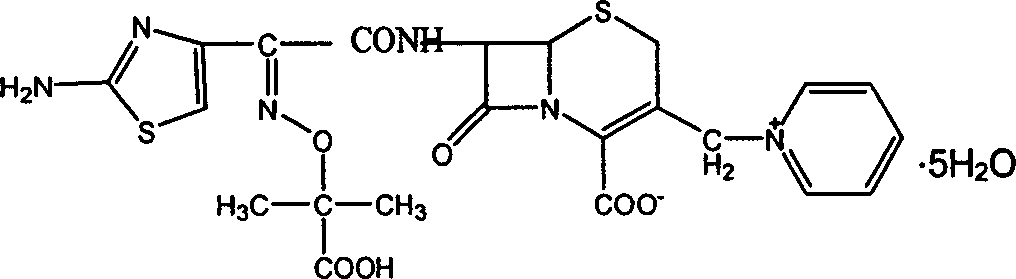
Ceftazidime pentahydrate purifying method
A ceftazidime and purification method technology, which is applied in the field of ceftazime pentahydrate, can solve the problems of low recovery rate and failure to obtain ceftazidime pentahydrate crystals, etc., and achieve the effects of low cost, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] For unqualified ceftazime pentahydrate, the content of ceftazime determined by high performance liquid chromatography is 90.1wt.%, and the content of polymer determined by illuminating column chromatography is 8.5wt.%.
[0022] Suspend 20.0 g of the unqualified ceftazidime pentahydrate in 100 ml of water, cool to 0-5° C. in an ice-water bath, and add 36 wt.% concentrated hydrochloric acid dropwise while stirring until the material dissolves to obtain an aqueous solution of ceftazidime. Under stirring condition, in the ceftazidime aqueous solution, dropwise add 2N NaOH solution, adjust pH to 2.5, separate out off-white precipitate, filter off precipitate (precipitate wet weight 1.99g, HPLC detection contains ceftazidime 0.25g), obtain filtrate. Then, the resulting filtrate was heated up to 5-10°C in an ice-water bath, and 2N NaOH solution was added dropwise with stirring to make the pH 4.0, ceftazidime pentahydrate crystals were precipitated, and the temperature was adjus...
Embodiment 2
[0024] 20.0 g of the unqualified ceftazime pentahydrate of Example 1 was suspended in 100 ml of water, cooled to 0-5° C. with an ice-water bath, and 99 wt.% acetic acid was added dropwise while stirring until the material was dissolved to obtain a clarified aqueous solution of ceftazime. Then, 0.5 g of activated carbon was added to the obtained aqueous solution of ceftazidime, stirred and decolorized for 30 minutes, and then filtered to remove the activated carbon to obtain a filtrate. The resulting filtrate was kept in an ice-water bath to keep the temperature at 0-5°C. Under stirring, 22 wt.% ammonia water was added dropwise to adjust the pH to 2.0. An off-white precipitate was precipitated, and the precipitate was filtered off to obtain the filtrate. Then, the resulting filtrate was heated to 20-25°C in a water bath, and 22wt.% ammonia water was added dropwise under stirring, so that the pH was 4.2, and ceftazidime pentahydrate crystals were precipitated, and then the temper...
Embodiment 3
[0026] 20.0 g of the unqualified ceftazime pentahydrate of Example 1 was suspended in 30 ml of water, cooled to 0-5° C. with an ice-water bath, and 36 wt.% hydrochloric acid was added dropwise while stirring until the material was dissolved to obtain a clarified aqueous solution of ceftazime. Then, 0.5 g of activated carbon was added to the obtained aqueous solution of ceftazidime, stirred and decolorized for 30 minutes, and then filtered to remove the activated carbon to obtain a filtrate. The resulting filtrate was kept in an ice-water bath to keep the temperature at 0-5°C. Under stirring, 22 wt.% ammonia water was added dropwise to adjust the pH to 2.0. An off-white precipitate was precipitated, and the precipitate was filtered off to obtain the filtrate. Then, the resulting filtrate is heated to 20-25°C in a water bath, and 22wt.% ammonia water is added dropwise under stirring, so that the pH is 3.8, and ceftazidime pentahydrate crystals are precipitated, and then the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com