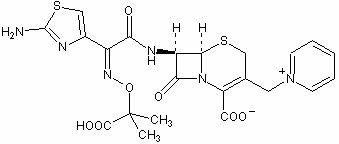
A kind of synthetic method of ceftazidime
A technology of ceftazidime and a synthesis method, applied in the field of drug synthesis, can solve the problems of unstable ceftazidime crystal quality, by-product pollution, long reaction steps and the like, and achieve the effects of being beneficial to production control, easy to handle and low in cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] [Example 1] Synthesis of 7-APCA
[0055] Add 1.0 g of 7-ACA (7-aminocephalosporanic acid) to 5.0 g of dichloromethane, then add 1.0 g of hexamethyldisilazane, stir and heat under reflux for about 5 hours at 35-45°C. Under the catalysis of xylidine, 0.05 g of trimethylchlorosilane and 1.0 g of trimethyl iodosilane were added, the reaction was continued for 5 hours, 1 g of pyridine was added, and the reaction progress was detected by high performance liquid phase. When the 7-ACA residue is measured to be ≤0.5%, the reaction is over, add methanol and dilute hydrochloric acid, separate layers, add activated carbon to the water phase for decolorization for 20 minutes, and filter. Then add 0.5 to 2.0 grams of isopropanol to the filtrate as a dispersant, adjust the pH to 1.0 to 1.2 with triethylamine, stir until a large amount of crystals are precipitated, and continue to add dropwise until the final pH reaches 2.8 to 3.0. After suction filtration and washing with 0.5 g of is...
Embodiment 2
[0056] [embodiment 2] the synthesis of ceftazidime tert-butyl ester
[0057] Add 1.0 g of 7-APCA and 1.8 g of α-(2-aminothiazol-4-yl)-α-[(tert-butoxycarbonyl)isopropoxyimino]acetate to 5 g dichloromethane and 0.7 g methanol as a solvent, stirring and cooling down to 4°C. After maintaining the temperature for 5 minutes, 0.7 g of triethylamine was added, and the temperature was controlled at 0-10°C. After 10 hours, the 7-APCA residue was detected, and the reaction was considered to be over when the 7-APCA residue ≤ 2 mg / ml was monitored by high performance liquid chromatography. Lower the temperature to 0-3°C and grow the crystal for 3 hours. Filter, wash the filter cake with dichloromethane, filter dry and vacuum dry for 4 hours to obtain 1.482 g of ceftazidime tert-butyl ester. The mass yield is 148.2%. The purity of ceftazidime tert-butyl ester in the tested product was 98.1%.
Embodiment 3
[0058] [embodiment 3] the synthesis of ceftazidime dihydrochloride
[0059] Dissolve 1.0 g of ceftazidime tert-butyl ester in a mixed solution of 2 g of hydrochloric acid and formic acid (the weight ratio of hydrochloric acid: formic acid is 2:1 to 3:2), and react at room temperature until the residue of ceftazidime tert-butyl ester is ≤0.5%. The reaction is over. Slowly add acetone to the filtrate to carry out solvent crystallization. When the crystallization liquid appears slightly turbid, stop adding solvent, slowly stir and grow the crystal for 0.5 hours, continue to add the remaining solvent, and then stir and grow the crystal for 1 hour. After suction filtration and washing, the wet product is vacuum Dry to obtain 0.91 g of ceftazidime dihydrochloride. The mass yield is 91%, and the product purity of ceftazime dihydrochloride in the product is 98.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com