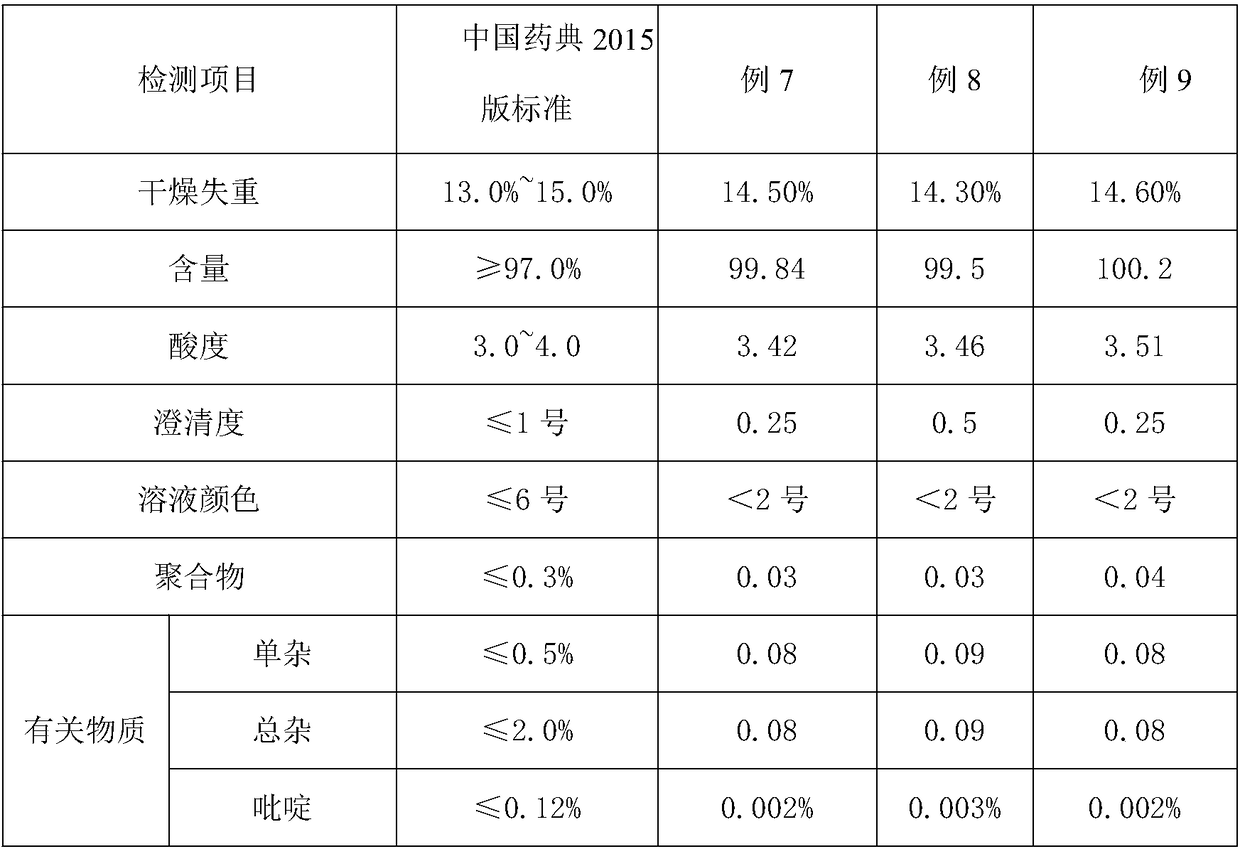
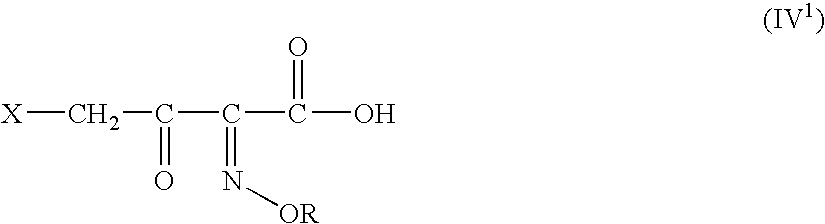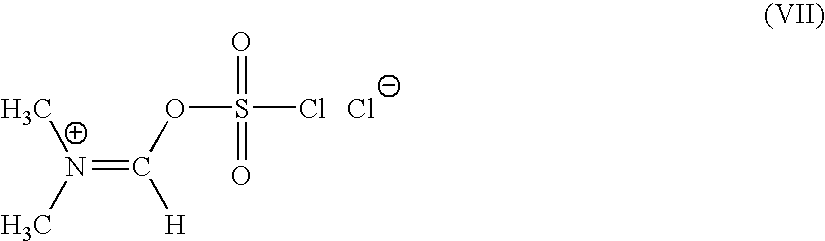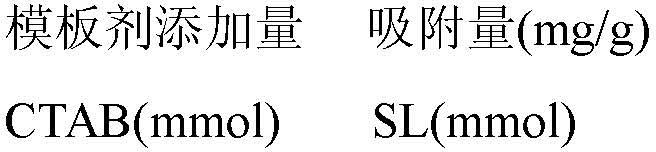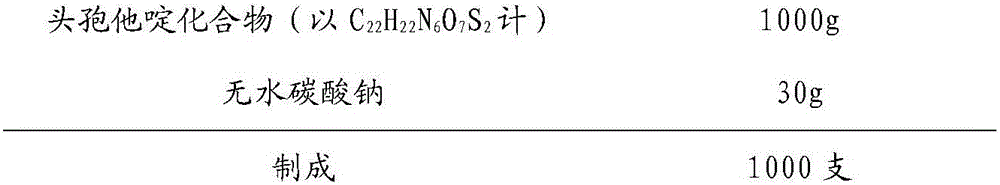Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
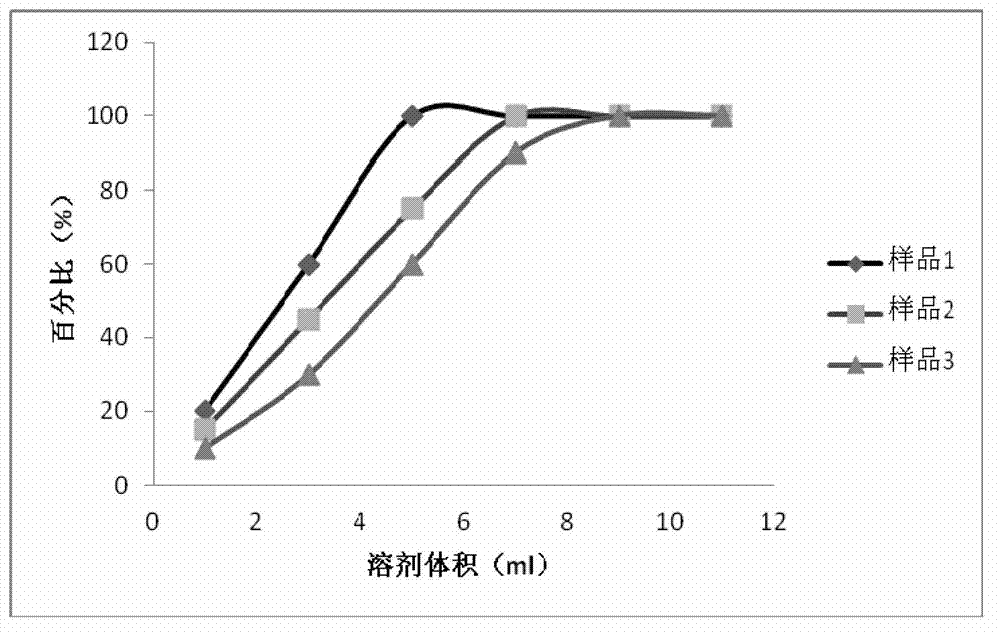
131 results about "Ceftazidime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
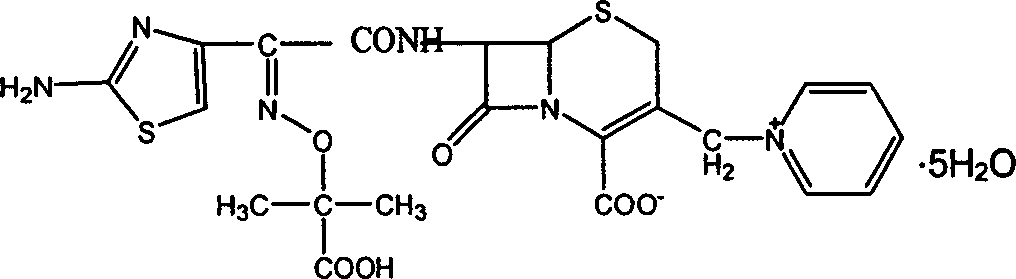
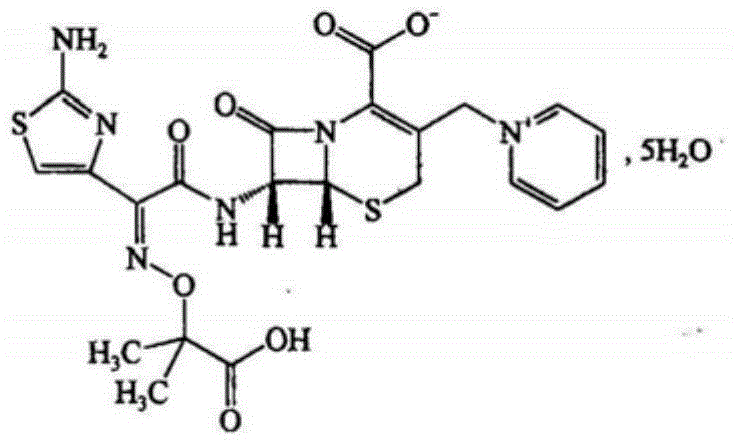
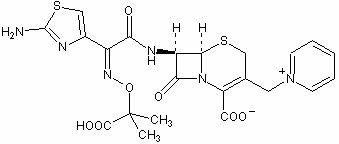
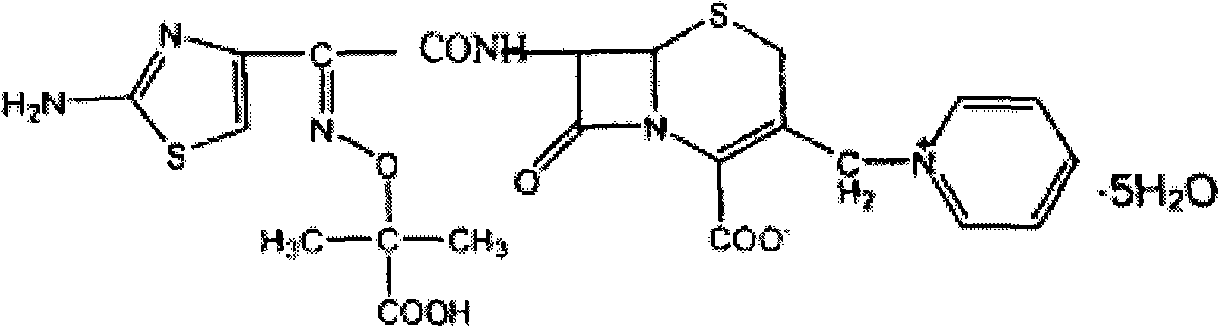
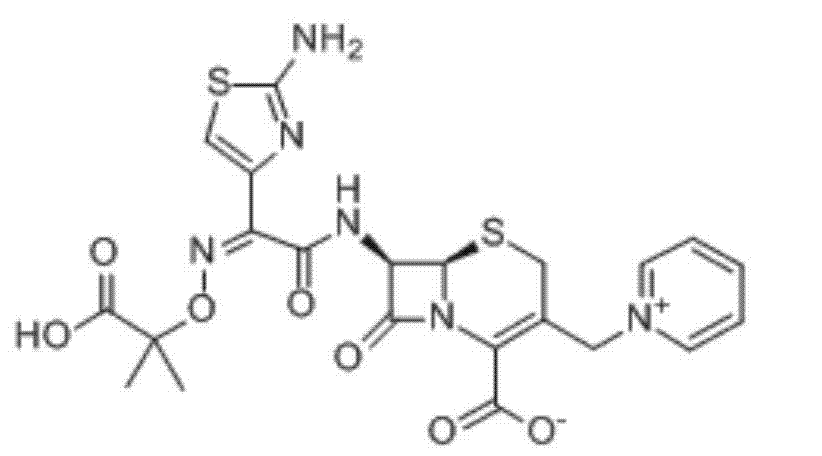
Ceftazidime is used to treat a wide variety of bacterial infections.
Beta-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Β-lactamase inhibitors
Substituted bicyclic beta-lactams of Formula I: (I), are β-lactamase inhibitors, wherein a, X, R1 and R2 are defined herein. The compounds and pharmaceutically acceptable salts thereof are useful in the treatment of bacterial infections in combination with β-lactam antibiotics. In particular, the compounds can be employed with a β-lactam antibiotics (e.g., imipenem, piperacillin, or ceftazidime) against microorganisms resistant to β-lactam antibiotics due to the presence of the β-lactamases.
