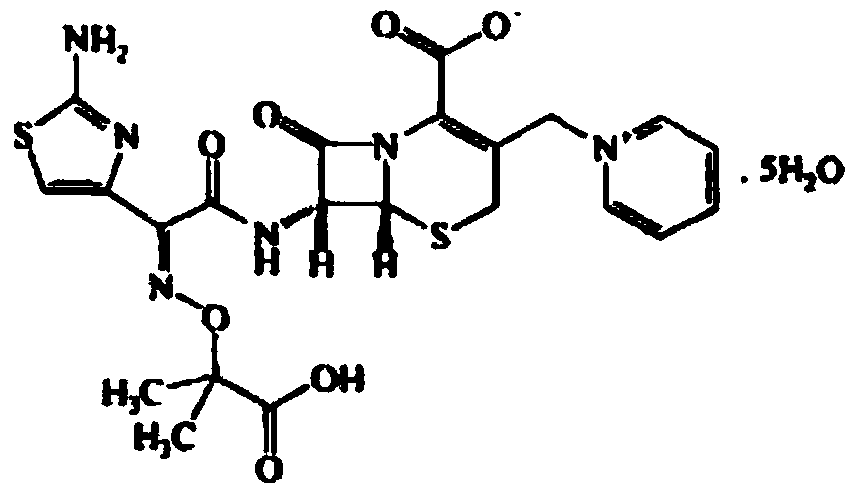
Ceftazidime compound and pharmaceutical composition thereof
A ceftazidime and compound technology, applied in the directions of antibacterial drugs, organic chemistry, pharmaceutical formulations, etc., can solve the problems of poor ceftazidime fluidity, affect the accuracy and uniformity of dispensing, and be unsuitable for industrial production, and achieve good bioavailability, Improved medication safety and little change in polymer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
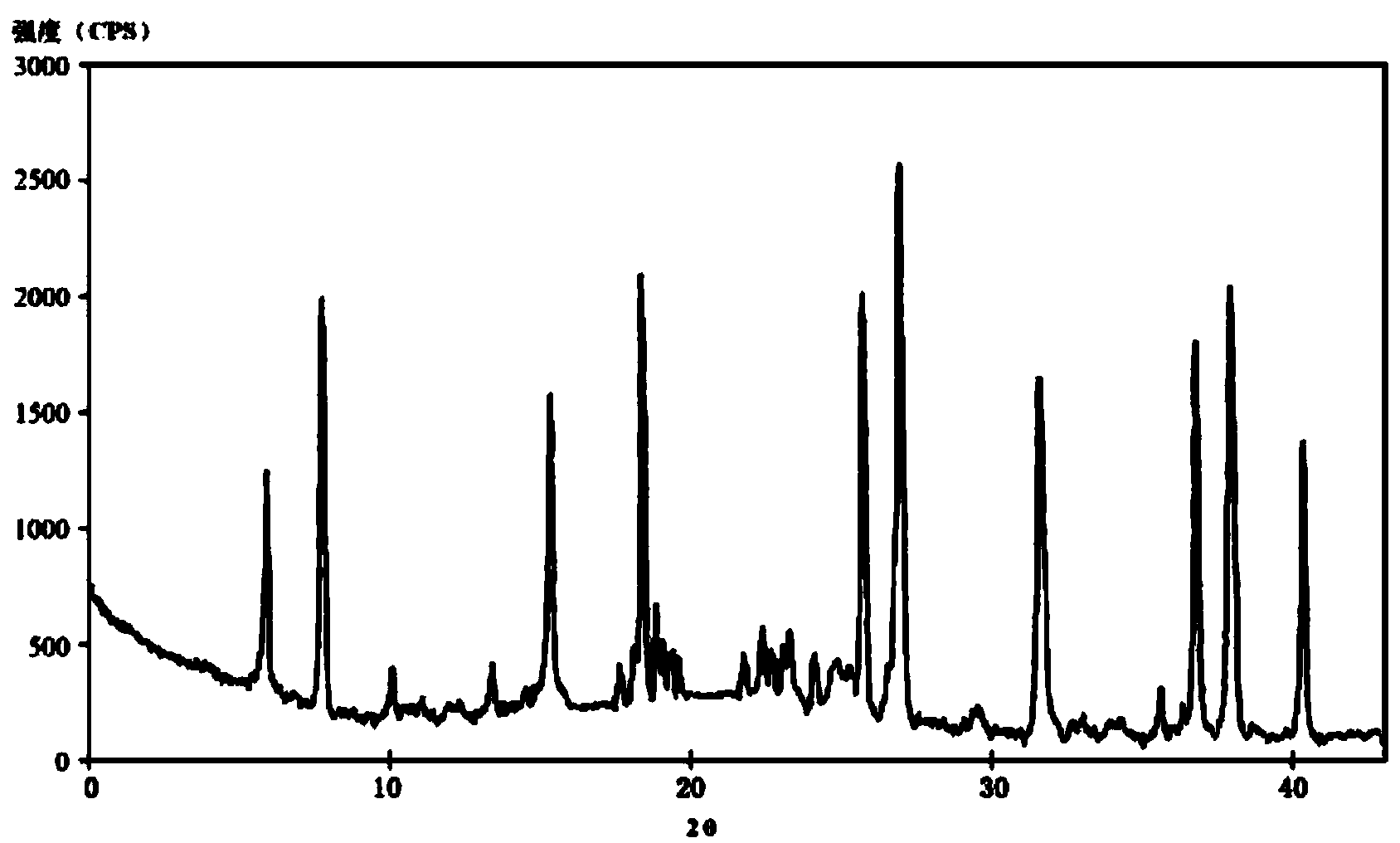
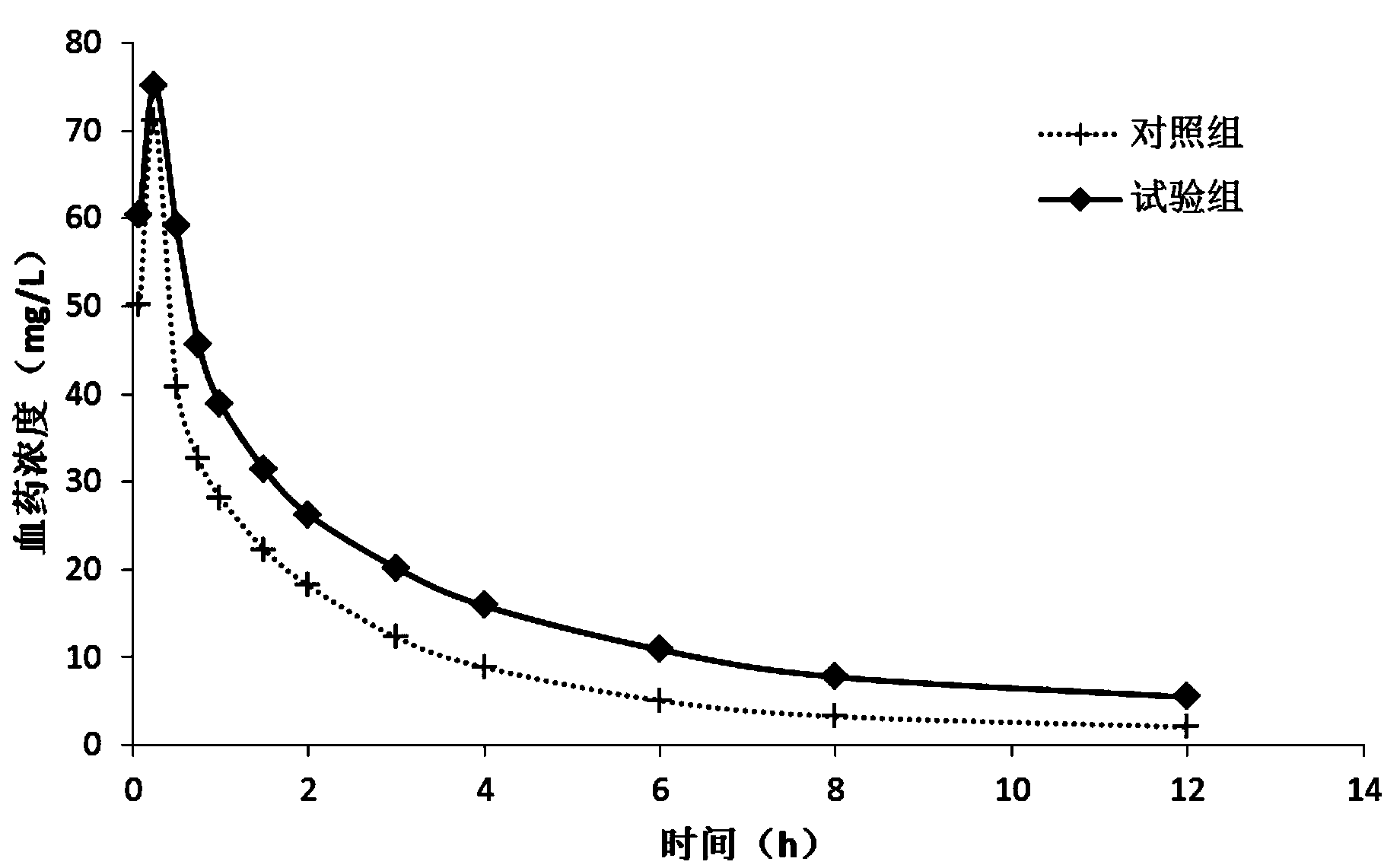
Image
Examples
Embodiment 1
[0046] [embodiment 1] the preparation of ceftazidime compound
[0047] 1) Preparation of crude product solution: Add 20g of crude ceftazidime into 110ml of mixed solvent prepared from dimethyl sulfoxide and methanol (the volume ratio of dimethyl sulfoxide and methanol is 6:1), control the temperature at 22°C, and add ceftazidime 0.01% active carbon decolorization of crude product weight, filter, obtain ceftazidime crude product solution, standby;
