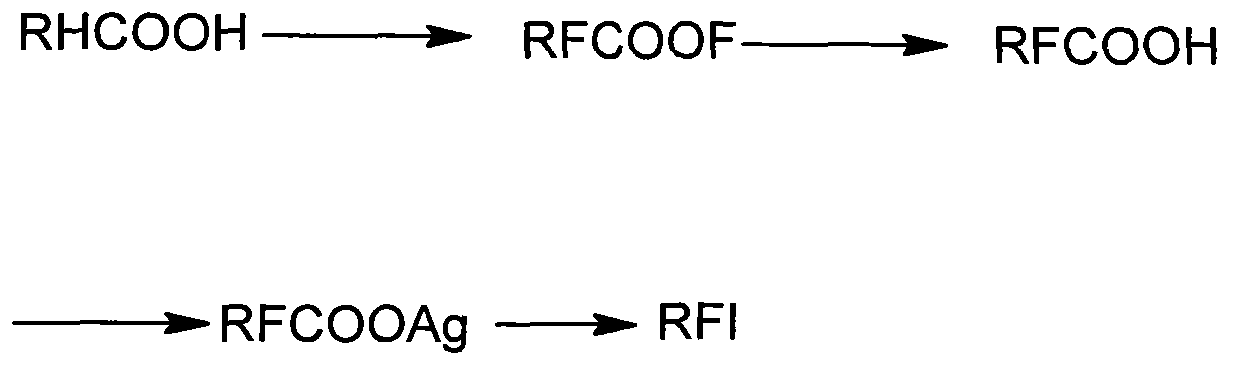
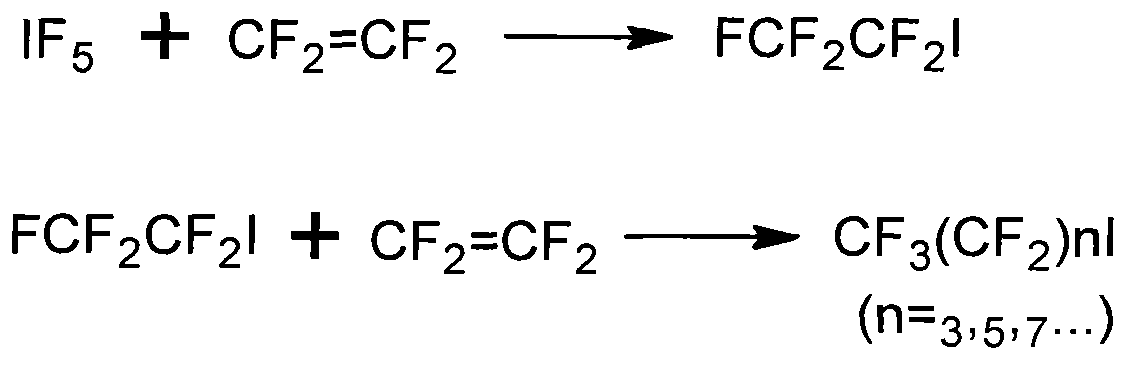
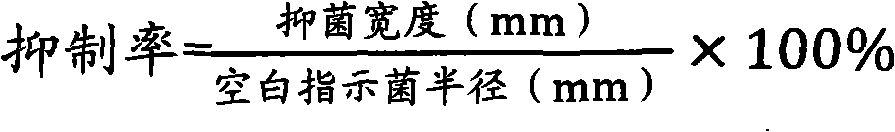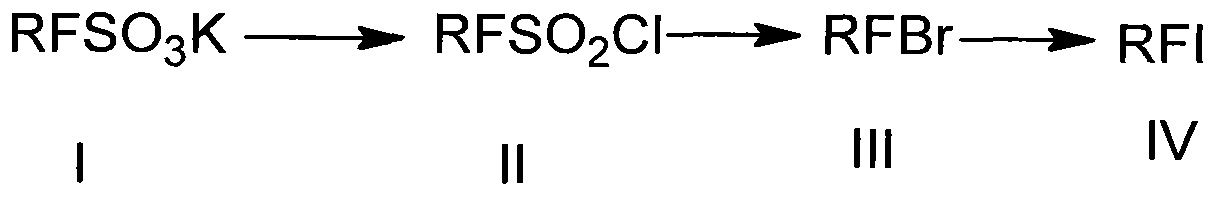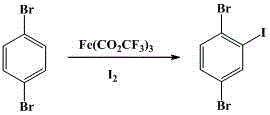Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
134 results about "Iodination reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
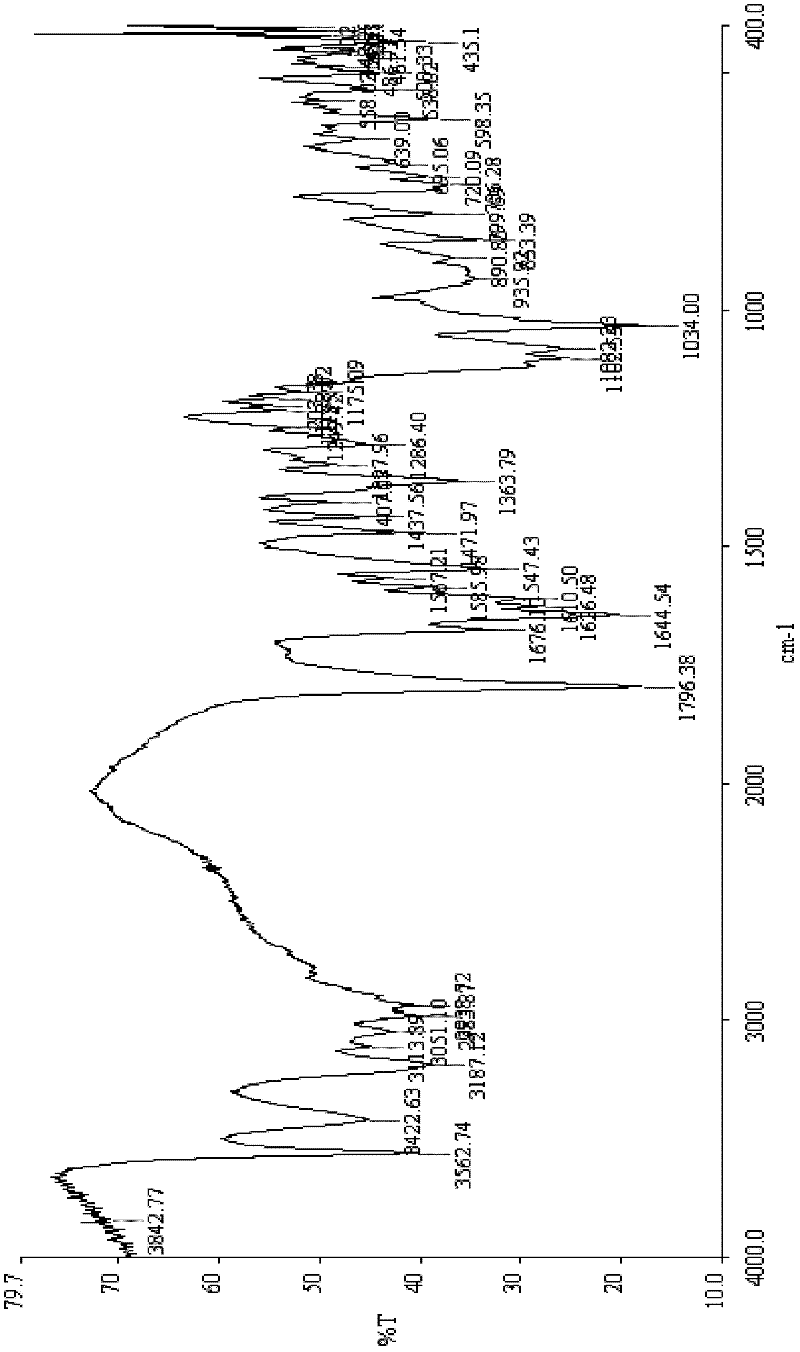
Hydrazone iodination is an organic reaction in which a hydrazone is converted into a vinyl iodide by reaction of iodine and a non-nucleophilic base such as DBU .