Owner:MERCK SHARP & DOHME LLC
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
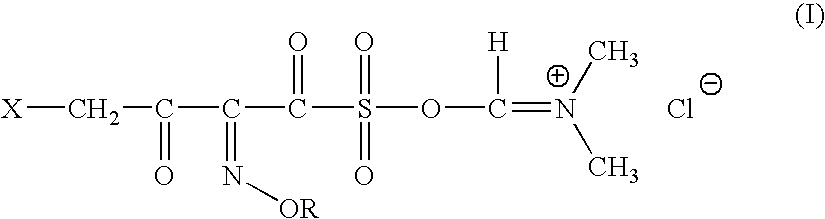
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
Synthetic methods of ceftazidime intermediate and ceftazidime
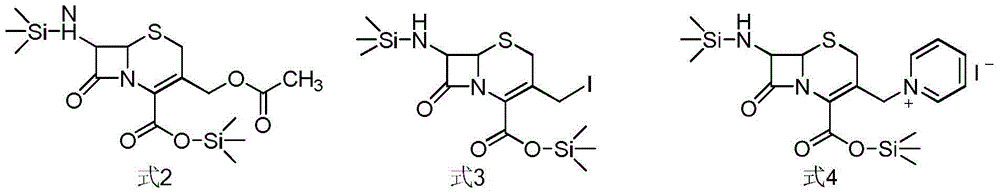
The invention relates to a synthetic method of ceftazidime intermediate; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the ceftazidimeintermediate (6R, 7R)-7-amino-3-pyridine methyl-ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing ceftazidime by using the obtained intermediate halogen acid salt. The ceftazidime intermediate and ceftazidime prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Ceftazidime pentahydrate purifying method
The method relates to a method for purifying ceftazidime, more concretely relating to a method for preparing high quality ceftazidime pentahydrate by impure, high-polymer impurity content ceftazidime through crystallization. It prepares impure, high-polymer impurity content ceftazidime pentahydrate, ceftazidime Hclú¼ceftazidime hydrobromide or ceftazidime overdue or recovered from the market, into a ceftazidime water solution, then regulates the pH value of the ceftazidime water solution to 1.5-2.5 by alkali or acid, here the impurity ceftazidime polymer is separated out with a little ceftazidime, and the separated-out matter is filtered, and the pH value of the filtrate is regulated to 3.5-4.8 by alkali so that the ceftazidime pentahydrate crystals are separated out. The method is simple and convenient to operate, low-cost, good-safety and high-yield. The obtained ceftazidime pentahydrate crystals are high purity and the polymer is low-content, reaching pharmacopoeia specified requirements.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD +1
High-purity ceftazidime powder injection and preparation method thereof
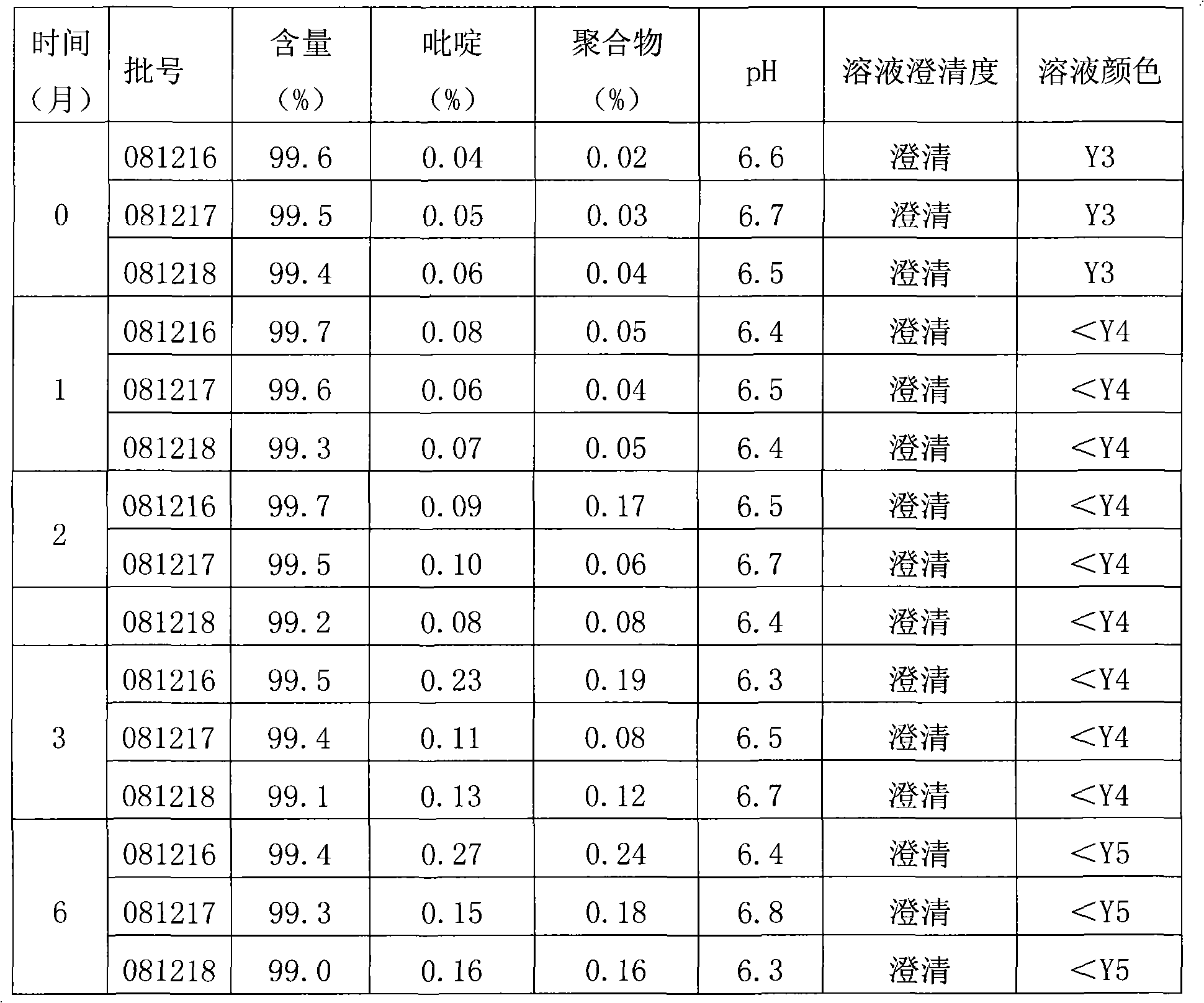
ActiveCN105560194AHigh antibacterial activityImprove stabilityAntibacterial agentsPowder deliveryCeftazidimeAntibacterial activity
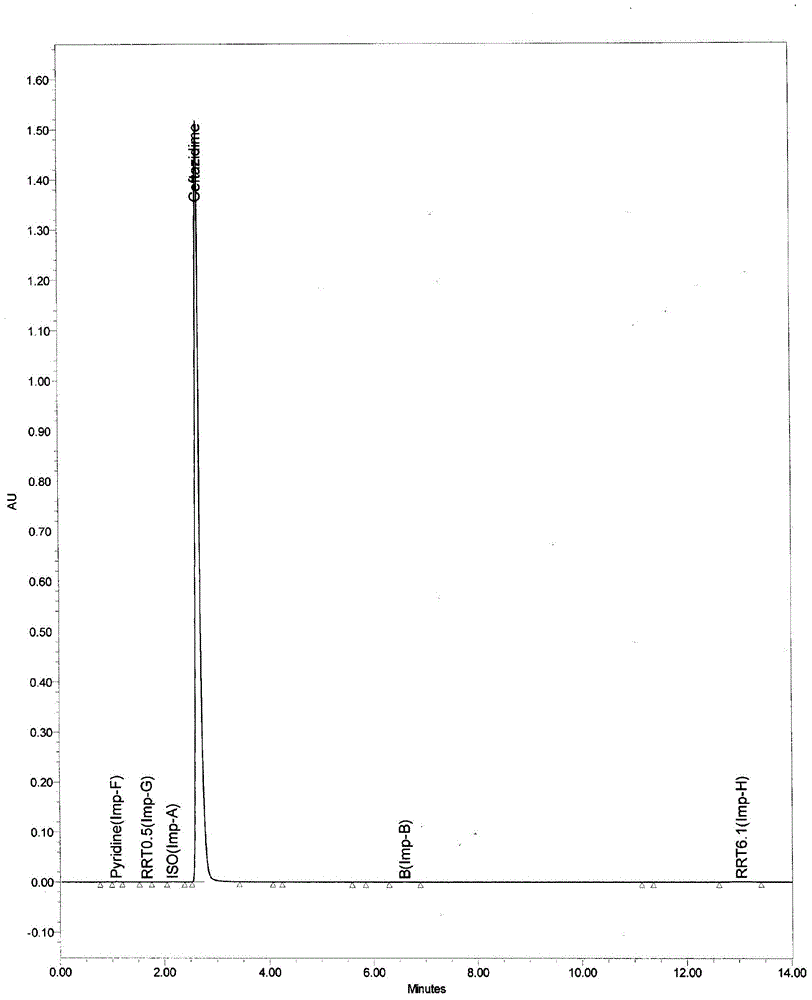
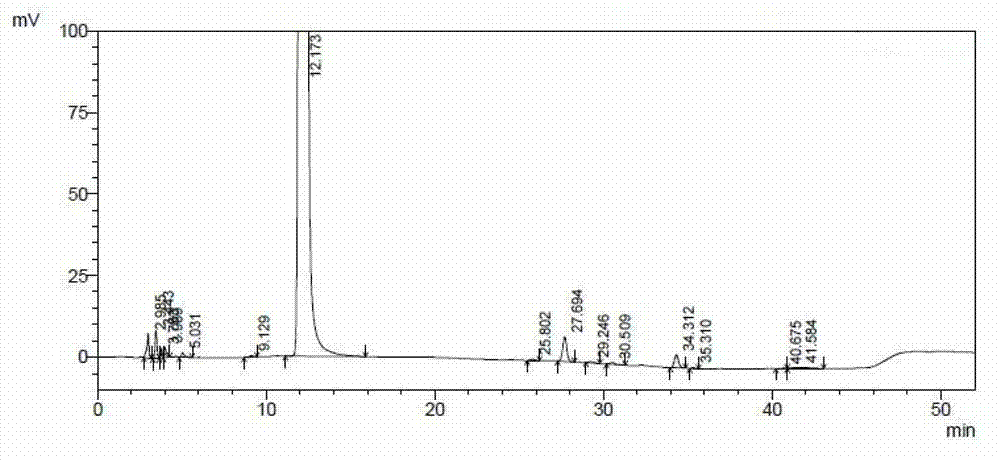
The invention belongs to the field of medicinal chemistry, and particularly relates to a high-purity ceftazidime powder injection and a preparation method thereof. The high-purity ceftazidime powder injection can have the dosage of 0.5g, 1g or 2g, and contains ceftazidime crude drug, wherein in the ceftazidime crude drug, the content of ceftazidime with the structural formula (I) is 99.70-99.95wt%, the content of impurity pyridine is 0.001-0.05wt%, the content of impurity ceftazidime polymer is 0.001-0.05wt%, the content of impurity ceftazidime methyl ester is lower than 0.05wt%, and the total content of other impurities is 0.03-0.30wt%. The high-purity ceftazidime powder injection has extremely high stability, high safety and high antibacterial activity, and the lifetime thereof is longer than that of existing ceftazidime powder injection by 50% or above. Furthermore, the preparation method of the high-purity ceftazidime powder injection, provided by the invention, is easy and convenient to operate, low in cost and high in safety.
