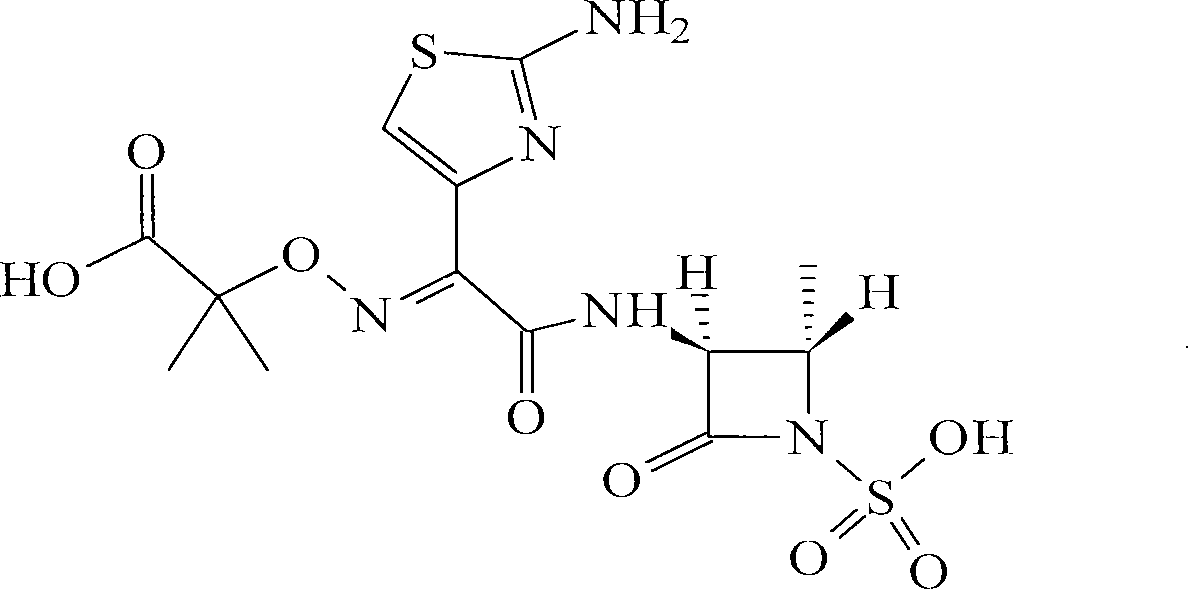
Method for producing aztreonam amino acid salt
A technology of amino acid salt and aztreonam, which is applied in the field of preparation of aztreonam amino acid salt, can solve the problems of low reaction yield, long freeze-drying process, low water solubility of aztreonam, etc., and achieve high reaction yield, Simple operation, good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of aztreonam arginine salt
[0031] Step (1): Prepare aztreonam solution
[0032] Get 20.0 grams of aztreonam crude product (moisture content 12.5%) and be dissolved in 350ml ethanol and stand-by;
[0033] Step (2): Preparation of L-arginine solution
[0034] Get 14.0 grams of L-arginine and dissolve it in water to make a 15% aqueous solution for later use;
[0035] Step (3): Preparation of aztreonam arginine salt
[0036] Add the aqueous amino acid solution in step (2) dropwise to the aztreonam solution in step (1) until the pH of the reaction solution is 4.5, react for 1 hour at 0°C, cool the resulting reactant, filter, After drying, 23.7 g of the obtained aztreonam arginine salt was obtained, and the yield was 70.7%.
Embodiment 2
[0037] Embodiment 2: the preparation of aztreonam lysine salt
[0038] Step (1): Prepare aztreonam solution
[0039] Get 25.0 grams (moisture content 14.3%) of aztreonam crude product and be dissolved in 150ml ethanol for stand-by;
[0040] Step (2): Preparation of L-lysine solution
[0041] Get 15.5 grams of L-lysine and dissolve in water to prepare a 24% aqueous solution for use;
[0042] Step (3): preparation of aztreonam lysine salt
[0043] Add the aqueous solution of lysyl acid in step (2) dropwise to the aztreonam solution in step (1), until the pH value of the reaction solution is 5.5, react for 120 minutes at 20°C, and cool the reactant obtained, After filtering and drying, 28.5 g of the obtained aztreonam lysine salt was obtained, and the yield was 72.0%.
Embodiment 3
[0044] Embodiment 3: the preparation of aztreonam arginine salt
[0045] Step (1): Prepare aztreonam solution
[0046] Get 45.0 grams (moisture content 11.5%) of aztreonam crude product and be dissolved in 270ml methanol for stand-by;
[0047] Step (2): Preparation of L-arginine solution
[0048] Get 31.9 grams of L-arginine and dissolve in water to prepare a 34% solution for use;
[0049] Step (3): Preparation of aztreonam arginine salt
[0050] Add the above L-arginine aqueous solution dropwise to the aztreonam solution until the pH value of the reaction solution is 4.7, react for 2 hours at 40°C, cool, filter, and dry to obtain aztreonam arginine Salt 65.3g, the yield is 91.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com