Purification method for insulin crystal or insulin analogue crystal
A technology for insulin analogs and purification methods, which is applied in the field of purification of insulin crystals or insulin analog crystals, can solve problems such as poor volatility, and achieve the effects of low solvent residue, uniform particles, and simple and quick operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
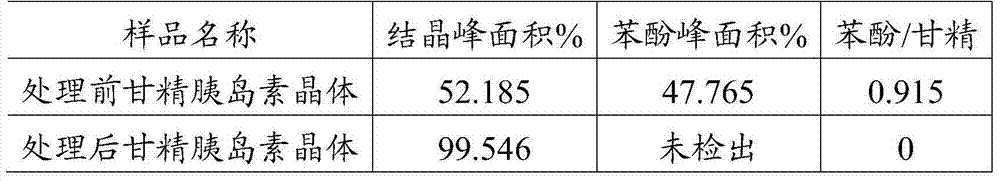
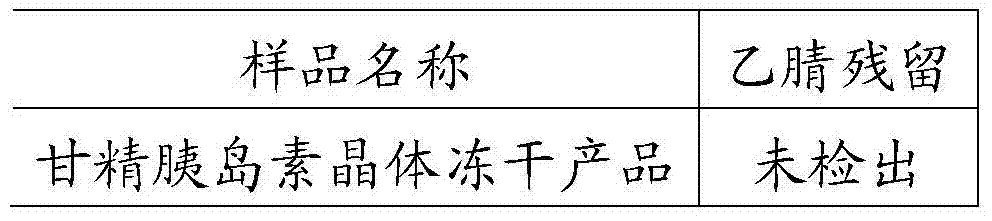
[0031] Take insulin glargine crystals prepared with phenol, zinc chloride, etc., add about 10 times the volume of acetonitrile to wash the crystals for 10 minutes, stir to completely disperse the crystals, turn on the vacuum pump to filter, and then rinse with about 8 times the volume of acetonitrile 5min. After rinsing, drain completely, remove the crystals, and freeze-dry them in a freeze-drying tray. The phenol content in the insulin glargine crystals before and after the acetonitrile treatment was detected by HPLC, and the results are shown in Table 1. And the residual acetonitrile dissolved in the final insulin glargine crystalline freeze-dried product was detected by gas chromatography with reference to the national standard product, and the results are shown in Table 2.
[0032] Table 1 Phenol content in insulin glargine crystals before and after acetonitrile treatment
[0033]
[0034] The content of acetonitrile in the freeze-dried product of insulin glargine cry...
Embodiment 2
[0038] Take the insulin glargine crystals described in Example 1, add about 8 times the volume of absolute ethanol to rinse the crystals for 20 minutes, stir to completely disperse the crystals, turn on the vacuum pump for suction filtration, and rinse with about 5 times the volume of absolute ethanol for 8 minutes at the same time , after rinsing, drain completely, take out the crystals, and freeze-dry them in a freeze-drying tray. The phenol content in insulin glargine crystals before and after treatment with absolute ethanol detected by HPLC, the results are shown in Table 3, and the ethanol residue in the final freeze-dried product of insulin glargine crystals was detected by gas chromatography with reference to the national standard product, the results are shown in Table 3 4.
[0039] Table 3 Phenol content in insulin glargine crystals before and after absolute ethanol treatment
[0040]
[0041] Content of acetonitrile in the freeze-dried product of insulin glargine...
Embodiment 3
[0045] Take insulin crystals prepared with m-cresol and zinc chloride, add about 15 times the volume of 95% ethanol to wash the crystals for 15 minutes, stir to make them completely dispersed, turn on the vacuum pump to filter, and then use about 10 times the volume of 95% ethanol to rinse the crystals for 15 minutes. Rinse for 20 minutes, drain completely after rinsing, take out the crystals, put them in a freeze-drying tray, and soak in a small amount of ice water. The m-cresol content in insulin crystals before and after 95% ethanol treatment was detected by HPLC, and the results are shown in Table 5. The ethanol-dissolved residue in the final freeze-dried product of insulin crystals was detected by gas chromatography with reference to the national standard product, and the results are shown in Table 6.
[0046] Table 595% ethanol before and after the m-cresol content in the insulin crystals
[0047]
[0048] Table 6 Content of ethanol in the freeze-dried product of ins...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com