Owner:GUANGZHOU HC PHARM CO LTD
Method for preparing ceftazidime pentahydrate
InactiveCN101607966ASimple and fast operationSuitable for industrial productionAntibacterial agentsOrganic chemistryWastewaterCeftazidime
The invention discloses a method for preparing ceftazidime pentahydrate, which comprises the following steps: 1, filling water for injection in a dissolving tank, adding a ceftazidime hydrochloride into the dissolving tank, putting active carbon into the dissolving tank for decolorization after the ceftazidime hydrochloride is dissolved and filtering the solution to obtain filtrate for later use; 2, filling water for injection in another dissolving tank, adding the ceftazidime hydrochloride into the dissolving tank, putting active carbon into the dissolving tank for decolorization after the ceftazidime hydrochloride is dissolved, filtering the solution and transferring filtrate into a crystallizing tank; 3, dripping alkaline solution into the crystallizing tank in the step 2 to adjust the pH value of the filtrate to 4.0 to 6.0, dripping the filtrate obtained by the step 1 into the crystallizing tank to adjust the pH value of the filtrate back to 3.6, keeping the temperature at 0 to 10 DEG C and stirring the mixed solution for crystallization; and 4, growing crystals for 3 to 4 hours, filtering the mixed solution, washing a filter cake with cold water and acetone respectively and drying the filter cake under vacuum for 2 to 3 hours to obtain the ceftazidime pentahydrate. The method has the advantages of saving capital, simplifying process, reducing discharged waste gas and water, along with simple and convenient operation, suitability for industrial production, high purity and high yield.
Owner:SHANGHAI NEW ASIA PHARMA
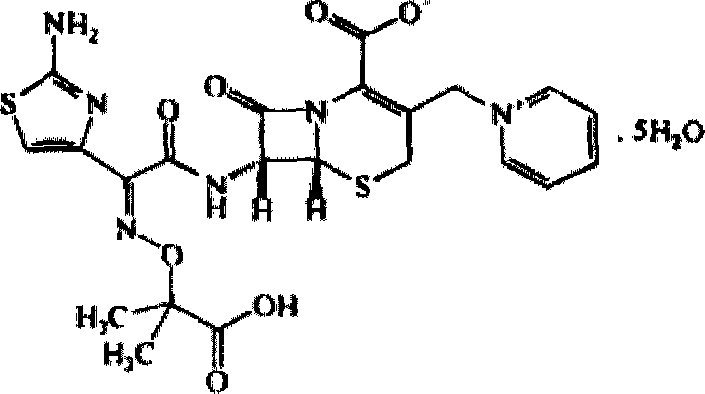
Ceftazidime compound and pharmaceutical composition thereof
ActiveCN103864819AHigh lattice energyEasy to packAntibacterial agentsOrganic active ingredientsCeftazidimeX-ray
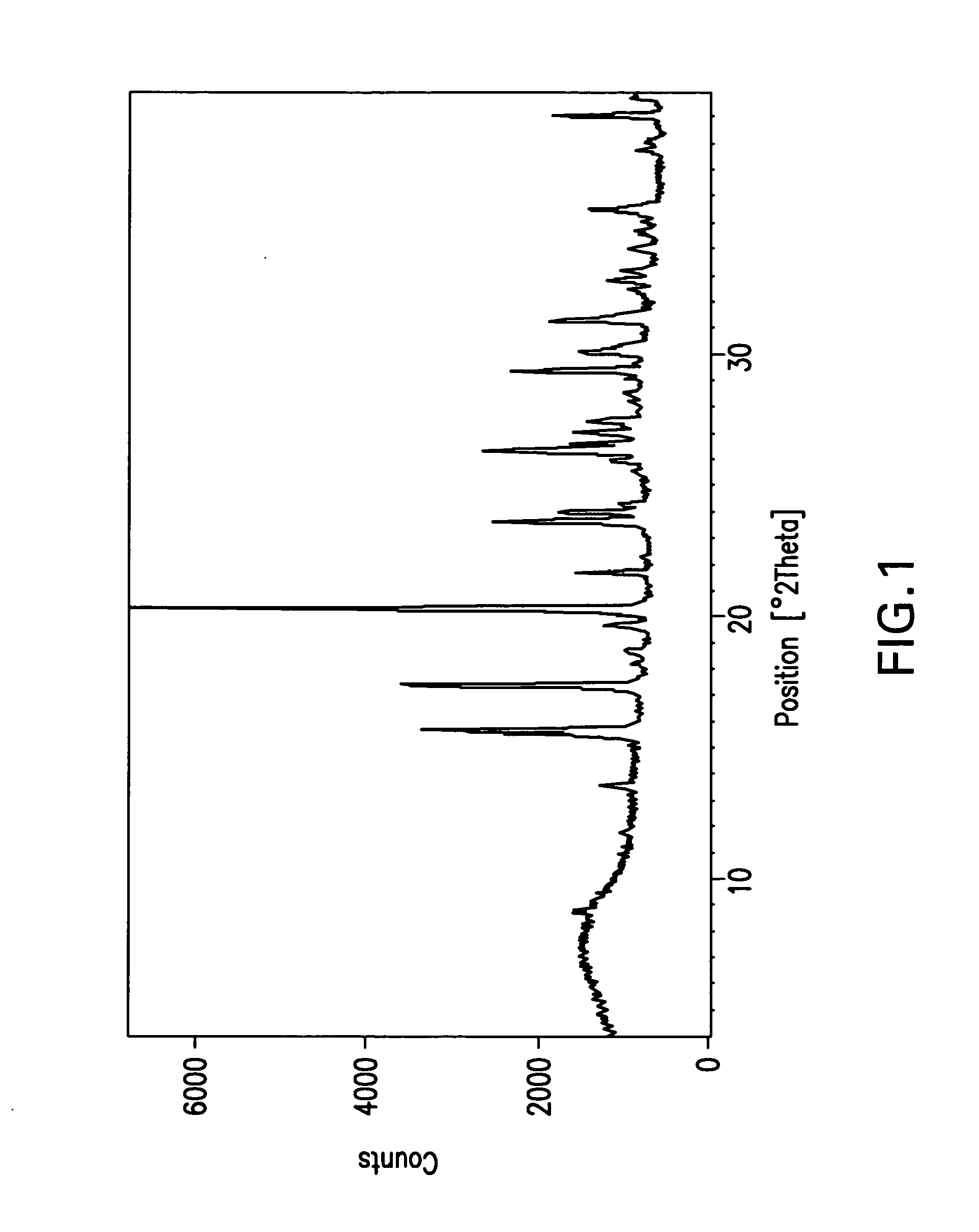
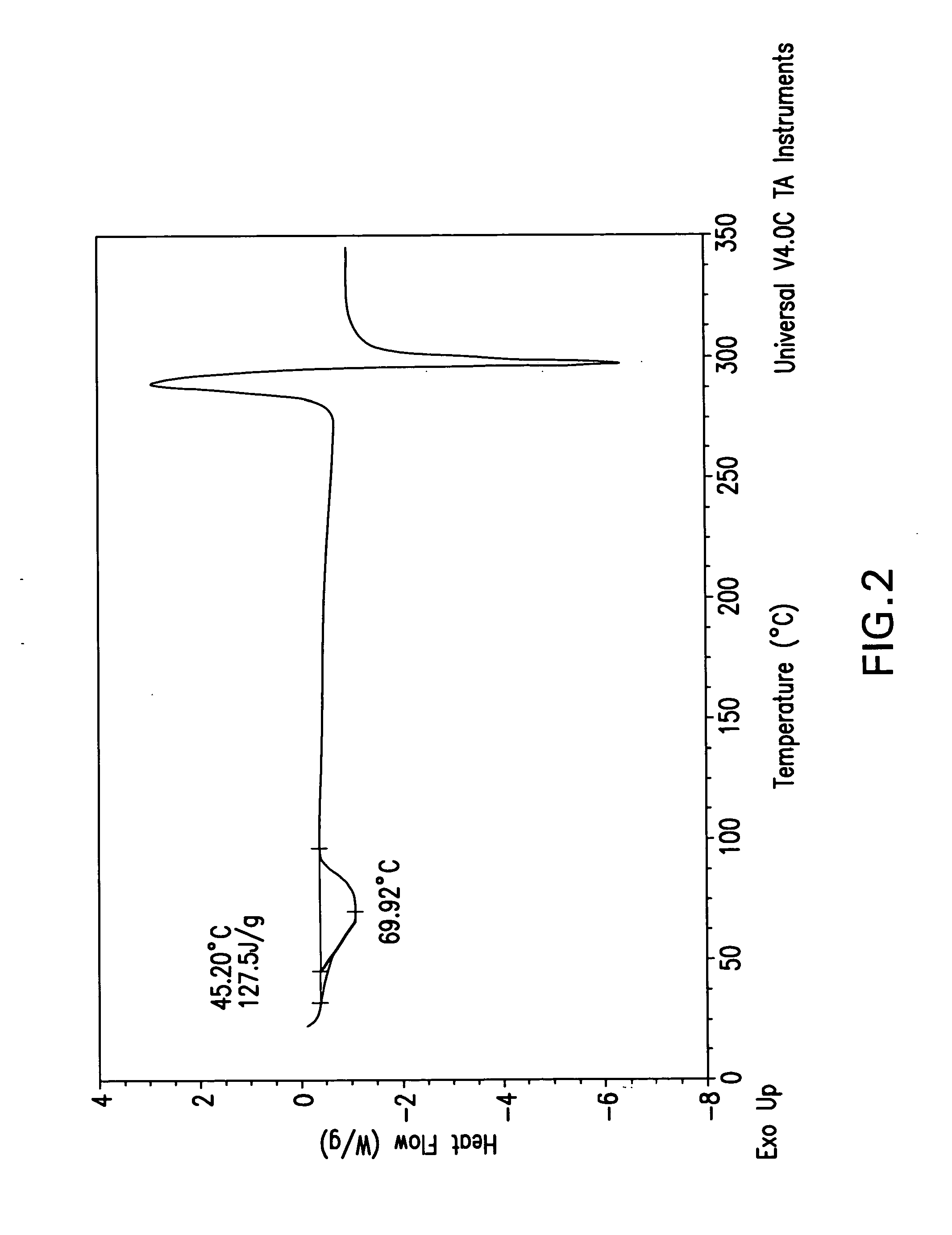
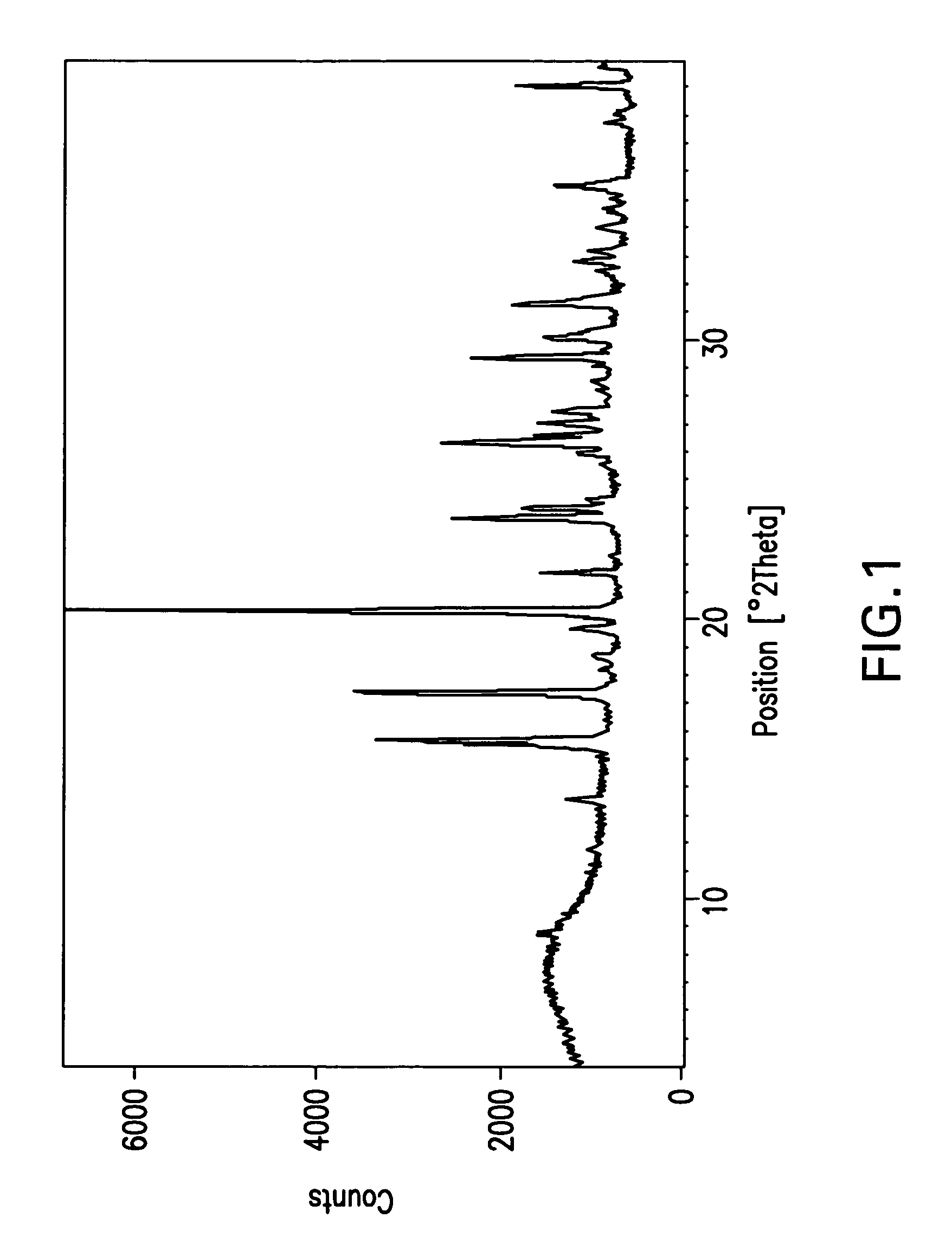
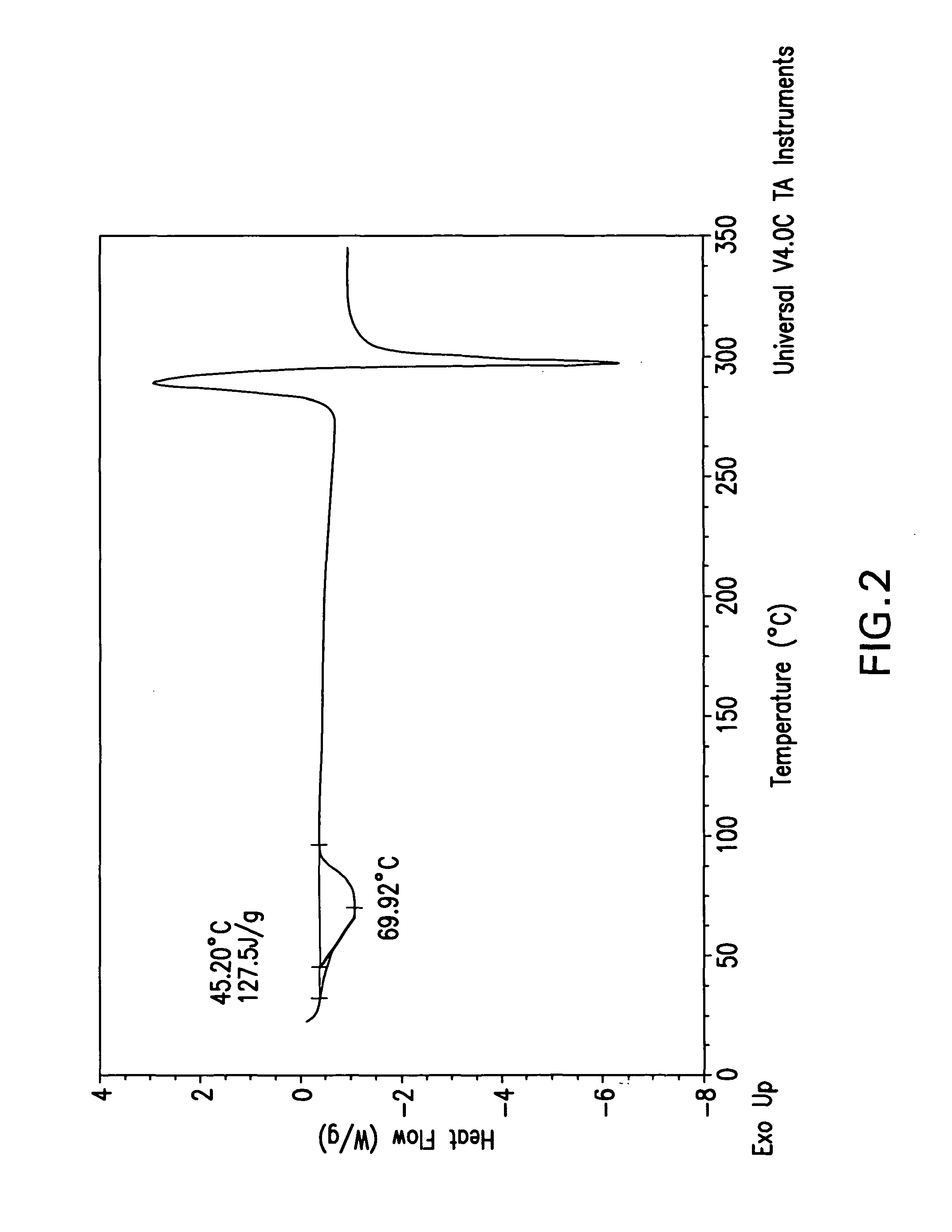
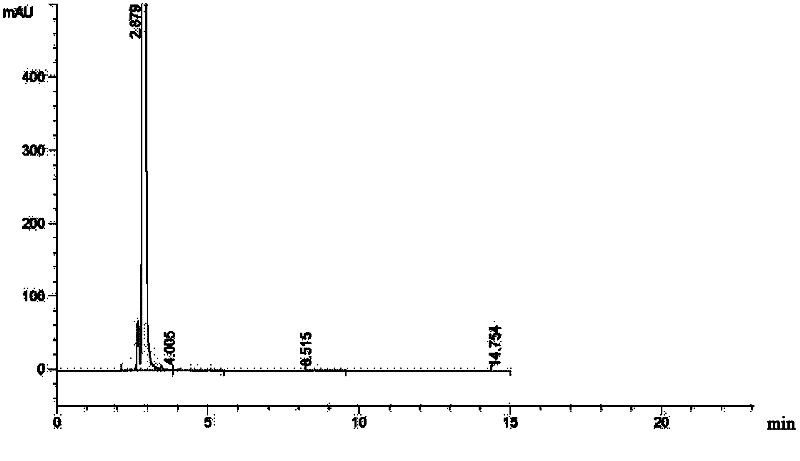
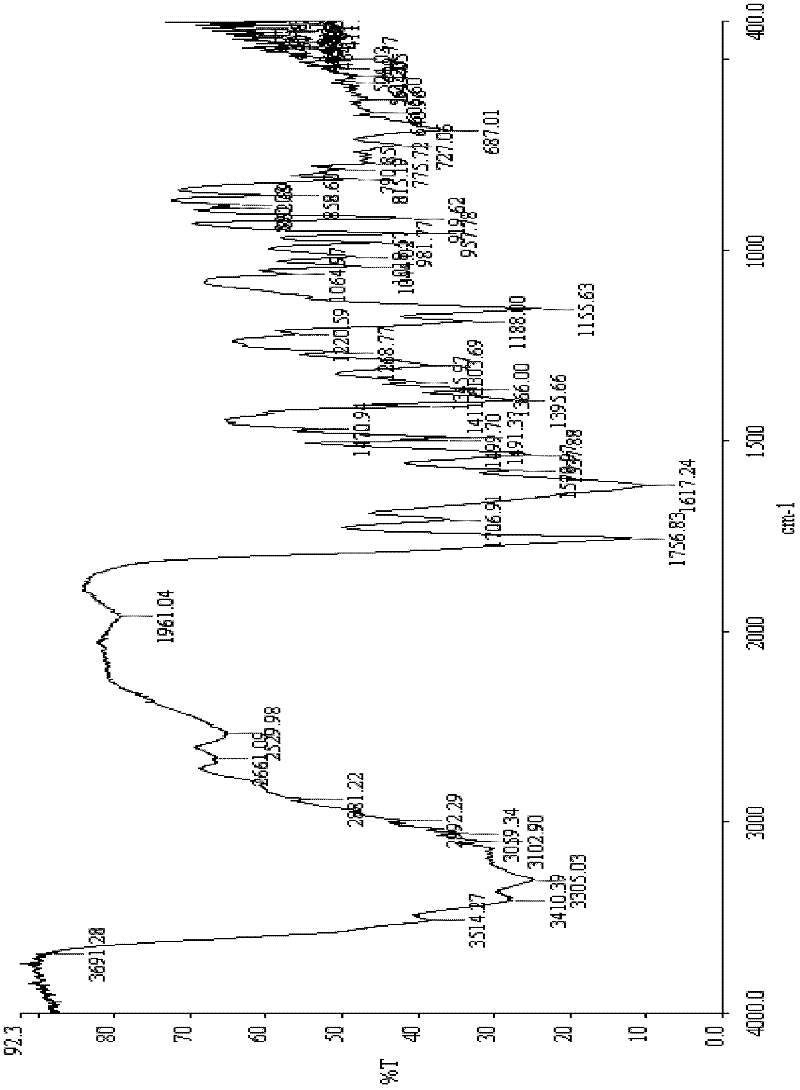
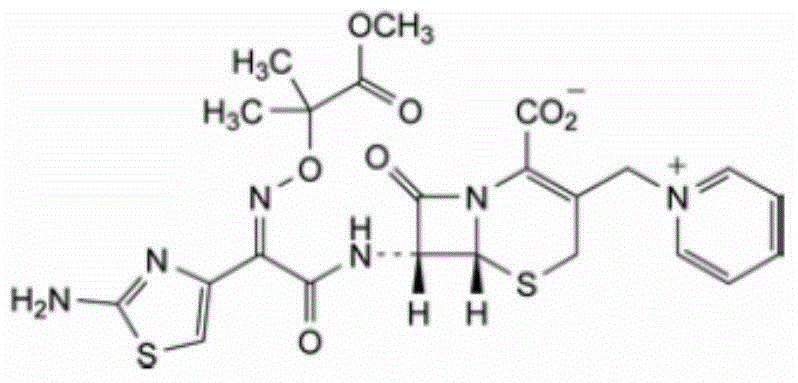
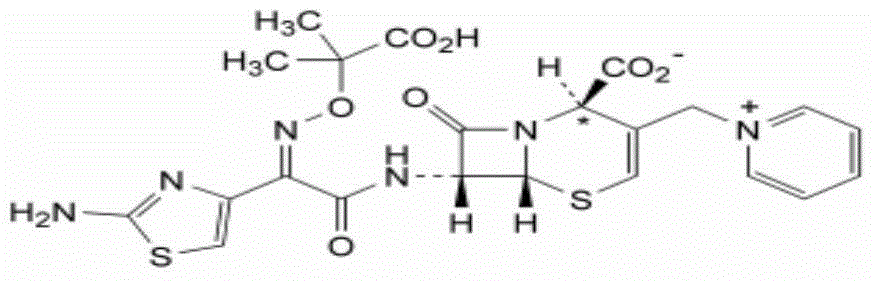
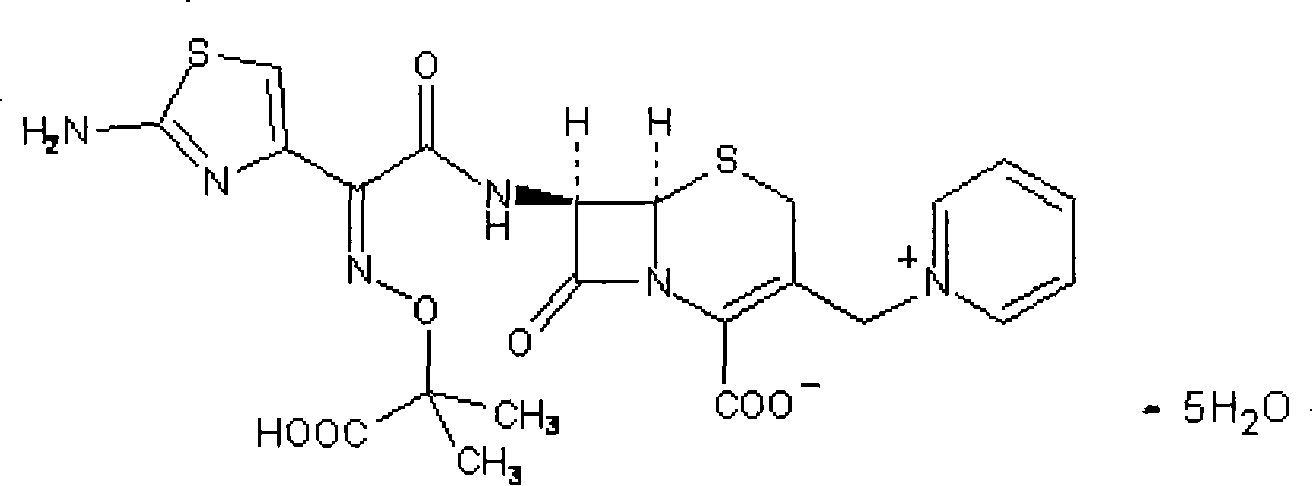
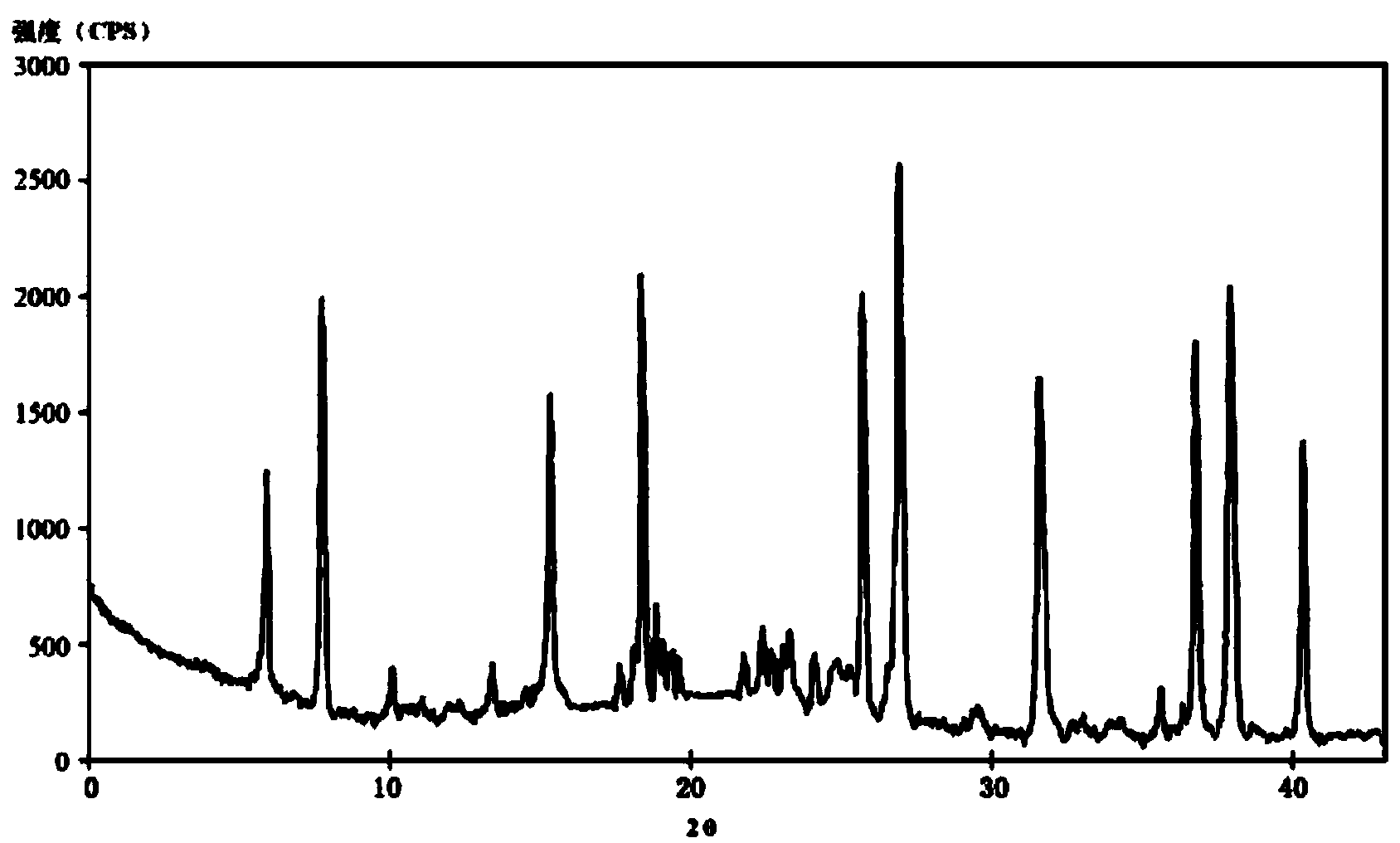
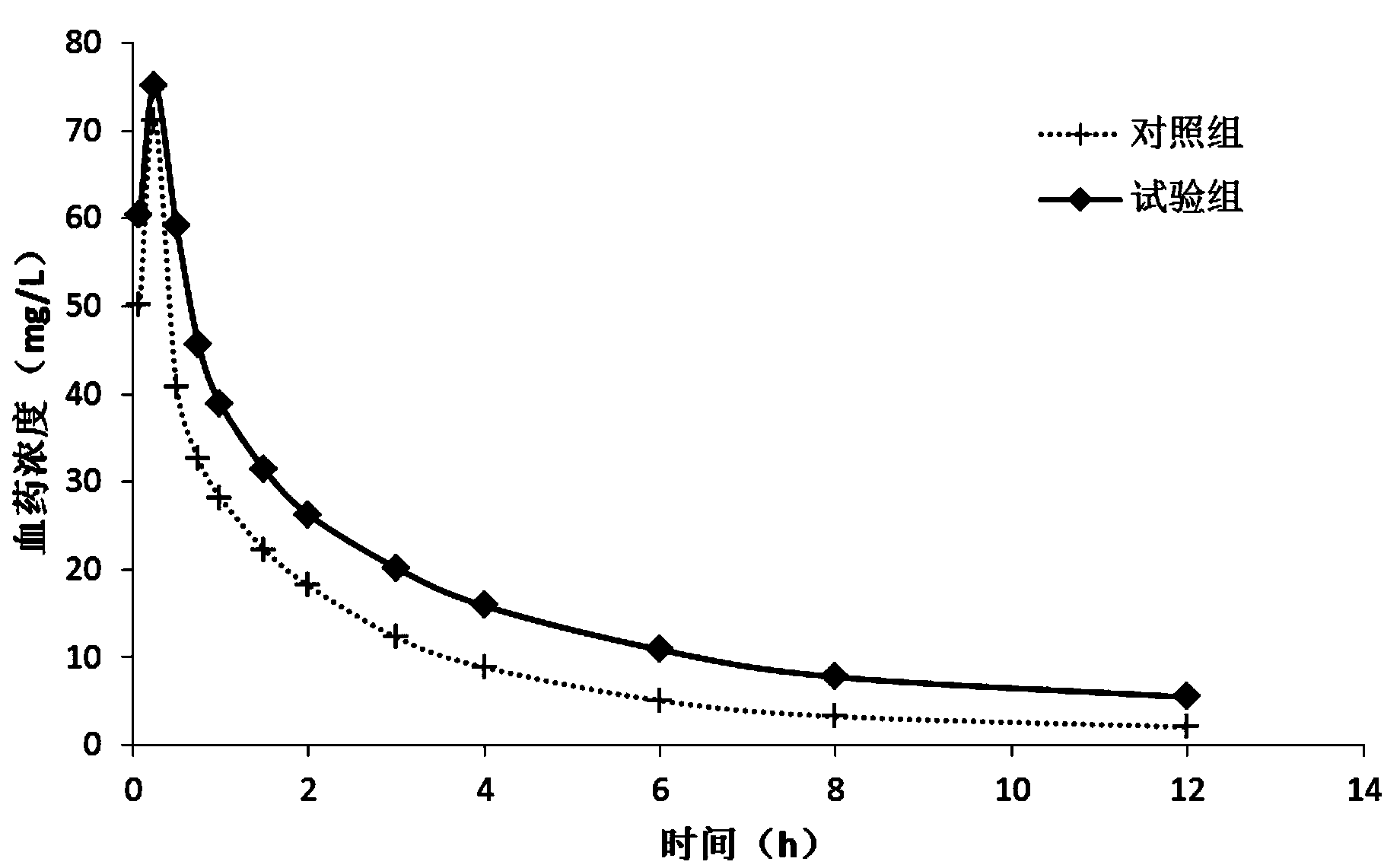
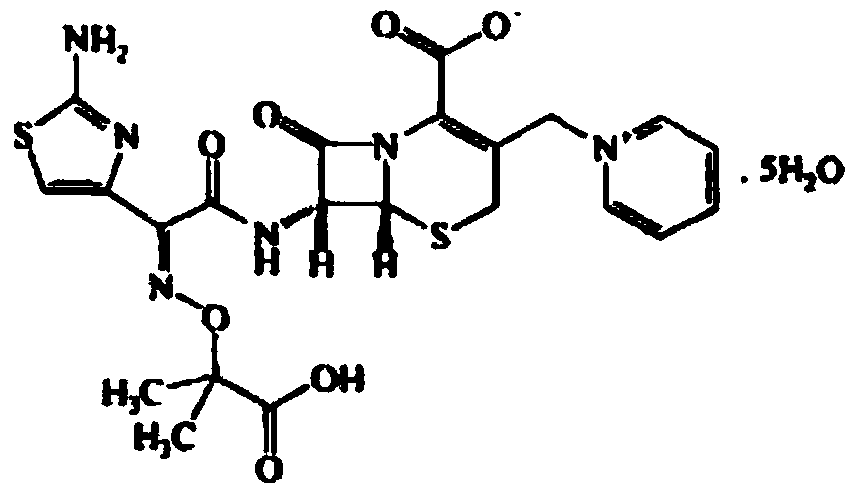
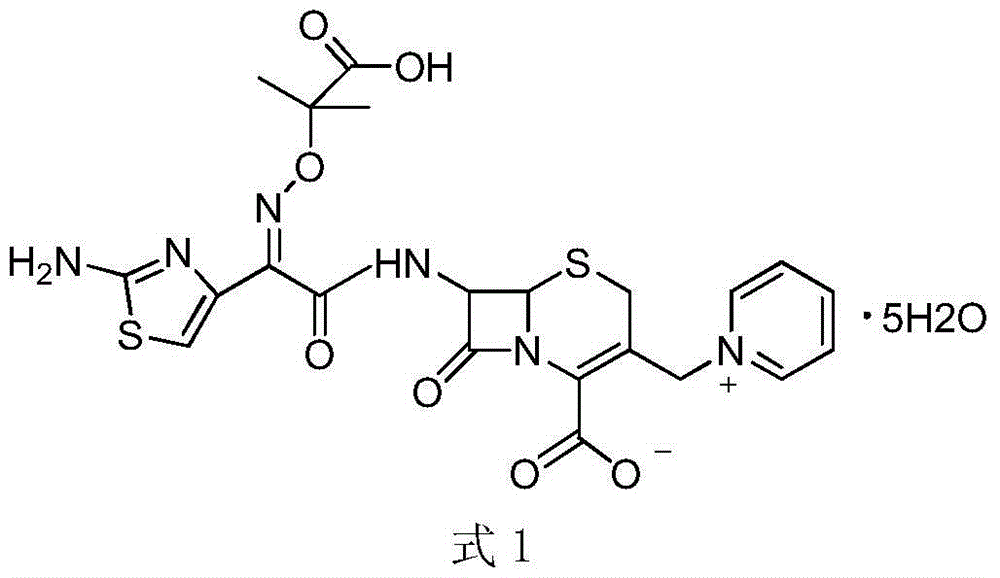