Synthetic methods of ceftazidime intermediate and ceftazidime
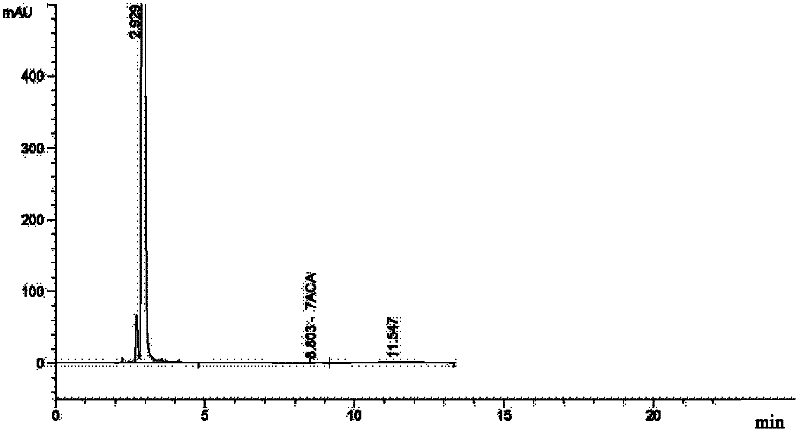
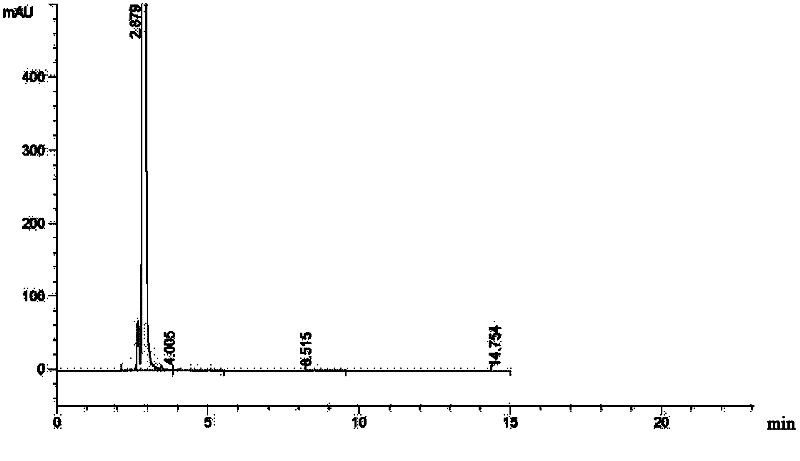
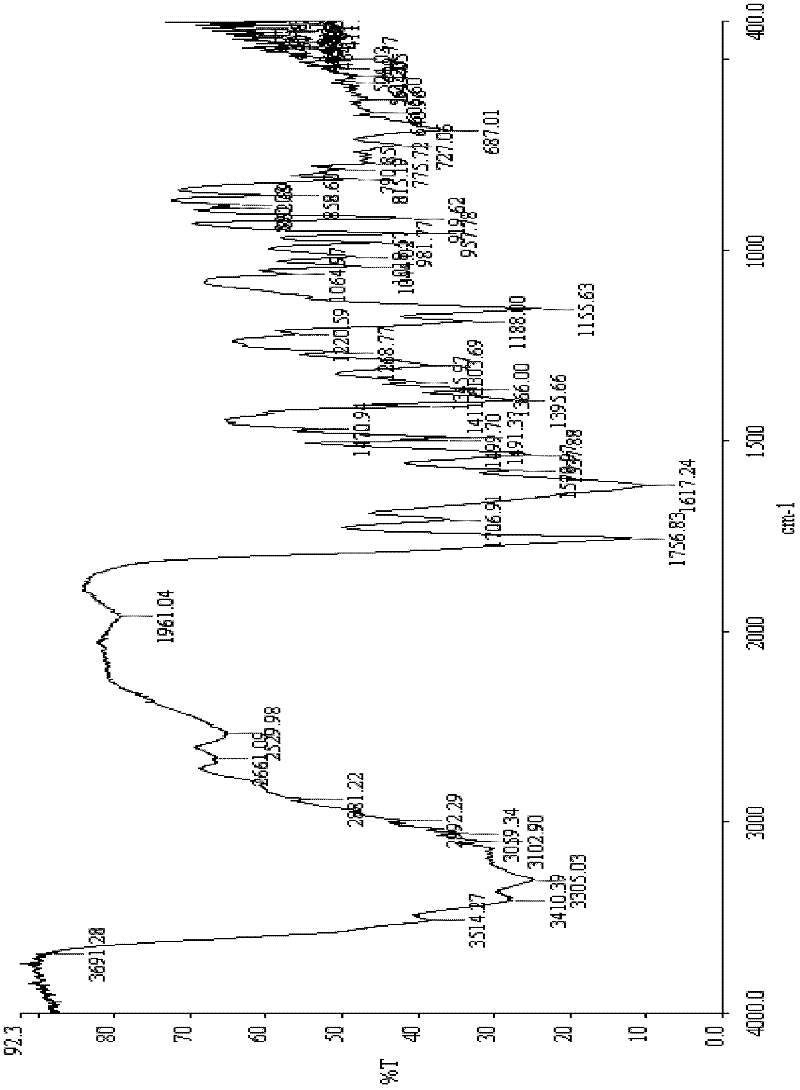
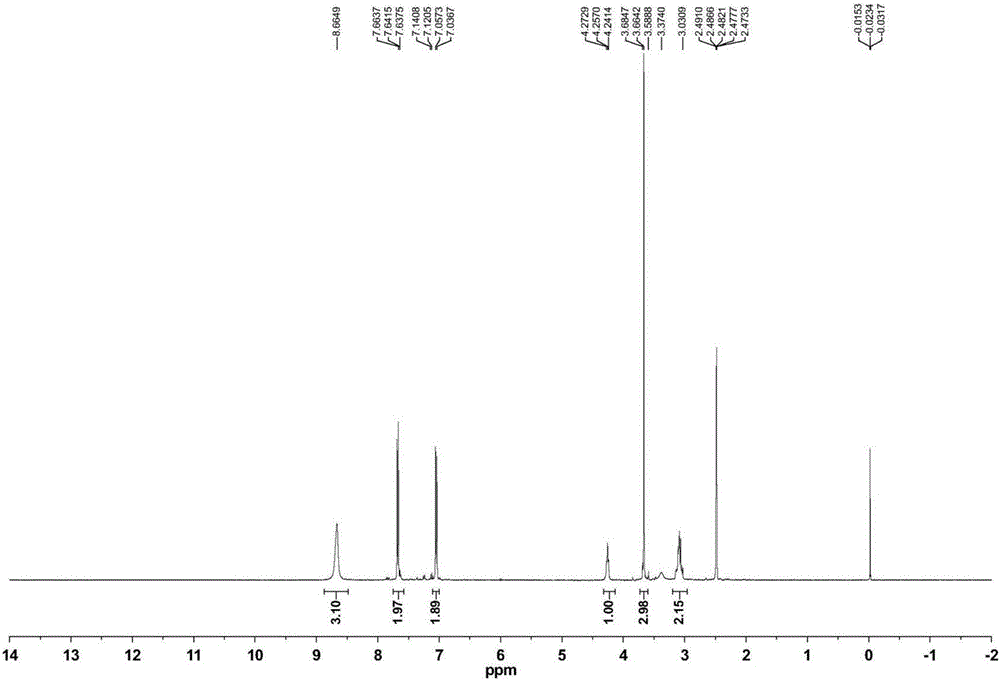
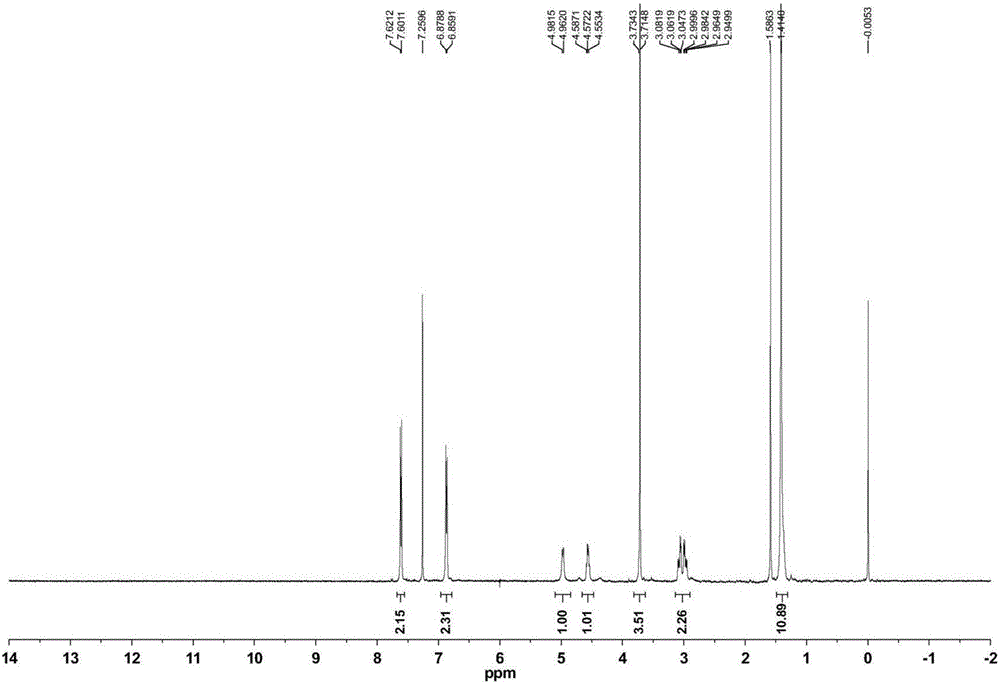
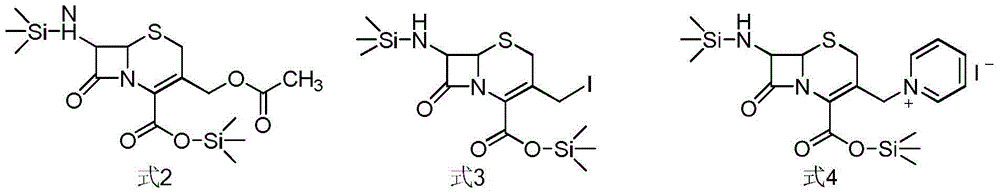
The invention relates to a synthetic method of ceftazidime intermediate; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the ceftazidimeintermediate (6R, 7R)-7-amino-3-pyridine methyl-ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing ceftazidime