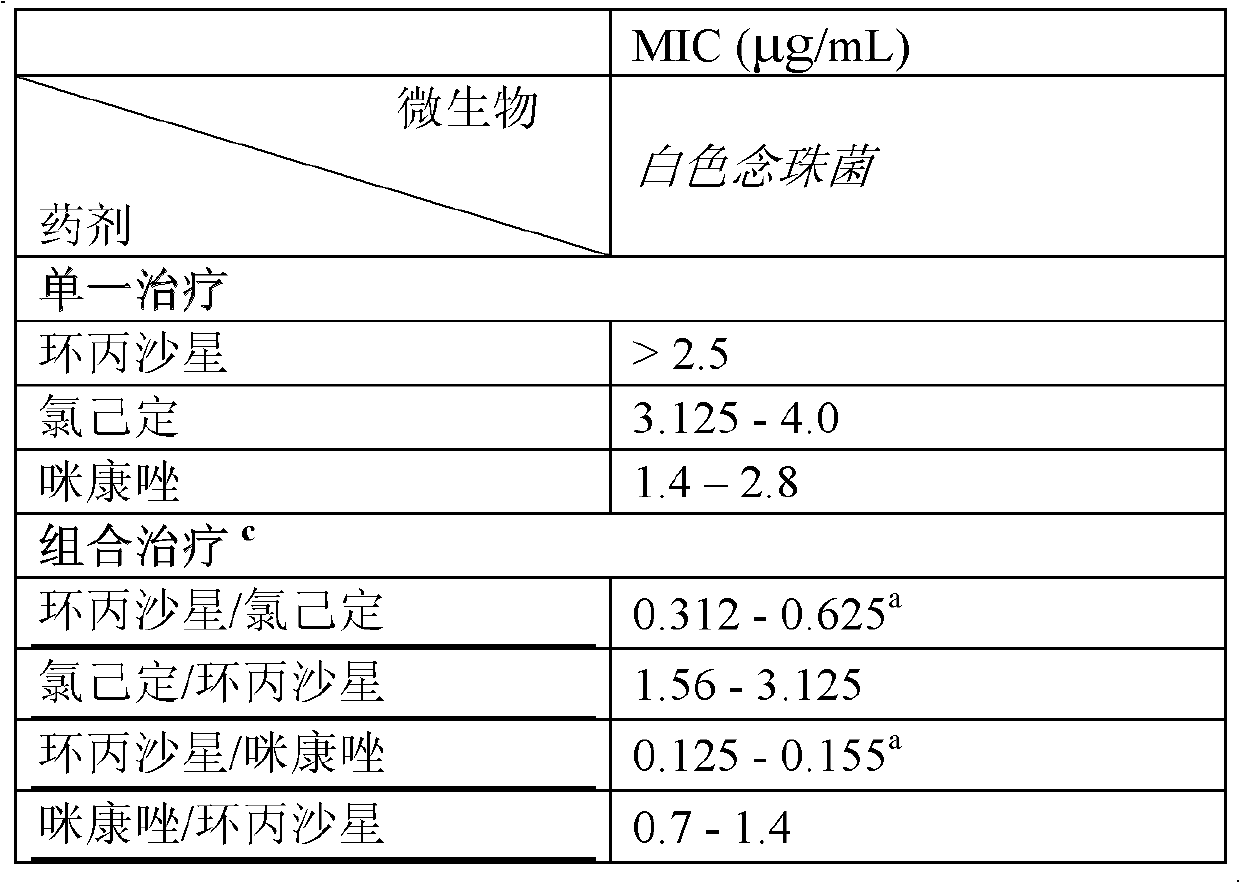
Compositions comprising the combination of a membrane active biocide and a second agent selected from fluoroquinolones, ammoglycodies, ss-lactams, glycopeptide antibiotics, sufonamides and antifungal azoles and their use in the treatment or prevention of bacterial or fungal infections Preferred membrane active biocides are selected from chlorohexidme, polymyxin B-nonapeptide, bacitracin, aztreonam, benzlakomum salts and metal chelators The active ingredients can be in either monomer or polymeric form.