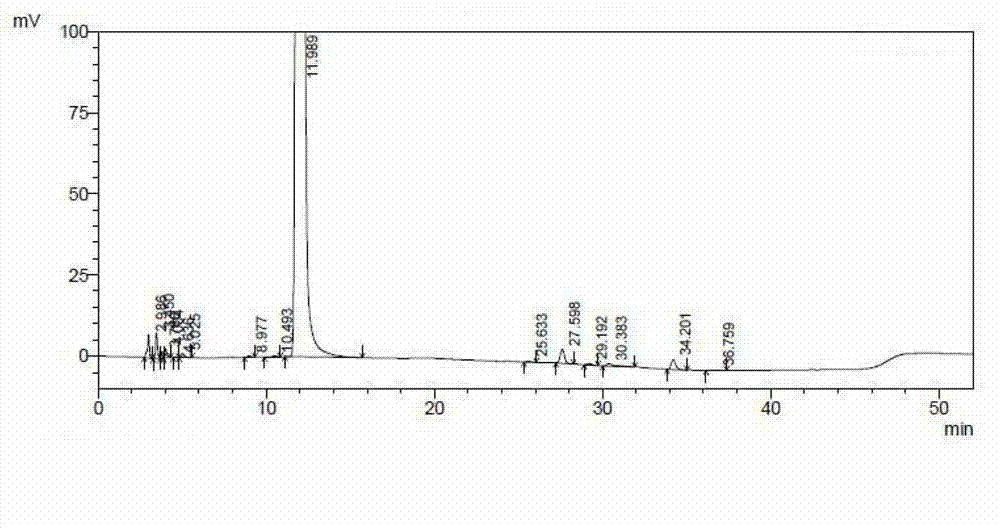
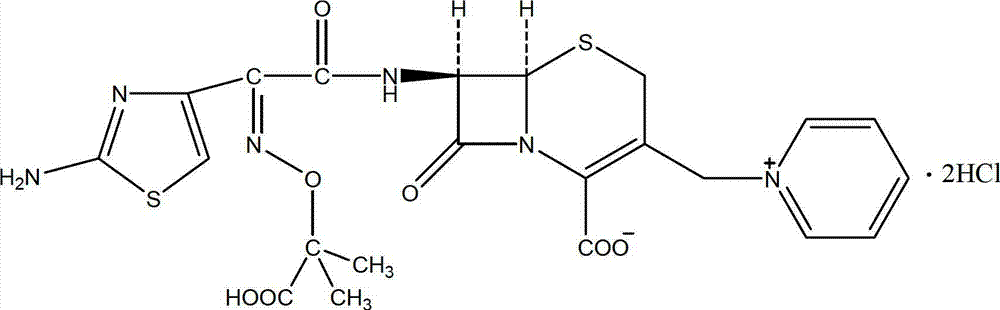
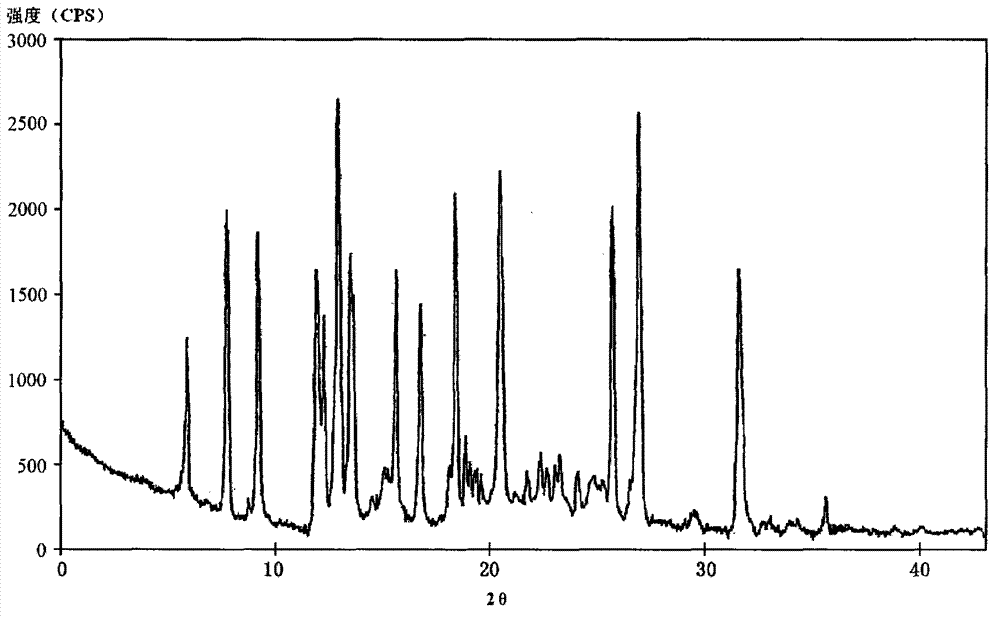
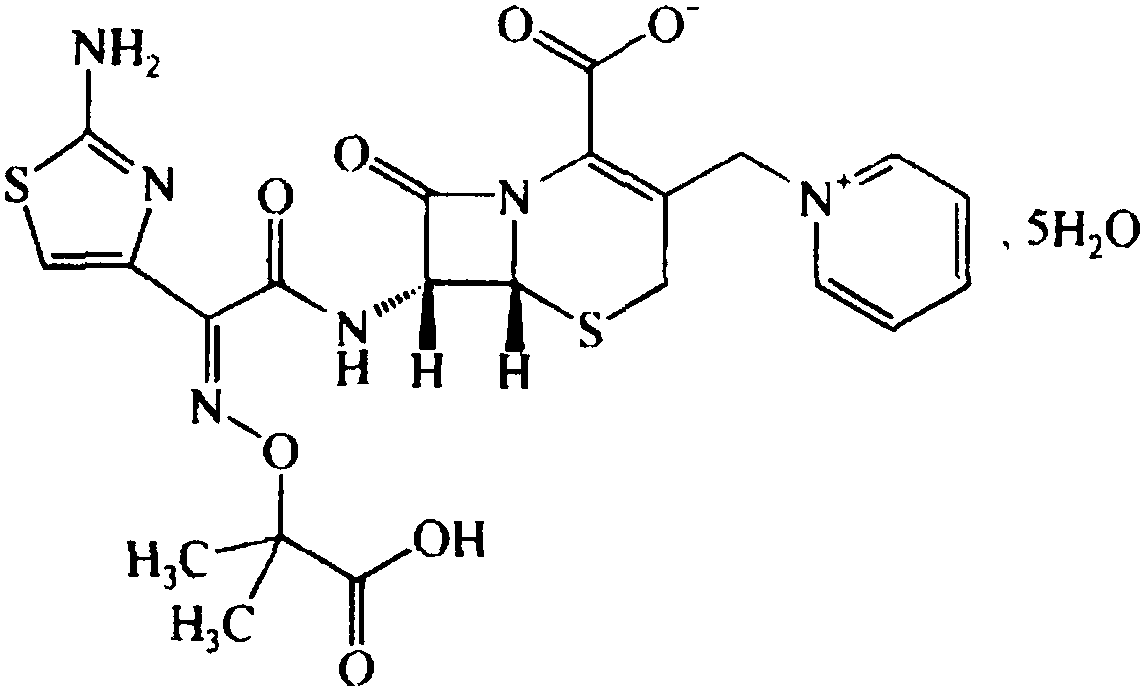
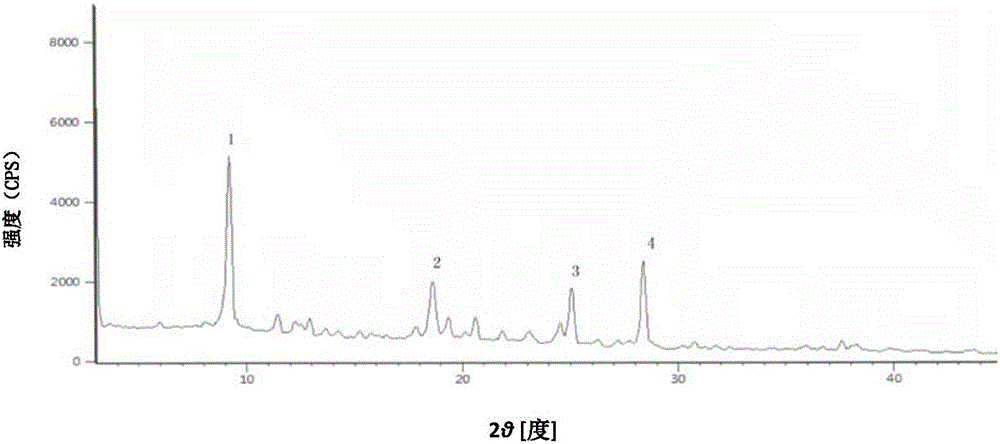
The invention relates to a ceftazidime compound and a pharmaceutical composition thereof. The structural formula of the ceftazidime compound is shown in a formula (I) described in the specification. The compound is determined by a powder X-ray diffraction determination method, an X-ray powder diffraction pattern represented by a diffraction angle of 2theta+ / -0.2 degrees is shown in the figure 1. The compound not only has good thermal stability, but also has excellent mobility. Pharmacokinetic study surprisingly finds out that the ceftazidime compound disclosed by the invention has good bioavailability.
Owner:YOUCARE PHARMA GROUP
A kind of synthetic method of ceftazidime
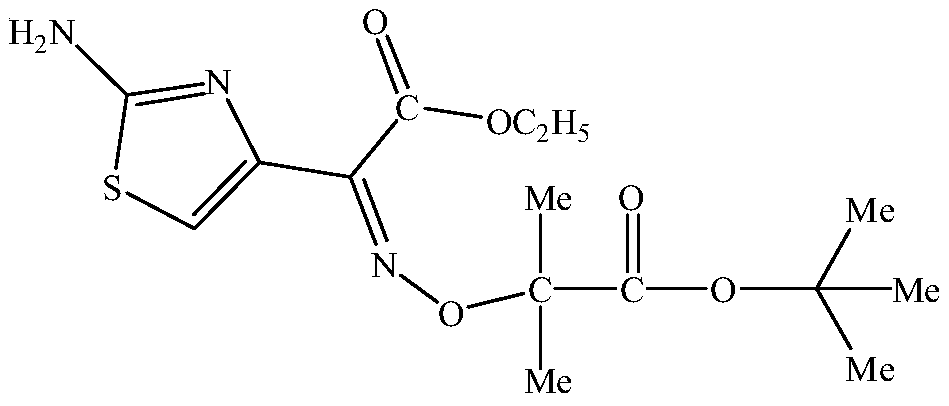
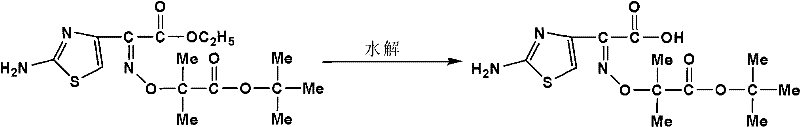
The invention relates to a method for preparing ceftazidime. The synthesis method comprises the following steps: by taking 7-aminocephalosporanic acid (7-ACA) as a starting raw material, introducing a pyridine ion to 3-position methylene of 7-ACA so as to obtain 7-amino-3-(1-picolyl)-cephem acid (7-APCA) hydrochloride; then introducing a side chain with a thiazole ring on 7-position amino of the 7-APCA hydrochloride through acylation reaction; and carrying out hydrolyzing reaction, refining reaction and the like so as to obtain ceftazidime. The synthesis method for preparing the ceftazidime inthe invention is simple to operate and high in yield, and is suitable for industrial production.
Owner:哈药集团股份有限公司 +1
Treating method of ceftazidime mother liquor
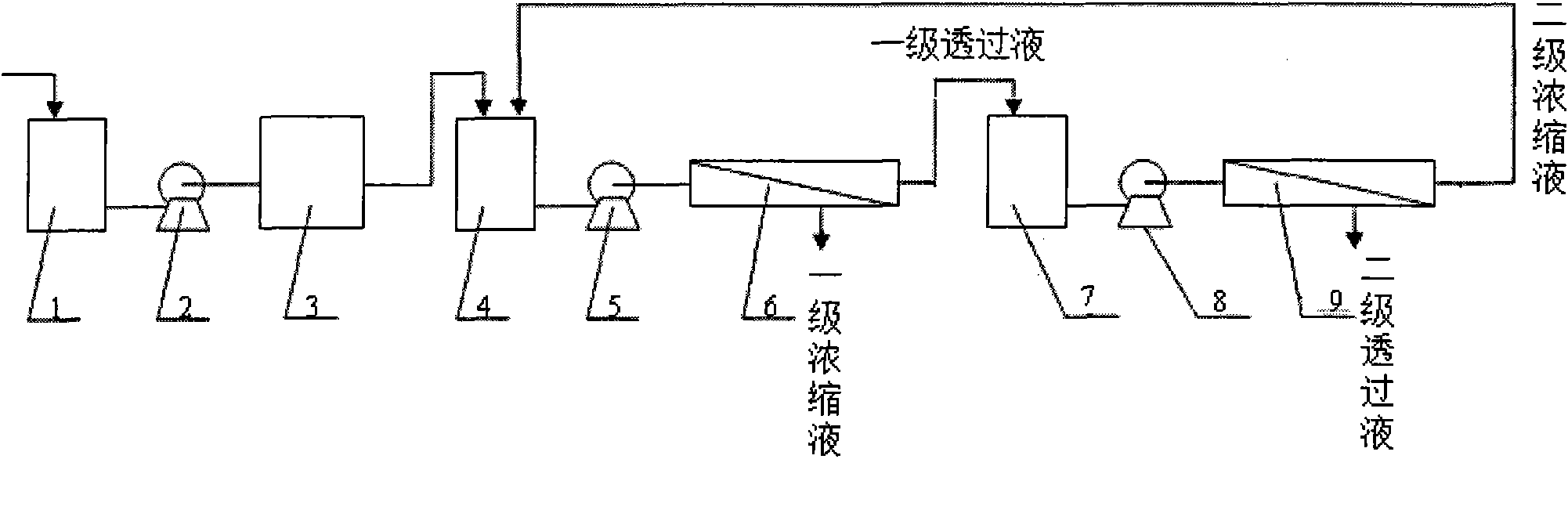
InactiveCN101774709AConcentrated recovery is goodEfficient ConcentrationWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentSocial benefitsSeparation technology
The invention discloses a treating method for concentrating and separating ceftazidime mother liquor by using a membrane separation technology. The treating method mainly comprises the following steps: (1) carrying out pretreatment on the ceftazidime mother liquor to be treated, and removing fine suspended matters in the mother liquor; and (2) under the condition that the operation pressure is 0.5 to 4.0MPa, and the temperature is 10 to 80 DEG C, carrying out concentration and separation to a nanofiltration component for the ceftazidime mother liquor after pretreatment, obtaining concentrated liquor with the mass fraction of ceftazidime of more than 10 percent, collecting and then carrying out crystallization treatment; and the obtained permeate is discharged into a sewage treatment station for treatment. The treating method has the advantages of: effectively concentrating the ceftazidime mother