by using the obtained intermediate halogen acid salt. The ceftazidime intermediate and ceftazidime prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
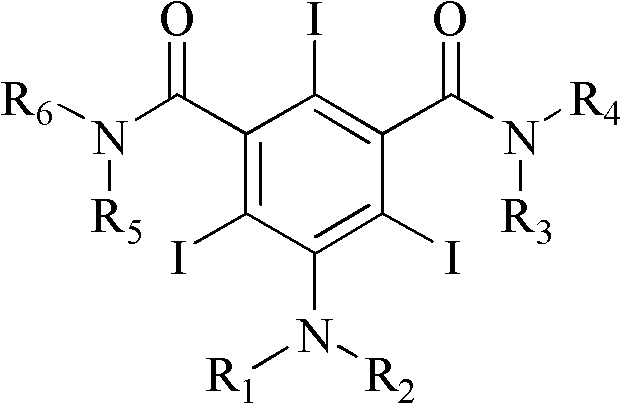
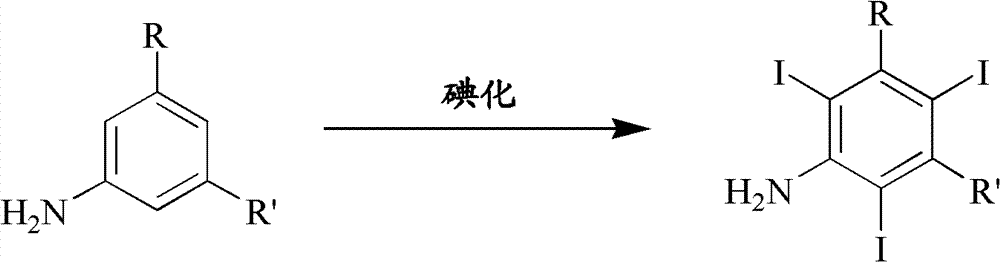
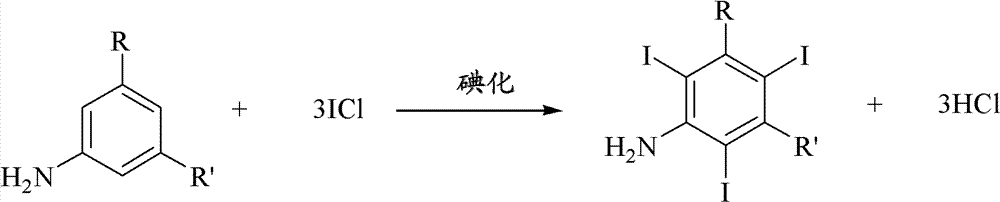
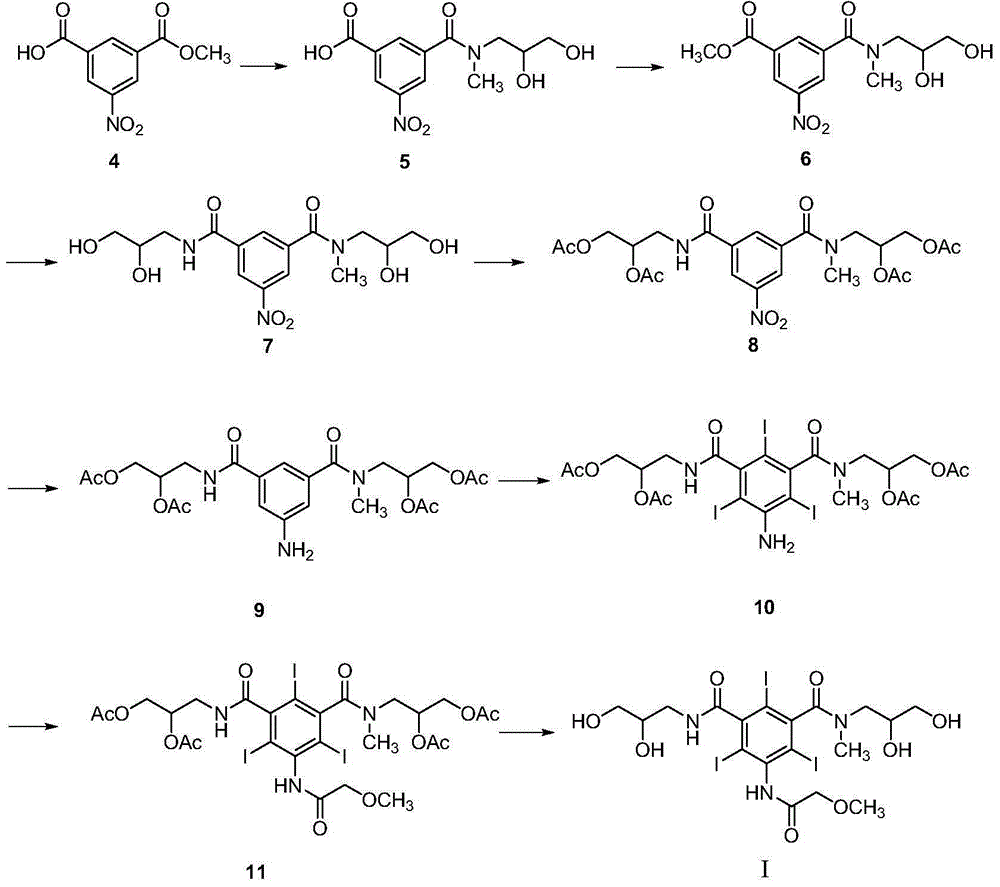
Iodination method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine compound
InactiveCN103086915AOrganic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
The invention discloses an improved method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by a formula (II), wherein R1 and R2 are defined in the instruction, and the compound represented by the formula (II) is a key intermediate for synthesizing iopamidol, iohexol, iodixanol and a series of non-ionic contrast agents. The method comprises: adopting a chlorine-free iodination reagent and a 3,5-disubstituted aromatic amine compound to carry out an iodination reaction to obtain the 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by the formula (II), wherein a mole yield of the iodination reaction can be 89%.