Owner:INTERFACE BIOLOGICS INC
Inhalable aztreonam lysinate formulation for treatment and prevention of pulmonary bacterial infections
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam lysinate delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Method for producing aztreonam amino acid salt
InactiveCN101172974AComplete salt reactionHigh reaction yieldOrganic chemistrySolubilityOrganic solvent
The invention discloses a synthesis method for aztreonam salt. The method comprises the steps as follow: aztreonam is dissolved into organic solvent; the water solution of amino acid is dropped in the mixture; and the aztreonam salt is obtained by cooling and filtering the solution after full reaction. The invention is characterized in that the organic solvent is used as reaction solution, thereby ensuring the salt making reaction of aztreonam to be more completely with the high reaction yield; the used solvent can be recycled, thereby enabling the aztreonam amino acid salt to have better water-solubility and to be more easily to be made into preparations; and the salt is no longer mixed with the amino acid, thereby being more convenient for medicine use. The invention has the advantages of easy operation, high purity, low cost, and being suitable for mass production.
Owner:PKU HEALTHCARE CORP LTD
Sub-micro emulsion frozen preparation of aztreonam
InactiveCN101548956ASafety proofImprove stabilityAntibacterial agentsPowder deliveryEmulsionAztreonam
The invention provides a sub-micro emulsion frozen preparation of aztreonam and a preparation method thereof. The frozen preparation is prepared mainly with the following components according to parts by weight: 1-20 portions of aztreonam, 1-40 portions of biodegradable polymer, 1-20 portions of emulsifying agent, 10-50 portions of skeleton supporting agent and 0.5-20 portions of stabilizing agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for producing beta crystal system anhydrous aztreonam
The invention relates to a production method of anhydrous beta-type aztreonam, which comprises dissolving alpha-crystal-system aztreonam in the mixture of absolute ethyl alcohol and acetone at -10-+25DEG C, filtering without germ, heating the solution to 30-55DEG C, or seriously mixing to obtain anhydrous beta-type aztreonam crystals.
Owner:BEIJING GUANGFENG XIANFENG BIOTECH
Process for manufacturing bulk solutions and a lyophilized pure alpha-aztreonam lysinate
InactiveUS20050063912A1Improve stabilityHigh purityAntibacterial agentsPowder deliveryManufacturing technologyAZTREONAM LYSINE
A process for manufacturing bulk solutions and lyophilized α-aztreonam lysinate for large scale production of an inhalable aztreonam. A purified α-aztreonam lysinate for inhalation. Dry powder or lyophilized α-aztreonam lysinate composition for dry powder or aerosol inhalation.
Owner:GILEAD SCI INC
Synthesis method for aztreonam
The invention provides a synthesis method for aztreonam. The synthesis method for the aztreonam particularly comprises the following steps: preparing 2-[[(Z)-1-(2-amino-4-thiazolyl)-2-chlorine-2-oxo ethylidene]amino]oxygen-2-methyl propionic acid hydrochloride (IV) by using (2-amino thiazolyl-4-yl)-2-(tert-butyl oxycarbonyl)-isopropoxy iminodiacetic acid (III) as a starting material under the action of a BTC / TPPO system and HCl gas; and performing condensation on aztreonam parent nucleus (II) to directly obtain the aztreonam. Compared with the traditional process, the new process has the characteristics as follows: (1) an acyl chloride method is adopted, so damage to beta-lactam ring by strong acid in the traditional process is avoided from the source; (2) residue of 2-mercaptobenzothiazole (M) of the product is eliminated from the source; (3) the used reaction reagent triphenylphosphine oxide (TPPO) can be recycled and is environment-friendly; and (4) in the aztreonam product, the yield is high and the purity is over 99 percent, so important industrialized application prospect is achieved.
Owner:ZHEJIANG HUAFANG PHARMA
Method for synthesizing aztreonam compound
ActiveCN102127068AReduce pollutionReduce manufacturing costOrganic chemistry2-methylpropanoic acidSolvent
The invention relates to a method for synthesizing an aztreonam compound. The method comprises the following steps of: (1) adding water and a water-soluble non-aqueous solvent into a reaction container, adding trans-3(S)-amino-4-methyl-2-keto-1-azetidine sulfonic acid, triethylamine and (Z)-2-[(2-aminothiazole-4-radical)-(benzothiazole-2-sulfenyl carbonyl) methylamine oxygroup]-2-methylpropanoic acid tert-butyl ester for reacting, and adjusting the pH with acid and separating crystals out to obtain tertiary butyl aztreonam; and (2) subjecting the tertiary butyl aztreonam and acid-water mixed liquor to reaction and performing post-treatment to obtain aztreonam. By adopting the method, the reaction time can be shortened, the reaction speed can be increased, the production cost can be lowered, and environmental pollution can be reduced.