liquor, not only recovering effective ingredient in the mother liquor, namely the ceftazidime, ensuring the quality of the ceftazidime, but also reducing the indexes of COD and BOD and the like discharged by the mother liquor, reducing the pollution and energy consumption, and having obvious economical and social benefits. The treating method can be widely applied in occasions needing to concentrate and recover the ceftazidime.
Owner:UNIV OF JINAN
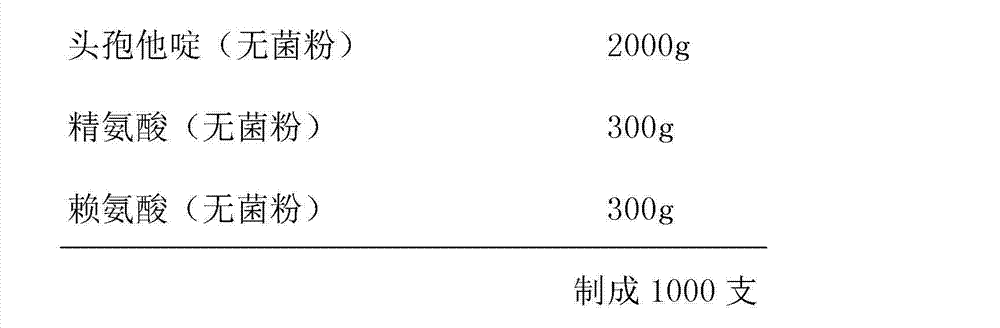
Ceftazidime medicinal composition for injection and preparation method thereof
ActiveCN101810623AImprove solubilityQuality improvementAntibacterial agentsOrganic active ingredientsSolubilityAntioxidant
The invention relates to a ceftazidime medicinal composition for injection and a preparation method thereof. The ceftazidime medicinal composition for injection comprises the following components in part by weight: 1 part of ceftazidime, 0.05 to 0.5 part of arginine and 0.0001 to 0.001 part of antioxidant. The ceftazidime medicinal composition for injection provided by the invention has the advantages of good dissolubility, good quality, stable color and luster and low polymer content, and the polymer content cannot be increased due to the increment of storage time, so that the medication safety is improved.
Owner:HEFEI YIFAN MEDICINE MARKETING
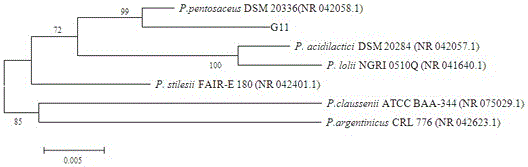
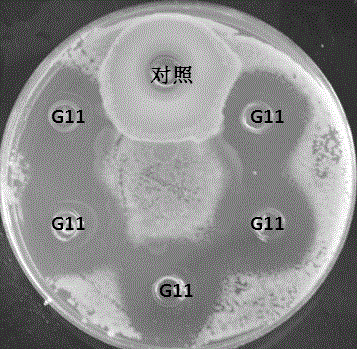
Pediococcus pentosaceus G11 strain as well as screening and applications thereof
ActiveCN106190894AToleratedHas antibacterial activityAntibacterial agentsBacteriaSynechococcusAnimal mortality
The invention relates to a pediococcus pentosaceus G11 strain as well as screening and applications of the pediococcus pentosaceus G11 strain. The pediococcus pentosaceus G11 strain is preserved in the China Center for Type Culture Collection, and is assigned with the accession number of CCTCC NO:2016248. The pediococcus pentosaceus G11 strain has tolerance to compound sulfamethoxazole and ceftazidime, has antibacterial activity for vibrio parahaemolyticus, aeromonas hydrophila, vibrio alginolyticus, staphylococcus aureus and beta streptococcus, has certain stress resistance and enzyme producing ability, is free of hemocyte solubility, and has no drug tolerance for most antibiotics; the dipping bath experiment proves that the pediococcus pentosaceus G11 strain is harmless to the host, can improve the immunity of the body, has wide application prospects, can be applied to probiotic preparations, and also can be prepared into bacterial powder which is then added into a feed as a feed additive, further, the growth of pathogenic bacteria in the bodies of the animals is inhibited, so that the death rate of animals is reduced, and the pediococcus pentosaceus G11 strain is a probiotic brain worthy of development.