Owner:上海亿脉利医药科技有限公司
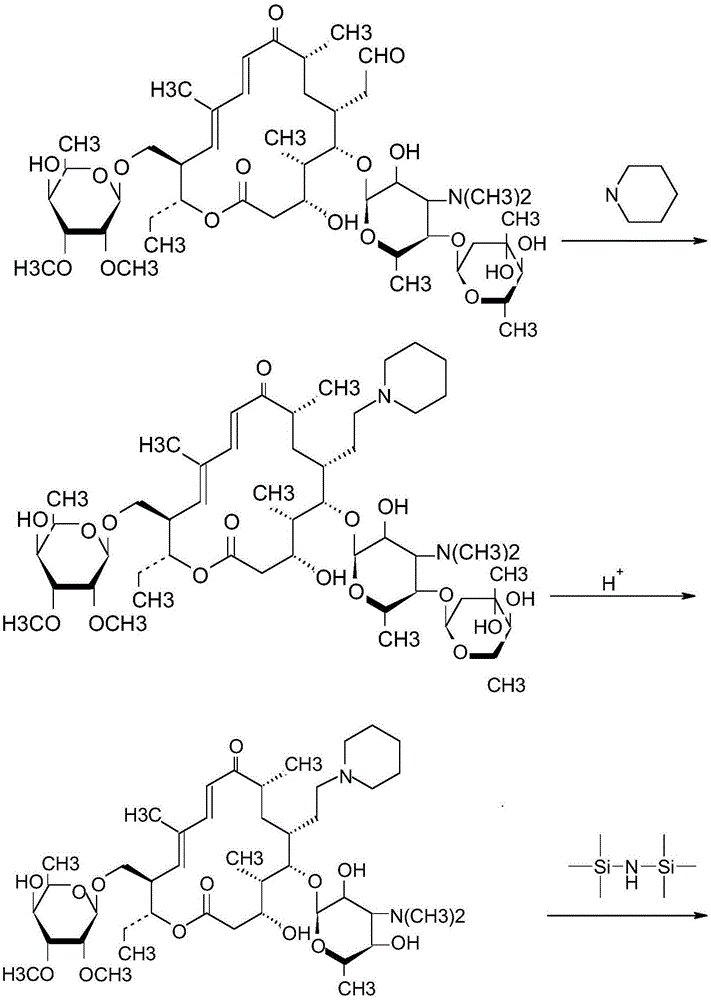
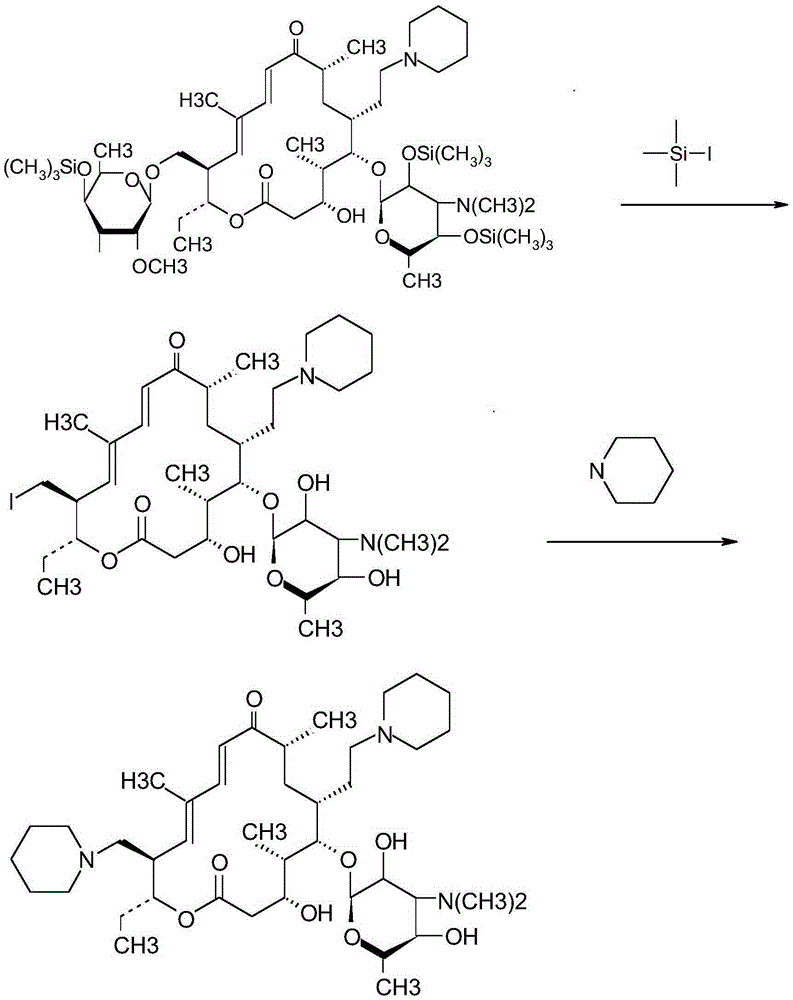
Synthetic method for tildipirosin
InactiveCN105254693AReduce consumptionFew reaction stepsSugar derivativesSugar derivatives preparationIodination reactionAlkyl transfer
The invention discloses a synthetic method for tildipirosin. The synthetic method comprises the steps that 20-piperidyl-5-O-mycaminose base-tylosin lactone silicon is subjected to alkylation protection and then has an iodination reaction with iodotrimethylsilane, and 20-piperidyl-23-I-5-O-mycaminose base-tylosin lactone is prepared and then synthesized with piperidine to obtain tildipirosin. The whole process route is simplified, reaction conditions are mild, the reaction is easy to control, the 23-digit allose base does not need long-time hydrolysis, the reaction time is shortened, the overall yield is increased and reaches up to more than 85%, and the product quality completely meets the related standard requirement.
