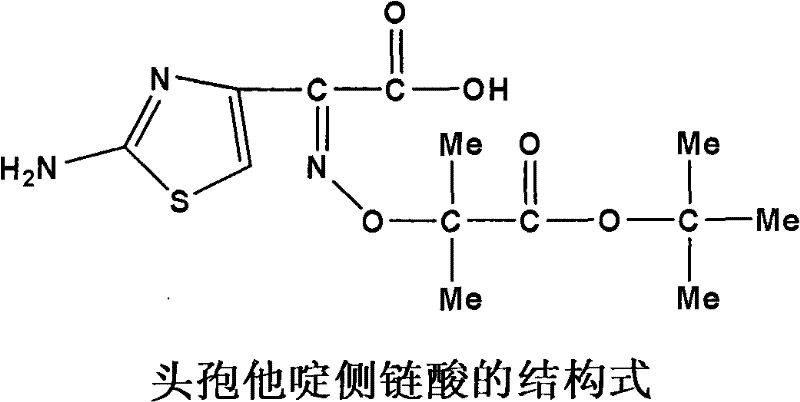
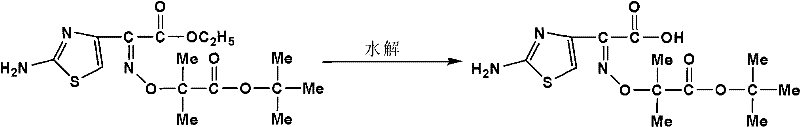
Synthetic process of ceftazidime intermediate
A technology of ceftazidime and synthesis process, applied in the field of synthesis technology of ceftazidime intermediates, can solve the problems of low yield, difficult post-processing, long synthesis steps and the like, and achieve the effects of simple synthesis and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
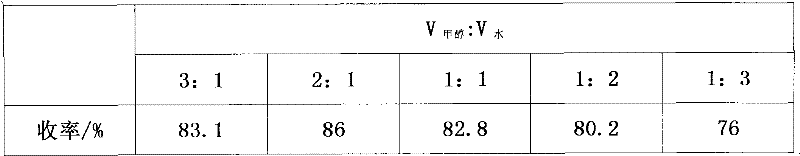
[0014] The synthesis steps of the present invention are: add 35-40 g of the compound ceftazime side-chain acid ethyl ester to a four-necked flask equipped with a condenser tube and mechanical stirring, the reaction solvent is a mixed solvent of methanol and water, and the volume ratio is V 甲醇 :V 水 =3:1~1:3, add NaOH, m 头孢他啶侧链酸乙酯 :m NaOH =1:1~1:2, stir and react at 15~60°C for 8h; track the reaction with TLC; after the end, add 2g of activated carbon and stir for 1h, filter out insoluble matter; cool the filtrate to 20°C, adjust until the pH is 7; distill under reduced pressure at 40-45°C to recover methanol, dissolve the residue in 75ml of water, adjust the pH to 3 with 1mol / L hydrochloric acid; cool down to 5°C, continue stirring for 30min, filter with suction, and water 50mL in turn 1. Wash the filter cake with 50 ml of acetonitrile, and dry in vacuo to obtain a pale yellow solid.
[0015] The present invention selects solvent composition, alkali consumption, reaction tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com