Owner:SHANTOU UNIV
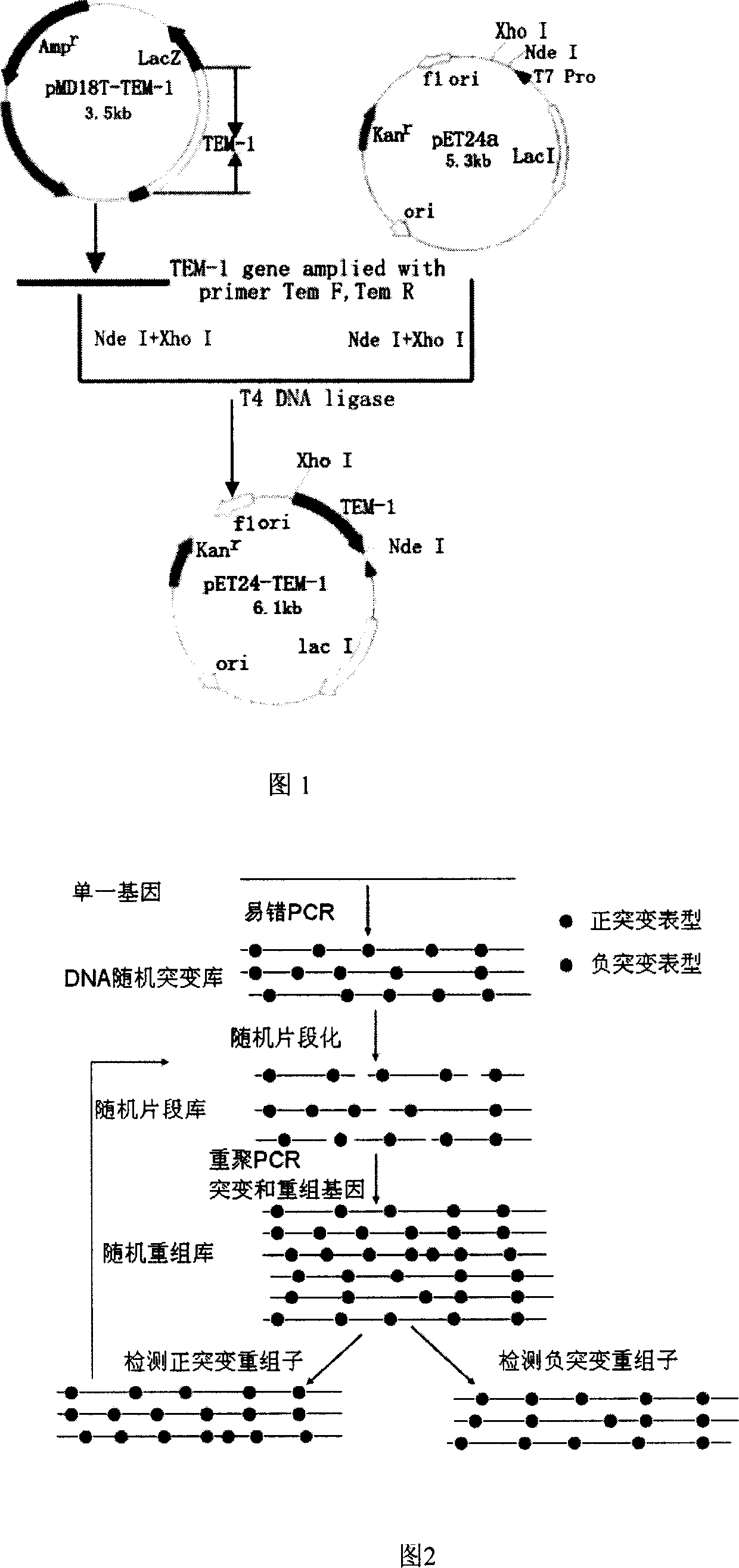
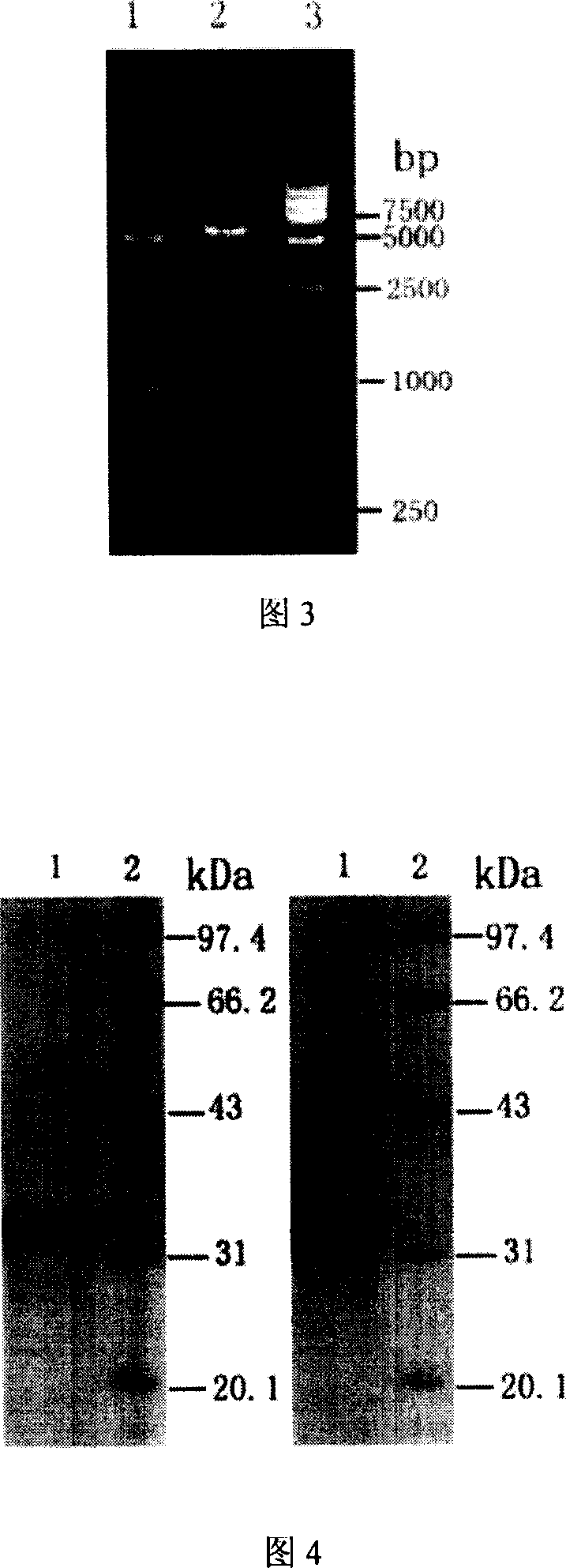
Method for obtaining high-vitality superspectrum beta-lactam enzyme by directional anagenesis in vitro
InactiveCN101104858ABroad substrate spectrumIncrease productionBacteriaHydrolasesBiotechnologyCefotaxime
The method to prepare high active extended spectrum beta-lactamase through directed evolution in vitro of the invention belongs to medical bioengineering technical field. In particular, the invention adopts two directed evolution strategies of error-prone PCR and DNA shuffling to transform a lab strain plant which contains TEM-1 gene. The substrate spectrum enzymes of the mutated strain plant and the wild strain plant, and the activity of the substrate spectrum enzymes are compared, showing that the original substrate spectrum is enlarged because of mutation and the degradability of the third representative generation of beta-lactam antibiotics ceftazidime (CAZ) and the cefotaxime (CTX) is improved from zero to a specific activity of 43IU / mg to 78IU / mg.
Owner:WENZHOU MEDICAL UNIV
Method for preparing ceftazidime by one-pot process
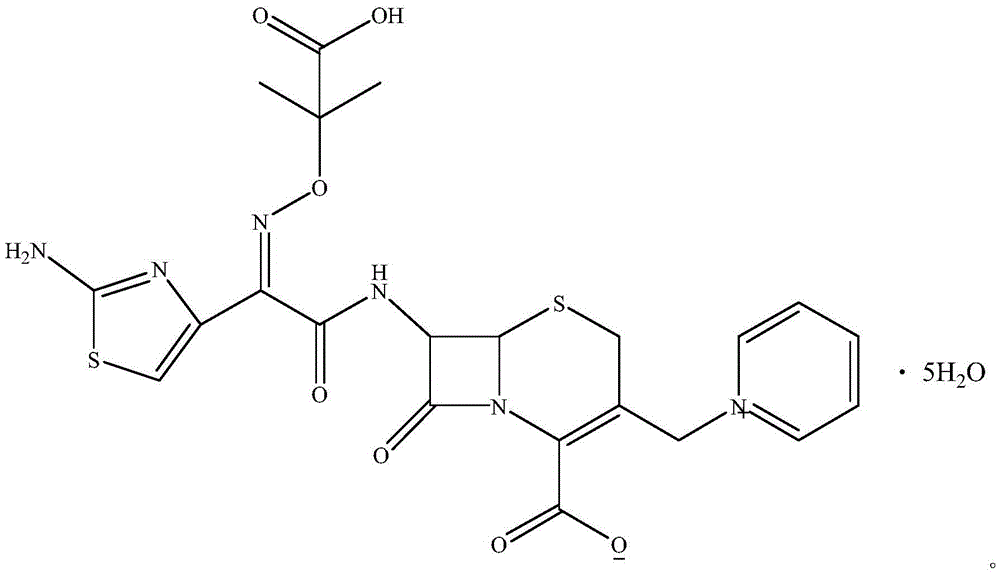
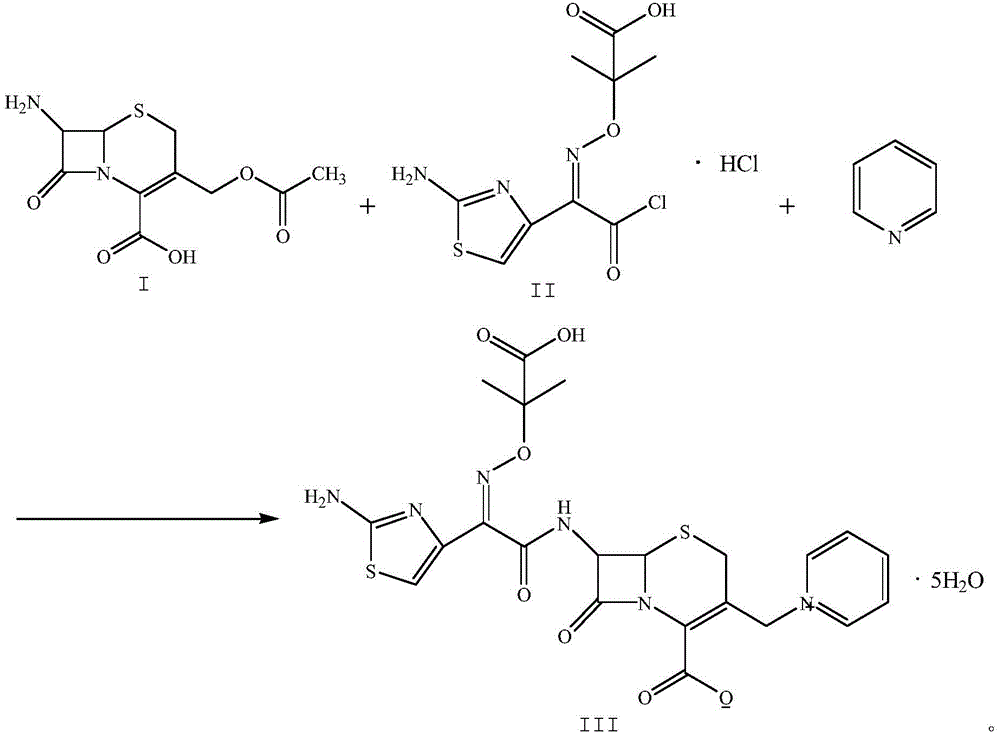
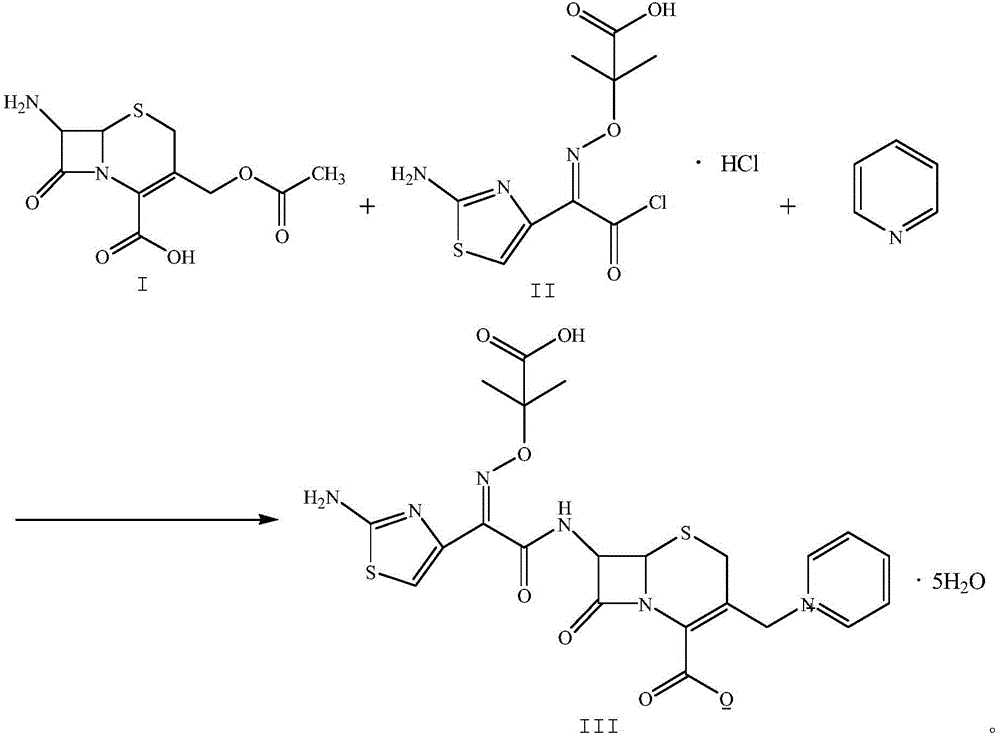
The invention relates to a method for preparing ceftazidime by a one-pot process. The method comprises the following steps: by using 7-aminocephalosporanic acid as the raw material, carrying out silanization reaction and iodination reaction, reacting with pyridine, directly adding the liquid into ceftazidime side chain acyl chloride hydrochloride to perform acylation reaction without separation to obtain ceftazidime iodate, adding the liquid into a concentrated hydrochloric acid-water mixed solution to perform deprotection, extracting to stratify, and regulating the pH value of the water phase with an alkaline solution to obtain ceftazidime (6R,7R)-7-[[(2-amino-4-thiazolyl)-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4,2,0]octyl-2-ene-3-methylpyridine pentahydrate. The method has the advantages of high yield, low cost, mild technological conditions, controllable technical process, high safety and low energy consumption, and is simple to operate.