Owner:周金华
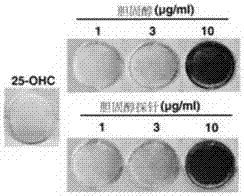
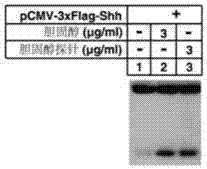
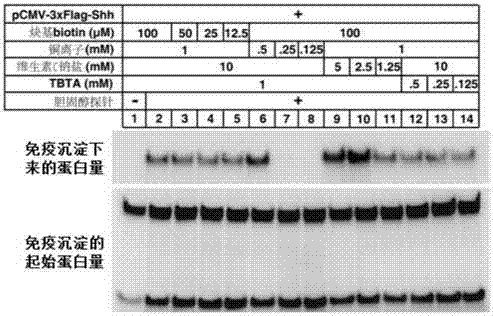
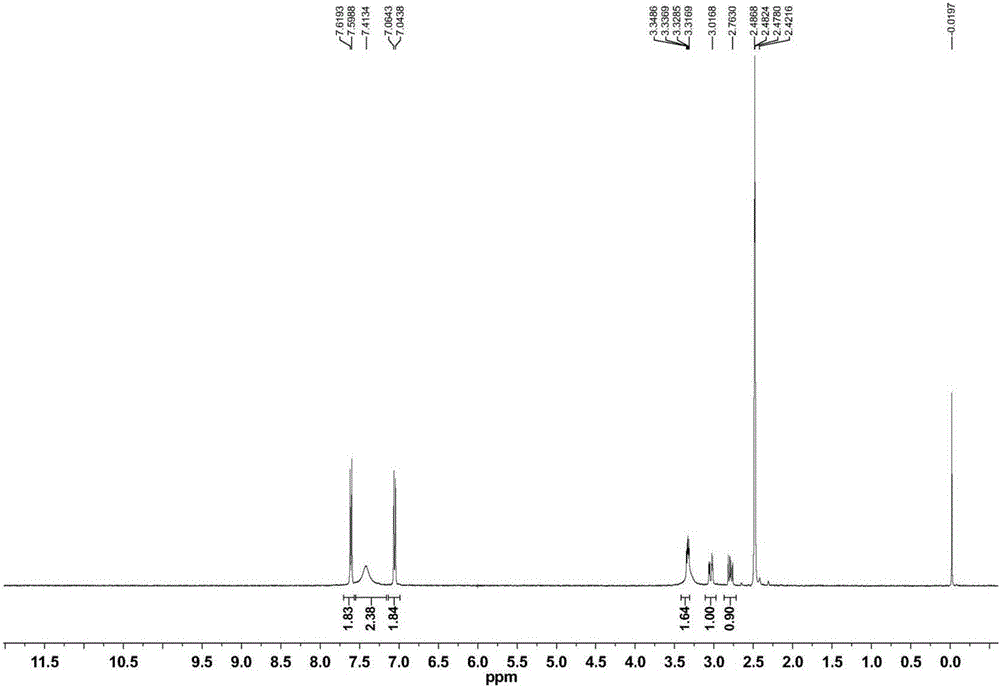
Cholesterol molecular probe as well as preparation method and application thereof
The invention discloses a cholesterol molecular probe and a preparation method thereof. The cholesterol molecular probe shown as a formula (I) is prepared by taking lithocholic acid as a raw material through esterification reaction, oxidization reaction, dehydrogenation reaction, carbonyl protection reaction, reduction reaction, hydroxyl protection reaction, reduction reaction, iodination reaction, substitution reaction and de-protection reaction. The invention further discloses application of the cholesterol molecular probe shown as the formula (I) to identification of cholesterol modified protein. The cholesterol molecular probe provided by the invention can be used for simulating normal cholesterol to promote cell growth, and prompting the shearing ripening of the cholesterol modified protein hedgehog, and can also be used for researching cholesterol modification of the protein.
Owner:WUHAN UNIV +1
Method for preparing Sacubitril intermediate of anti-heart-failure medicine
InactiveCN105753741AHigh reaction yieldEasy to purifyCarbamic acid derivatives preparationOrganic compound preparationWittig reactionSacubitril
The invention discloses a method for preparing a Sacubitril intermediate of anti-heart-failure medicine as indicated in the formula (VII).The method comprises the following steps of taking D-phenylalanine which is low in price and easy to obtain as the raw material, and conducting an iodination reaction, an esterification reaction, a Boc protection reaction, a negishi coupling reaction, a DIBAL-H reduction reaction and a wittig reaction, so that the Sacubitril intermediate is obtained through preparation.The method for preparing the Sacubitril intermediate is mild in reaction condition and environmentally friendly, compared with existing preparation methods, the yield is higher, and the method is economical, effective and suitable for large-scale industrialized production.
Owner:CHANGZHOU PHARMA FACTORY
Process for producing 5-iodo-2-methylbenzoic acid
InactiveCN1812954AImprove efficiencyExtended service lifeOrganic compound preparationOrganic chemistry methodsAcetic anhydrideDistillation
The present invention provides a process for producing 5-iodo-2-methylbenzoic acid by iodizing 2-methylbenzoic acid, which comprises, as essential steps, a reaction step in which 2-methylbenzoic acid is iodized in the presence of a microporous compound, iodine, an oxidizing agent, and acetic anhydride and a purification step in which sublimation, distillation, crystallization, or a combination of two or more of these is conducted. By the process, 5-iodo-2-methylbenzoic acid, which is useful in functional chemicals such as medicines, can be easily obtained as a high-purity compound in a high yield. The production steps comprising reaction and separation / purification are simple from the standpoint of process operation and the purification load is small. Furthermore, the microporous compound, e.g., a zeolite catalyst, separated and recovered from the liquid resulting from the reaction can be repeatedly used after a simple treatment. Consequently, the catalyst has a long life and the target compound can be produced by the efficient process.