Owner:SHANXI PUDE PHARMA CO LTD
Novel method for refining aztreonam
The invention relates to a method for refining aztreonam, comprising the following processing steps: 1) in the presence of an appropriate solvent or solvent mixture, processing raw material aztreonam by using alkali metal or alkaline-earth metal oxide under the condition of heating, regulating the pH value with proper acid, cooling, separating out aztreonam precipitate so as to obtain the primarily purified aztreonam; 2) absorbing the aztreonam with strong alkaline ion-exchange resin, then eluting, collecting eluent, and concentrating at reduced pressure, so as to obtain secondary purified aztreonam; and 3) regulating the pH value with proper acid, crystallizing, centrifugally washing precipitated crystals, and drying, thus three-stage purified aztreonam is obtained. The purity of the refined aztreonam obtained by the method is not less than 99.2%, the purity of most refined aztreonam is not less than 99.5%, flaming residues are few, and the content of heavy metal is extremely low.
Owner:HAINAN LINGKANG PHARMA CO LTD
Environmentally-friendly synthesis process of Aztreonam main ring (3S-trans)-3-amino-4-methyl-2-oxo-1-azetidinyl sulfonic acid
The invention provides an environmentally-friendly synthesis process of Aztreonam main ring (3S-trans)-3-amino-4-methyl-2-oxo-1-azetidinyl sulfonic acid. The synthesis process comprises: carrying out methyl esterification on L-threonine, carrying out ammonolysis on L-threonine methyl ester bisulfate, carrying out N-BOC (N-tert-butoxycarbonyl) protection on amino group of L-threoninamide, carrying out mesylation on N-BOC-L-threoninamide, carrying out sulfonylation on N-BOC-O-mesyl-L-threoninamide, carrying out cyclization reaction on sodium N-BOC-O-mesyl-L-threoninamide sulfonate, and de-protecting and acidifying sodium (3S-trans)-3-tert-butoxyformamido-4-methyl-2-oxo-1-azetidinyl sulfonate to obtain (3S-trans)-3-amino-4-methyl-2-oxo-1-azetidinyl sulfonic acid. The synthesis process has the advantages that simple post-treatment, high yield, high utilization rate of wastes, low cost and low pollution; moreover raw materials can be obtained easily.
Owner:CHONGQING NANSONG CHEMI TECH +1
Aztreonam/arginine medicinal composition lipid microsphere injection
InactiveCN101912356AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsArginineMicrosphere
The invention provides an aztreonam / arginine medicinal composition lipid microsphere injection. The aztreonam / arginine medicinal composition lipid microsphere injection is mainly prepared from the following components in part by weight: 1 part of aztreonam, 0.7 to 8 parts of arginine, 1 to 8 parts of poly-anhydride, 0.1 to 2 parts of propylene glycol, 0.2 to 5 parts of fatty acid sorbitan 80 and 0.9 to 6 parts of sodium chloride.
Owner:HAINAN LINGKANG PHARMA CO LTD
Prepn process of aztreonam
The present invention relates to the preparation process of Aztreonam as one kind of antibacterial medicine. The preparation process includes the reaction of Aztreonam with protected side chain and formic acid or its water solution to obtain Aztreonam.