Owner:QILU ANTIBIOTICS PHARMA
Method for preparing ceftazidime hydrochloride
The invention is suitable for the field of chemical pharmacy and provides a method for preparing ceftazidime hydrochloride. The method comprises a reaction of carrying out acylation to form ester and a hydrolysis reaction. The method for preparing the ceftazidime hydrochloride, which is disclosed by the invention, greatly simplifies the reaction process an reduces use of raw materials and energy by omitting steps of filtering, extracting, drying and the like after the esterification reaction, so that production cost is greatly reduced; and moreover, the method prevents loss of ceftazidime tert-butyl ester and improves production benefits.
Owner:SHENYANG SANJIU PHARMA
Breeding method for experimental macaque
ActiveCN104542475AImprove sexual functionImprove pregnancy rateAnimal feeding stuffAnimal housingCefotaximeCvd risk
The invention relates to the technical field of animal breeding, in particular to a breeding method for an experimental macaque. Main feed containing vitamin E is fed to the macaque in at least one of a suckling period, a growth period and an adult period; cefotaxime or ceftriaxone sodium or ceftazidime is added into the feed continuously for 5-7 days every 1-2 months in at least one of the growth period and the adult period; a young macaque is independently fed in the suckling period, feeding quality is further improved, the health condition of the macaque can be timely discovered and managed, the survival rate of the young macaque is improved, and the young macaque is normal in growth and development, flexible in limbs and physically strong. Moreover, the probability and the risk of bacterial dysentery infection of the macaque are reduced, and common health of the macaque and workers is ensured. A normalized experimental animal breeding standard is provided, and the breeding method is easy to popularize and use.
Owner:四川横竖生物科技股份有限公司
One-pot method for preparing ceftazidime
The invention discloses a one-pot method for preparing ceftazidime. The one-pot method includes the steps that 7-aminocephalosporanic acid, ceftazidime side chain acyl chloride hydrochloride and pyridine are weighed, placed in an ultrasonic wave reactor and ultrasonically oscillated for 2-3 h at the temperature of minus 18 DEG C-minus 15 DEG C and the power of 750-1000 W, the pH value is adjusted to 4.1-4.2, acetone is added into a reaction solution, and a white solid is separated, washed with cold water at the temperature of 0 DEG C-5 DEG C and dried to obtain ceftazidime. In the method, pyridine serves as an acid-binding agent and a catalyst for an acyl chloride and amidogen reaction, a reactant and reaction solvent, other reagents are not added any more, and the workload of impurity research in the later period of new drug research is greatly reduced. By means of environment-friendly ultrasonic oscillation, after the reaction is completed, the processing process is simple, the long crystal growing process is avoided, and meanwhile the possible influence on product quality by acetone residual amount due to acetone washing after cold water washing is avoided. The yield and the purity are high, and the method is suitable for industrial production.
Owner:上海博速医药科技有限公司
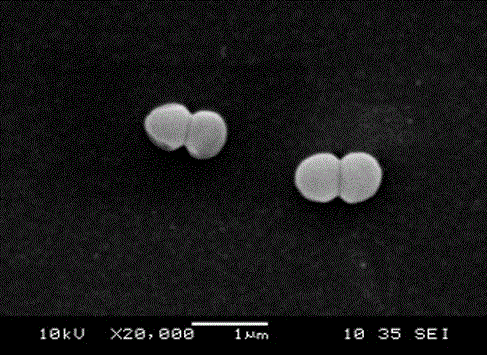
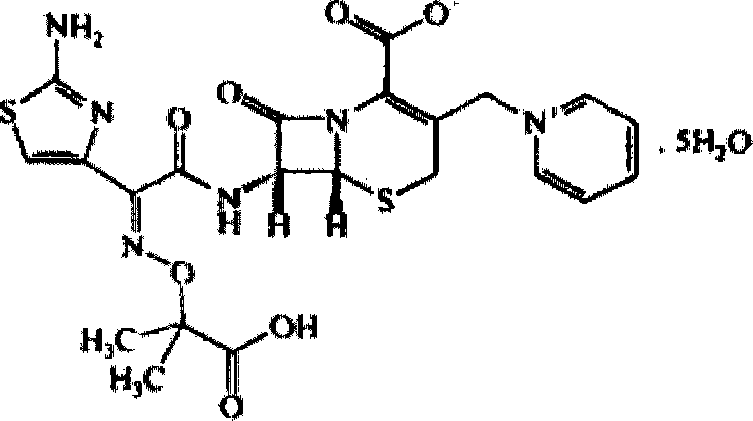
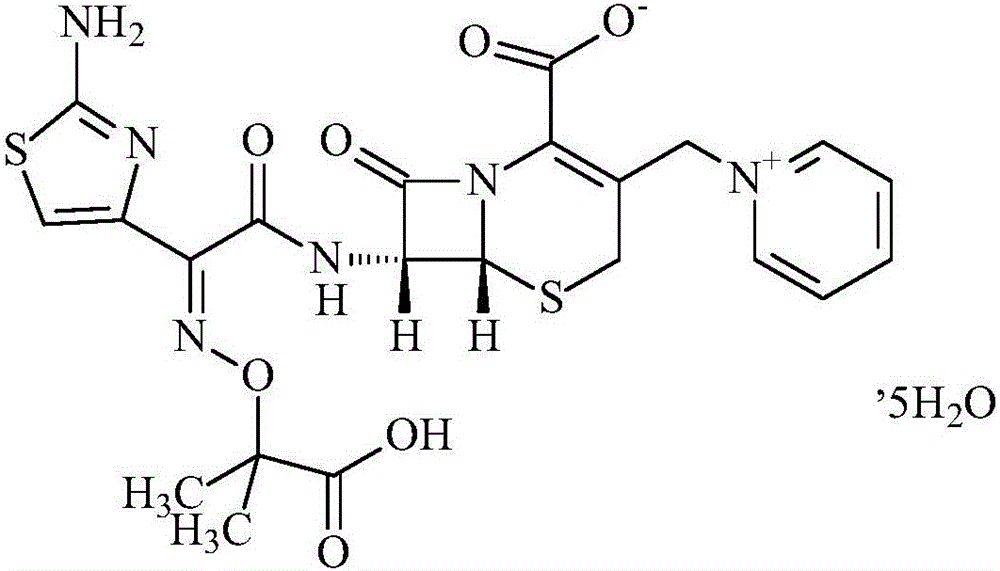
Ceftazidime crystal compound, preparation method of compound and pharmaceutical composition of compound in sterile mixed powder form
ActiveCN102924483BHigh purityImprove thermal stabilityAntibacterial agentsOrganic active ingredientsX-rayCeftazidime
Owner:HAINAN HERUI PHARMA
Preparation of modified magnetic zeolite imidazole framework material and adsorption of trace ceftazidime in water
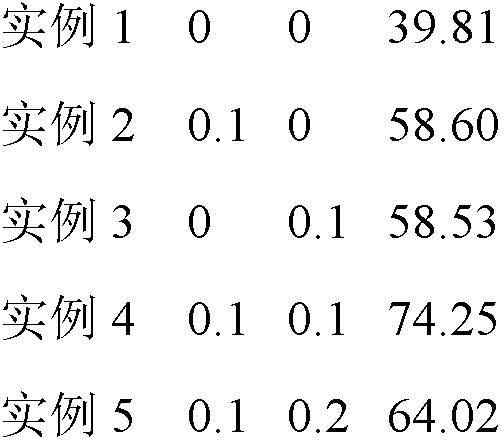
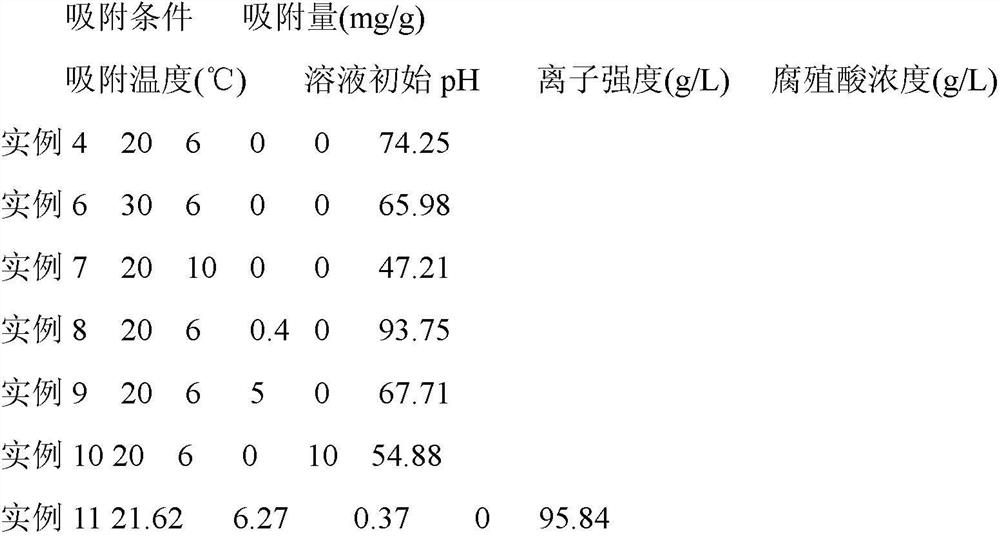
InactiveCN111889077AFlat surfaceUniform sizeOther chemical processesWater contaminantsAdsorption effectMethyl palmoxirate
The invention discloses a preparation method of a pore-channel-modified magnetic zeolite imidazole framework material adsorbent (ZIF-8@SiO2-Fe3O4). According to the method, zinc nitrate hexahydrate isused as a metal source, 2-methylimidazole is used as an organic ligand, methanol is used as a reaction solvent, cetyltrimethylammonium bromide (CTAB) and sodium laurate (SL) are used as template agents, and SiO2@Fe3O4 is used as a magnetic particle. After the template agents are added to adjust a pore diameter, the optimal adsorption pore diameter is 6.27 nm. As the influence of adsorption temperature, the initial pH value of a solution, ion strength and a humic acid concentration on the adsorption effect is taken into consideration, adsorption conditions after optimization are that the adsorption temperature is 294.62 K, the initial pH value is 6.27 and the ion strength is 0.37 g.L<-1>; and under the conditions, optimal adsorption effect is obtained, and the adsorption quantity of a ceftazidime solution reaches 96.84 mg.g<-1>. Repeated regeneration experiments also show that the ZIF-8@SiO2@Fe3O4 has good cyclic utilization, and the utilization rate of the ZIF-8@SiO2@Fe3O4 after fivecycles still reaches 90% or above. The adsorbent is prepared through a room-temperature stirring method, and the template agents are removed through calcination, so the method is simple; and the adsorbent is good in cyclic utilization and can serve as an adsorbent with excellent properties.
Owner:BEIJING UNIV OF CHEM TECH
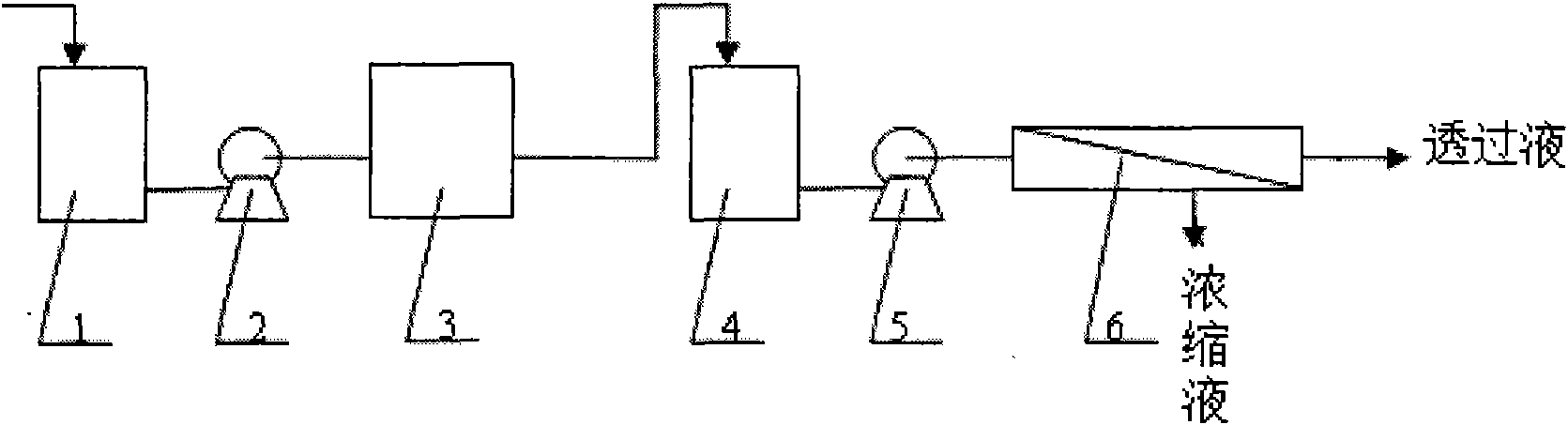
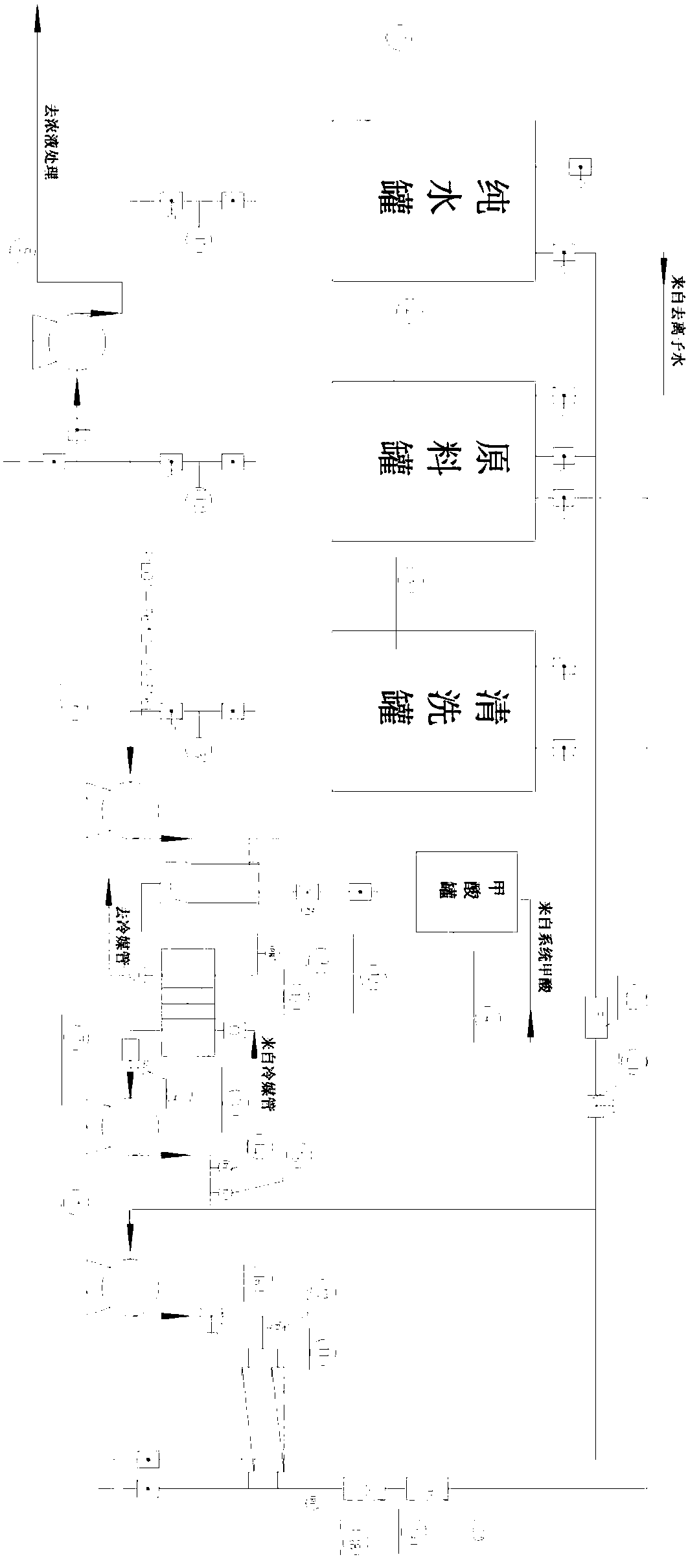
Membrane separation method and device for recycling ceftazidime mother liquor
InactiveCN107722040AStable qualityReduce labor costsOrganic chemistryReverse osmosisChemistryMother liquor
The invention belongs to the technical field of pharmacy, and relates to a membrane separation method and device for recycling ceftazidime mother liquor. Ceftazidime belongs to a third generation antibiotics and has wider application than other third generation cephalosporin antibiotics. In a present production process of ceftazidime, due to the salting-out effect of the mother liquor in the crystallization process, the yield of ceftazidime is only 82%, 18% of ceftazidime is still in the mother liquor, and therefore not only is the production cost increased, but also certain pollution to the environment is caused. According to the membrane separation method and device for recycling the ceftazidime mother liquor, by utilizing the membrane separation technology and the adjustment of the process, the products in the mother liquor can be recycled, the production cost can be saved greatly, and pollution to environment can be reduced; besides, the device is simple to operate so that industrial amplification can be achieved easily.