Owner:MITSUBISHI GAS CHEM CO INC
Method for preparing ceftazidime by one-pot process
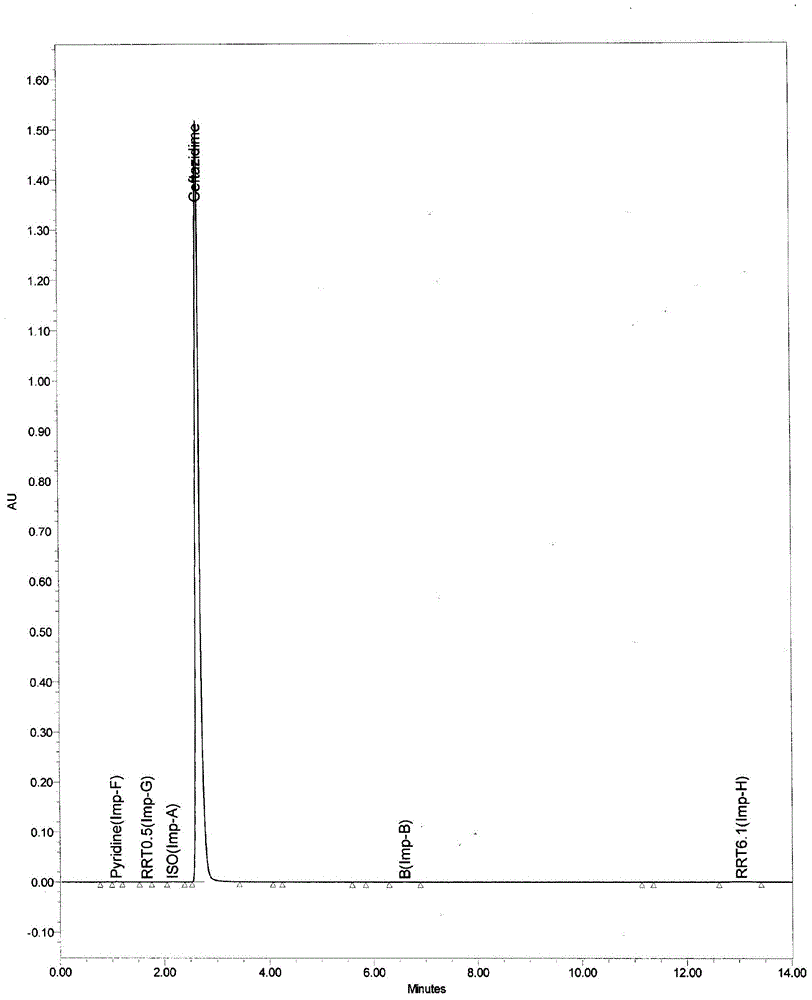
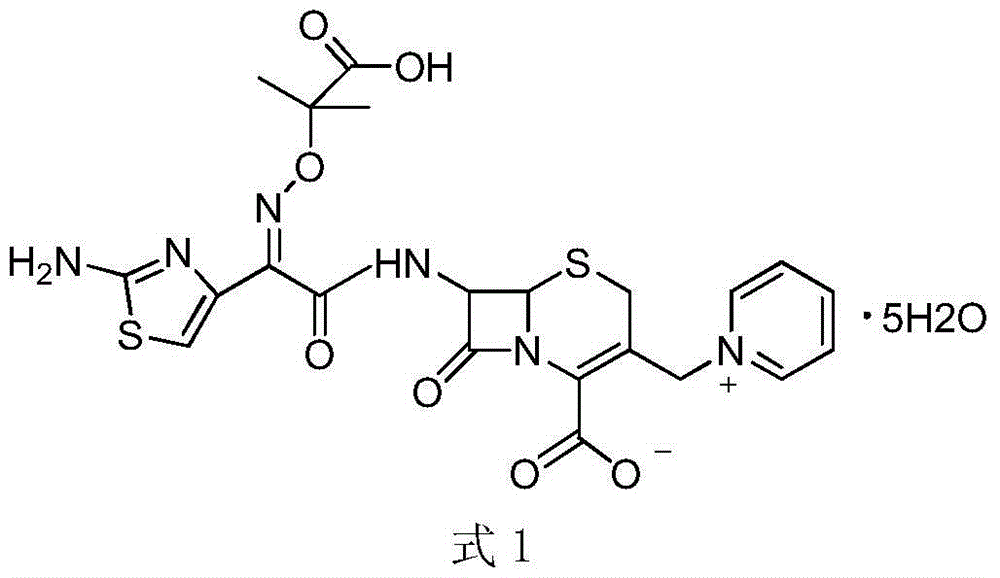
The invention relates to a method for preparing ceftazidime by a one-pot process. The method comprises the following steps: by using 7-aminocephalosporanic acid as the raw material, carrying out silanization reaction and iodination reaction, reacting with pyridine, directly adding the liquid into ceftazidime side chain acyl chloride hydrochloride to perform acylation reaction without separation to obtain ceftazidime iodate, adding the liquid into a concentrated hydrochloric acid-water mixed solution to perform deprotection, extracting to stratify, and regulating the pH value of the water phase with an alkaline solution to obtain ceftazidime (6R,7R)-7-[[(2-amino-4-thiazolyl)-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4,2,0]octyl-2-ene-3-methylpyridine pentahydrate. The method has the advantages of high yield, low cost, mild technological conditions, controllable technical process, high safety and low energy consumption, and is simple to operate.