Owner:CHINA RESOURCES SAIKE PHARMA
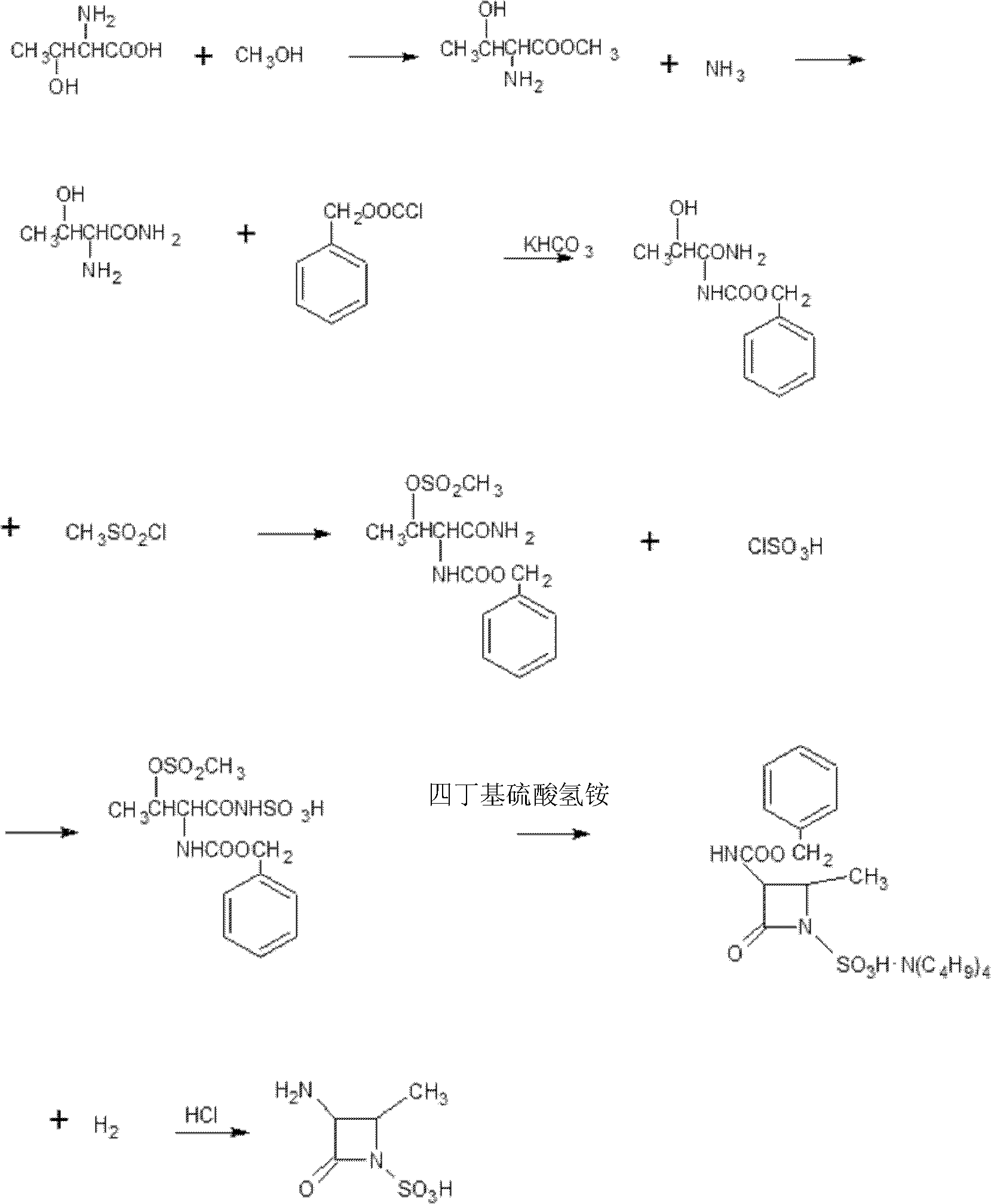
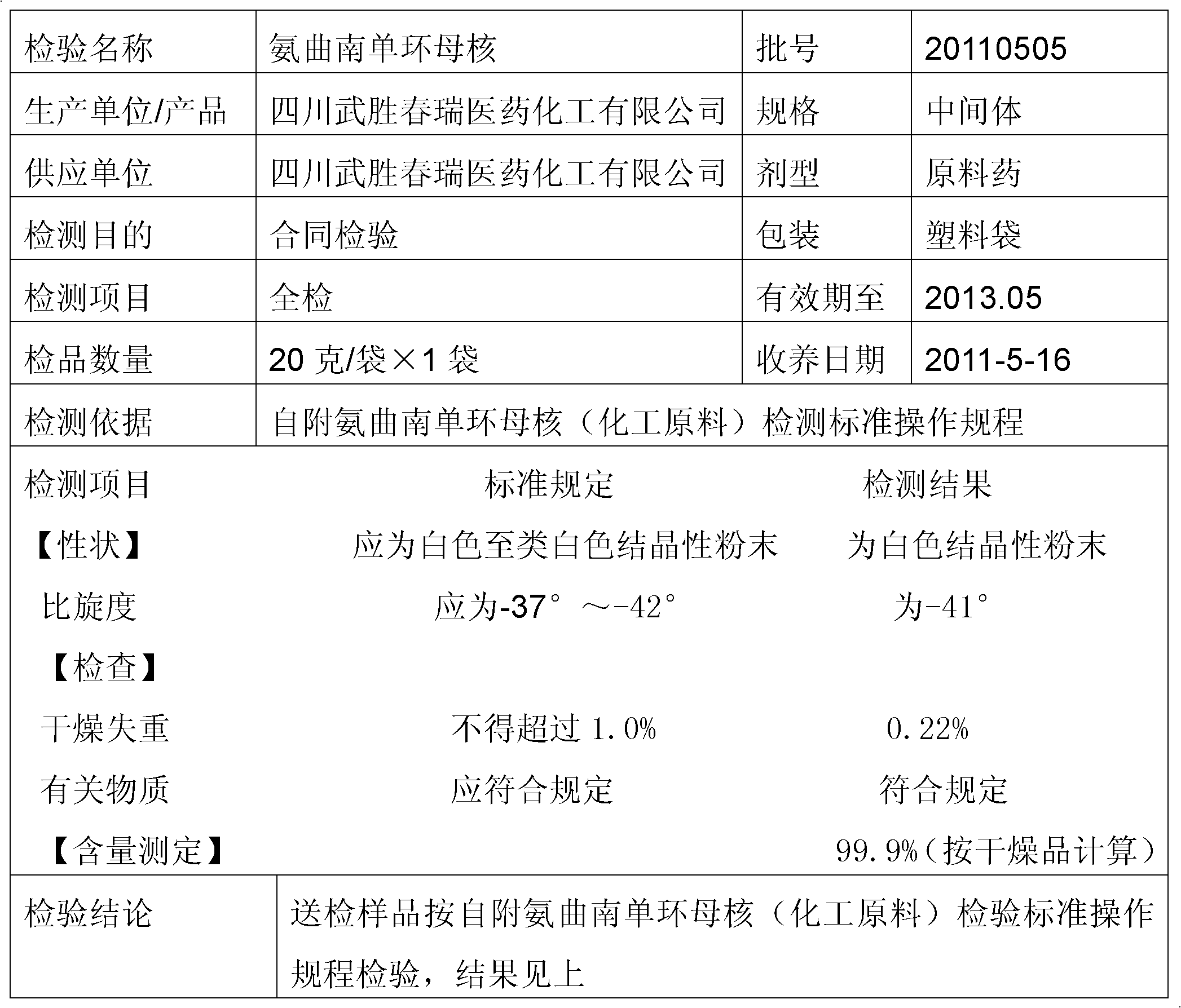
The preparation method of aztreonam monocyclic nucleus
ActiveCN102285907AGood water solubilityImprove product qualityOrganic chemistryBenzyl chloroformateAztreonam
The invention relates to a preparation method of aztreonam single-ring parent nucleus. The method uses benzyl chloroformate to protect under strong acidic conditions, and uses sodium carbonate to perform ring-closing reaction. The reaction is stable, the yield is greatly improved, and the total yield is For the 48% advantage.
Owner:SICHUAN WUSHENG CHUNRUI MEDICAL CHEM
Inhalable aztreonam lysinate formulation for treatment and prevention of pulmonary bacterial inections
A method and a composition for treatment of pulmonary bacterial infections caused by gram-negative bacteria suitable for treatment of infection caused by Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, Serratia marcescens as well as those caused by Burkholderia cepacia, Stenotrophomonas maltophilia, Alcaligenes xylosoxidans, and multidrug resistant Pseudomonas aeruginosa, using a concentrated formulation of aztreonam lysinate delivered as an aerosol or dry powder formulation.
Owner:GILEAD SCI INC
Method for preparing anhydrous beta-aztreonam
ActiveCN102311431ASolving Corrosion ProblemsGuaranteed sterilityOrganic chemistryOrganic acidAztreonam
The invention provides a method for preparing anhydrous beta-aztreonam. Based on preparation methods of amine-HCl-ethanol in the prior art, the method provided by the invention comprises steps of replacing inorganic acid with organic acid, carrying out neutralization, cooling and crystallizing, thus avoiding the corrosion of inorganic acid to equipment in clean areas and latent quality risk, guaranteeing the quality stability of the product and making the production to keep running normally.