Owner:南京志坤环保科技有限公司
New method for detecting compound ceftazidime and sulbactam sodium
InactiveCN101650356AComponent separationTesting medicinal preparationsAdditive ingredientCeftazidime
The invention provides a new method for detecting compound ceftazidime and sulbactam sodium, which can detect the content of two single ingredients and relative impurities in the ceftazidime and sulbactam sodium compound at the same time. The method prevents mutual interference and influence of two single ingredients, is characterized by simple and easy operation, strong specificity, high sensitivity, large linear range and excellent stability, and can be used for detecting compound preparation and raw materials.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine
ActiveCN105816511AReduce dosageAddressing drug resistanceAntibacterial agentsPlant ingredientsAntibiotic sensitivityAntibiotic Y
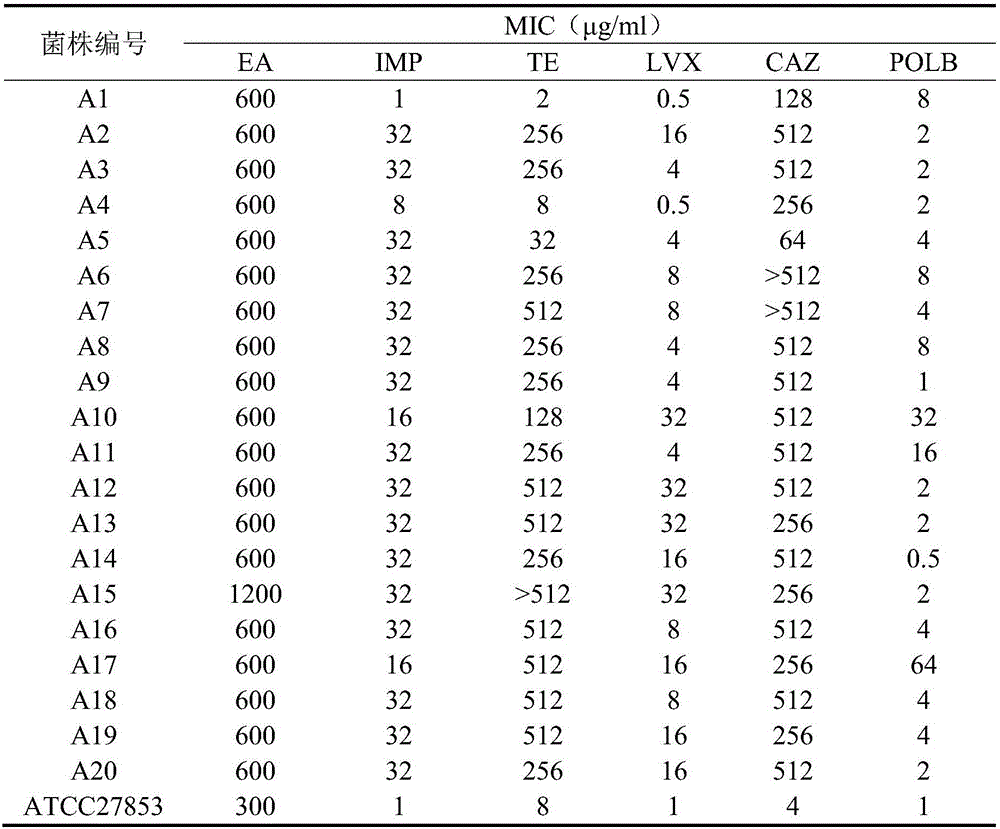
The invention discloses application of pithecellobium clypearia extracts to preparation of multi-drug resistant acinetobacter baumannii medicine. The pithecellobium clypearia extracts are prepared through the method includes the steps that pithecellobium clypearia coarse powder is extracted with water or an ethyl alcohol solution, the obtained extraction liquid is extracted with ethyl acetate, and the obtained extracts are target products. The antibacterial function of the pithecellobium clypearia extracts to multi-drug resistant acinetobacter baumannii and the sensitivity enhancing function of the pithecellobium clypearia extracts to similar antibiotics are disclosed for the first time. Tests prove that the pithecellobium clypearia extracts and imipenem or tetracycline or polymyxin B or ceftazidime or levofloxacin take effect together, and the use amount of antibiotics can be reduced by 50-87% compared with the mode that only the pithecellobium clypearia extracts are used. The pithecellobium clypearia extracts can serve as natural multi-drug resistant acinetobacter baumannii medicine or an antibiotic sensitivity-enhancing agent, and is applied to treatment of diseases caused by acinetobacter baumannii. A new means and alternative medicine are provided for solving the drug resistance problem of similar antibiotics.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
Original development quality ceftazidime and medicine preparation thereof
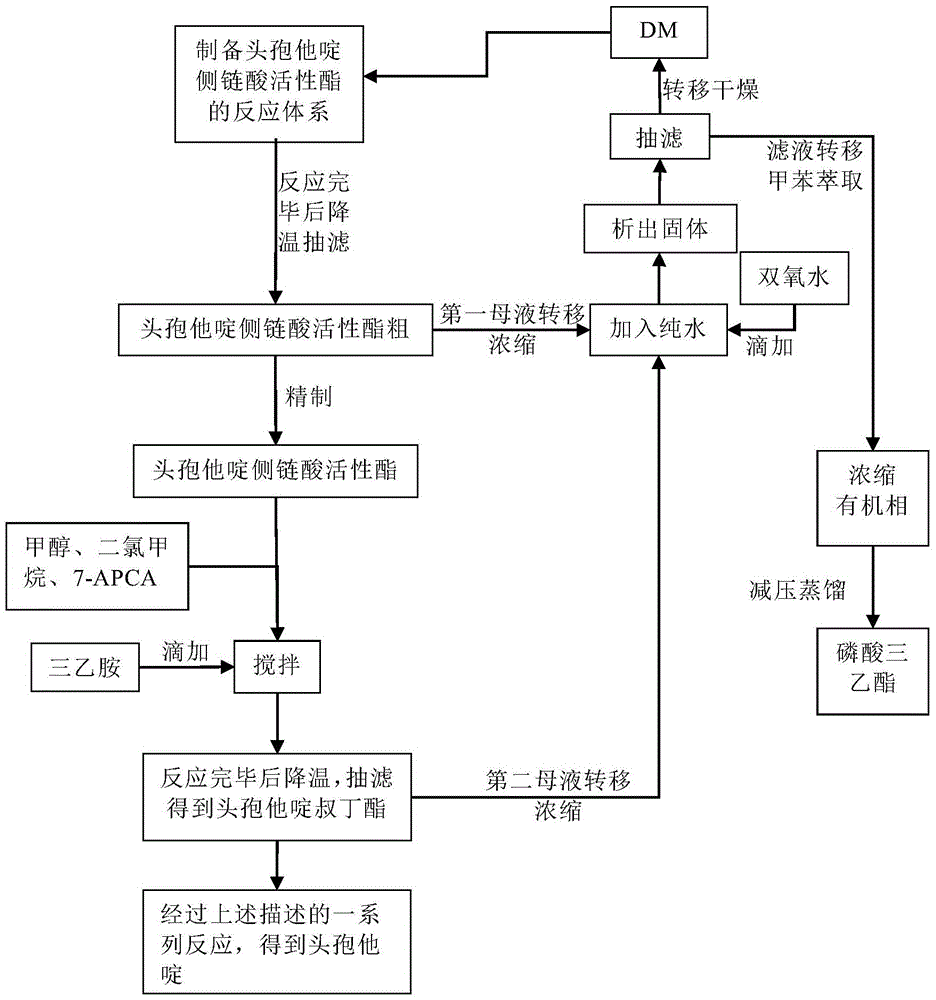
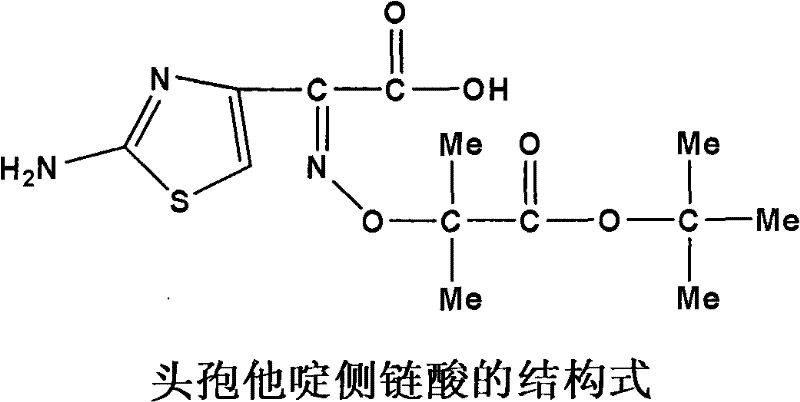
The invention discloses original development quality ceftazidime and a medicine preparation thereof. The third-generation cephalosporin antibiotics active ester midbody key technology and industrialization obtains the second prize of National Scientific and Technological Progress Award. The cephalosporin antibiotics active ester belongs to a key factor for influencing the internal quality of the cephalosporin. A preparation method comprises the following steps that (a) mixed solvents are added into ceftazidime side chain acid, dibenzothiazyl disulfide, aniline and 2-picoline; triethyl phosphate is dripped for reaction; (b) a coarse product is refined to obtain ceftazidime side chain acid active ester, and the first mother liquid is recovered; (c) the material is added into a mixed solvent for neutralizing 7-APCA; triethylamine is dripped; the temperature reduction is performed for crystal separation and filtering to obtain ceftazidime tert-butyl ester; the second mother liquid is recovered; (d) the ceftazidime tert-butyl ester is subjected to hydrolysis and purification, and then, the ceftazidime is obtained. The original development quality ceftazidime has the advantages that high-toxicity triphenylphosphine is not used; waste liquid and waste slag can be sufficiently recovered and reutilized; the method is safe; the cost is low; the yield is high; the industrial production is facilitated.
Owner:广东金城金素制药有限公司 +1
One-pot ceftazidime side-chain acid ethyl ester synthesis method
The invention relates to a preparation method of a pharmaceutical intermediate, in particular to a one-pot ceftazidime side-chain acid ethyl ester synthesis method. The method comprises the following steps that 1, ethyl 4-bromoacetoacetate is dissolved in water, a sodium nitrite water solution and 15%-25% of sulfuric acid are added dropwise, heat preservation reaction is performed after adding is completed dropwise, extraction is performed after the reaction is completed, the extracting solution is washed with a saturated potassium or sodium carbonate solution, and distillation is performed to obtain an oximation solution; 2, thiourea is added into a water and methanol mixed solution, and then the oximation solution is added dropwise to obtain an ethyl 2-(2-aminothiazole-4-yl)-2-hydroxyiminoacetate solution; 3, the pH of the ethyl 2-(2-aminothiazole-4-yl)-2-hydroxyiminoacetate solution is regulated, then tert-butyl alpha-bromoisobutyrate and a phase transfer catalyst are added for heat preservation reaction, and after the reaction is completed, cooling and suction filter are performed to obtain the ceftazidime side-chain acid ethyl ester. The simple processing of the product is simple, the purity and the yield are high, and the yield is up to 96.5% or above.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Compound prepn. contg. ceftazidime and tazobactam for injection use
InactiveCN1418632AEnhance clinical antibacterial effectImprove adverse reactionsAntibacterial agentsOrganic active ingredientsCeftazidimeResistant strain
Owner:海南瑞生堂制药有限公司
Ceftazidime composition for injection and preparation method for ceftazidime composition
InactiveCN103027894AImprove solubilityReduce dosageAntibacterial agentsPowder deliverySolubilitySide effect
The invention provides a ceftazidime composition for injection and a preparation method for the ceftazidime composition, and relates to the technical field of medicines and medicine preparation methods. The problems of side effects, low safety and low stability and dissolubility of the conventional ceftazidime composition for injection are solved. The composition comprises the following components in parts by weight: 1 part of ceftazidime, 0.10 to 0.30 part of arginine and 0.10 to 0.30 part of lysine. The arginine and the lysine are used as cosolvents, so that the using amount of the arginine is reduced, and the co-dissolving effect can be enhanced; furthermore, the low tolerance of liver and kidney patients to the medicines caused by excessive arginine is improved; anaphylactic reactions such as polypnea, pruritus, eczema, nausea, stomachache and blood chemical change are avoided; and the safety and the stability are effectively improved.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Synthetic process of ceftazidime intermediate
InactiveCN102452999AEasy to synthesizePost-processing is simpleOrganic chemistryDistillationSide chain
Owner:许婧
Ceftazidime compound prepared by adopting coupling crystallization technology and preparation thereof
InactiveCN106317080AHigh purityImprove color gradeOrganic active ingredientsOrganic chemistry methodsTechnology developmentCeftazidime
The invention discloses a ceftazidime compound and a preparation thereof, namely ceftazidime for injection. The ceftazidime compound is prepared by adopting a coupling crystallization technology. The 'high-end medicine product refining crystallization technology development and industrialization project' won the second award for national scientific and technological progress in 2015, and the coupling crystallization technology belongs to one of the high-end medicine product refining crystallization technologies. The ceftazidime compound has the advantages of being high in purity, good in color grade and good in stability.
Owner:海南顿斯医药科技有限公司 +1
Treatment method of ceftazidime mother liquid
The invention discloses a treatment method of ceftazidime mother liquid, and belongs to the technical field of medicine. The method comprises the step that ceftazidime mother liquid is subjected to freezing treatment at -1 to -21 DEG C. By the treatment method, ceftazidime concentrated mother liquid with high ceftazidime content is obtained; the COD discharged by the mother liquid is reduced; ceftazidime solid powder with high purity can also be obtained; the recovered ceftazidime concentrated mother liquid returns to an upstream process for use indiscriminately; the utilization rate of the ceftazidime mother liquid is improved. The treatment method also has the advantages that the quality of the recovered product is stable; the recovery method is simple; the recovery cost is low.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
New phloroglucinol compound and applications of new phloroglucinol compound in preparation of antibacterial drugs
ActiveCN106967085ANovel structureNovel skeleton structureAntibacterial agentsOrganic active ingredientsAmpicillinEnantiomer
The present invention discloses a new phloroglucinol compound and applications of the new phloroglucinol compound in preparation of antibacterial drugs, wherein the structure of the new phloroglucinol compound is represented by a formula I, the new phloroglucinol compound comprises two pairs of enantiomers, and the enantiomers are named (+) Myrtucyclitone A, (-) Myrtucyclitone A, (+) Myrtucyclitone B and (-) Myrtucyclitone B. According to the present invention, the compound has the novel structure, has significant antibacterial activity, can strongly resist Methicillin-resistant Staphylococcus aureus and Vancomycinintermediate-resistant staphylococcus aureus compared to ampicillin and ceftazidime, provides low cytotoxicity to normal cells so as to indicate the good medicinal prospect of the compound, and can be used for preparing antibacterial drugs. The formula I is defined in the specification.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com