[0048] 2) Crystal nucleation process: control the temperature of the crude ceftazidime solution within the range of 22°C, add deionized water to the solution at a rate of 11ml / min under stirring at a stirring speed of 150r / min, turbidity appears, and obtain cloudy solution;
[0049] 3) Nucleation and crystal growth process: put the turbid solution obtained in step 2) at a frequency of 4kHz and an intensity of 3W·cm -2 Under the ultrasonic field, control the temperature of the solution at 22°C, add 1210ml of chloroform to it at a r...
Embodiment 2
[0051] [embodiment 2] the preparation of ceftazidime compound
[0052] 1) Preparation of crude product solution: Add 20g of crude ceftazidime into 150ml of a mixed solvent prepared from dimethyl sulfoxide and methanol (the volume ratio of dimethyl sulfoxide and methanol is 4:1), control the temperature at 28°C, and add ceftazime 0.01% active carbon decolorization of crude product weight, filter, obtain ceftazidime crude product solution, standby;
[0053] 2) Crystal nucleation process: control the temperature of the crude ceftazidime solution within the range of 28°C, add 1050ml of deionized water to the solution at a rate of 14ml / min under stirring at a stirring speed of 180r / min, and turbidity appears. A cloudy solution was obtained;
[0054] 3) Nucleation and crystal growth process: put the turbid solution obtained in step 2) at a frequency of 4.8kHz and an intensity of 1W·cm -2 Under the ultrasonic field, control the solution temperature at 28°C, add 1500ml chloroform to...
Embodiment 3
[0056] [embodiment 3] the preparation of ceftazidime compound
[0057] 1) Preparation of crude product solution: Add 20g of crude ceftazidime into 130ml of a mixed solvent prepared from dimethyl sulfoxide and methanol (the volume ratio of dimethyl sulfoxide and methanol is 5:1), control the temperature at 25°C, and add ceftazidime 0.01% active carbon decolorization of crude product weight, filter, obtain ceftazidime crude product solution, standby;
[0058] 2) Crystal nucleation process: Control the temperature of the crude ceftazidime solution within the range of 25°C, add 1040ml of deionized water to the solution at a rate of 12ml / min under stirring at a stirring speed of 160r / min, turbidity appears, A cloudy solution was obtained;
[0059] 3) Nucleation and crystal growth process: place the turbid solution obtained in step 2) at a frequency of 5.0kHz and an intensity of 2W·cm -2 Under the ultrasonic field, control the temperature of the solution at 25°C, add 1170ml of chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com