Owner:HAINAN HAIYAO CO LTD +1
Formula and preparation process of aztreonam for injection
ActiveCN101953834AGood lookingQuality improvementAntibacterial agentsOrganic active ingredientsPolymer scienceFreeze-drying
The invention relates to a formula and a preparation process of aztreonam for injection. The preparation process comprises the following steps of: preparing; removing pyrogen; degerming, namely, filtering a prepared liquid medicament with a 0.22 mu m grade filter membrane within 8 hours for degerming; filling, namely, filling the degermed liquid medicament into a small bottle within 8 hours; performing freeze drying, namely, transferring the small bottle filled with the liquid medicament onto a shelf board of a freeze dryer, pre-freezing at the temperature of 40 DEG C below zero for 2 to 3 hours, setting the temperature of the shelf board to be between 0 and 8 DEG C at a primary drying stage, setting the vacuum degree to be between 5 and 15 Pa and drying for about 19 hours until the temperature of the product is close to the set temperature; and setting the temperature of the shelf board to be between 30 and 40 DEG C at a secondary drying stage, setting the vacuum degree to be between5 and 15 Pa, drying for 3 to 5 hours until the temperature of the product is more than 25 DEG C, restoring the pressure of a freeze drying box to atmospheric pressure, compressing a bottle plug and removing the small bottle from the freeze drier. The preparation process has the advantages of short period, low energy consumption and the like.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Aztreonam preparation method
The present invention relates to an aztreonam compound preparation method, which comprises the following operation steps: (1) dissolving and mixing a compound represented by a formula (I) and a compound represented by a formula (II) with an organic solvent, adding an alkali at a room temperature, carrying out a complete stirring reaction, cooling to a temperature of -20-10 DEG C, adding trifluoroacetic acid in a dropwise manner, stirring, filtering, and drying the filter cake to obtain a compound represented by a formula (III); and dissolving the compound represented by the formula (III) in formic acid, adding anisole, cooling to a temperature of -20-10 DEG C, adding hydrochloric acid in a dropwise manner, stirring, adding ethyl acetate, stirring, filtering, washing the filter cake with ethyl acetate, and drying to obtain the aztreonam compound represented by a formula (IV). Compared with the aztreonam compound preparation method in the prior art, the aztreonam compound preparation method of the present invention has characteristics of stable raw material source, low price, low cost, short reaction period, simple operation, high yield, less three-waste, environmental pollution reduction and high product purity, and is suitable for industrial production. The formula I, II, III and IV are as the follows.
Owner:FUAN PHARM (GRP) CO LTD +1
Aztreonam beta polymorph with very low residual solvent content
The invention relates to the β polymorph of Aztreonam, which contains less than 2.5% by weight residual solvent and to a process of making said polymorph.
Owner:TEVA PHARM USA INC +1
Method for preparing aztreonam powder aerosol
InactiveCN104784159AGood dispersionEfficient transpulmonary delivery efficiencyAntibacterial agentsOrganic active ingredientsDispersityPrill
The invention discloses a method for preparing an aztreonam (AZT) powder aerosol. The method comprises the following steps: accurately weighing a certain quantity of AZT or AZT and auxiliary materials; dissolving the weighed AZT or AZT and auxiliary materials in an appropriate solvent to obtain a basic solution; spray-drying the basic solution to manufacture corresponding dry powder particles, wherein the auxiliary material is amino acid and / or an absorption enhancing agent. According to the method, the particle diameters of the prepared AZT dry powder particles are within the inhalable range, so that favorable dispersity and efficient lung delivery efficiency are achieved.
Owner:SUZHOU HUIREN BIOLOGICAL SCI & TECH
Aztreonam liposomes freeze-dry preparations and method of preparing the same
InactiveCN100548295CHigh parcel rateLow costAntibacterial agentsOrganic active ingredientsFreeze-dryingAztreonam
The invention discloses a freeze-dried preparation of aztreonam liposomes, wherein aztreonam is encapsulated in antioxidant-containing liposomes made of neutral phospholipids, negative charge phospholipids and cholesterol. The freeze-dried preparation has stable quality. Additionally, the aztreonam is encapsulated to minimize toxicity and adverse effects without affecting drug effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for synthesizing aztreonam compound
ActiveCN102127068BReduce pollutionReduce manufacturing costOrganic chemistry2-methylpropanoic acidSolvent
The invention relates to a method for synthesizing an aztreonam compound. The method comprises the following steps of: (1) adding water and a water-soluble non-aqueous solvent into a reaction container, adding trans-3(S)-amino-4-methyl-2-keto-1-azetidine sulfonic acid, triethylamine and (Z)-2-[(2-aminothiazole-4-radical)-(benzothiazole-2-sulfenyl carbonyl) methylamine oxygroup]-2-methylpropanoic acid tert-butyl ester for reacting, and adjusting the pH with acid and separating crystals out to obtain tertiary butyl aztreonam; and (2) subjecting the tertiary butyl aztreonam and acid-water mixed liquor to reaction and performing post-treatment to obtain aztreonam. By adopting the method, the reaction time can be shortened, the reaction speed can be increased, the production cost can be lowered, and environmental pollution can be reduced.
Owner:SHANXI PUDE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com