Owner:QILU ANTIBIOTICS PHARMA
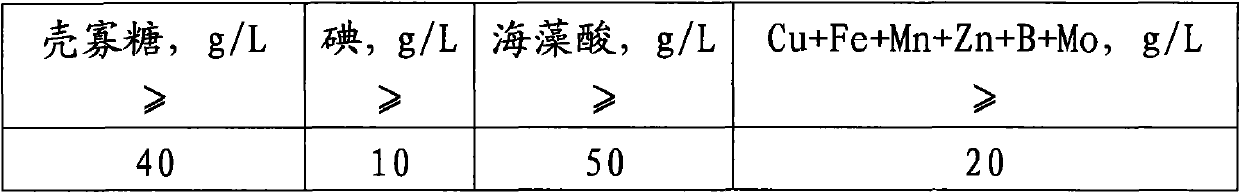
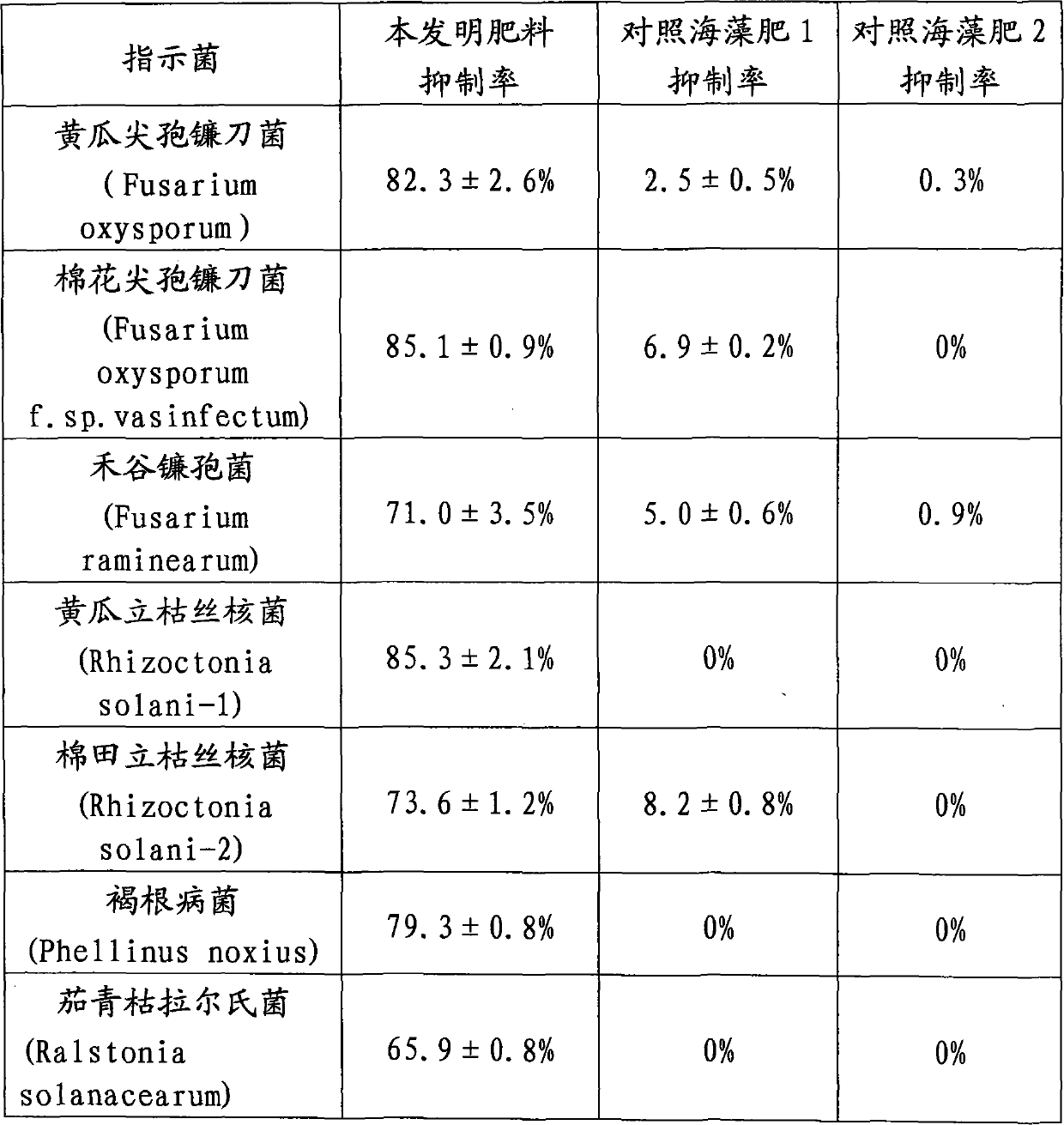
Preparation method of iodated chitosan oligosaccharide seaweed bio-fertilizer
InactiveCN103304304AGood water solubilityHigh activityFertilizer mixturesPotassium iodineIodination reaction
The invention relates to a preparation method of an iodated chitosan oligosaccharide seaweed bio-fertilizer. The preparation method comprises the following steps of: (1) carrying out enzymatic hydrolysis reaction on chitosan, namely, shearing and crushing chitosan, and carrying out enzymatic hydrolysis reaction by using non-special composite enzyme so as to obtain a chitosan oligosaccharide solution with a molecular polymerization degree of 4-8; (2) carrying out iodination reaction on the chitosan oligosaccharide, namely, adding a potassium iodide solution into the obtained chitosan oligosaccharide solution in a certain speed, so as to carry out the iodination reaction on the chitosan oligosaccharide and obtain an iodated chitosan oligosaccharide solution; and (3) carrying out chelation reaction on a seaweed extract, namely, taking the seaweed extract which is prepared by using an enzymatic hydrolysis method, carrying out chelation reaction on the seaweed extract and the iodated chitosan oligosaccharide solution according to a certain ratio, and compounding microelements of certain concentration, and finally obtaining the iodated chitosan oligosaccharide seaweed bio-fertilizer. The iodated chitosan oligosaccharide seaweed bio-fertilizer prepared by using the method has the remarkable functions of plant growth promoting, production increase, disease-resistance and health care, also has the strong and lasting sterilization function, is free of residue and pollution, and is a completely environmental-friendly bio-fertilizer that the medicine and the fertilizer are combined together.
Owner:青岛海大生物集团股份有限公司
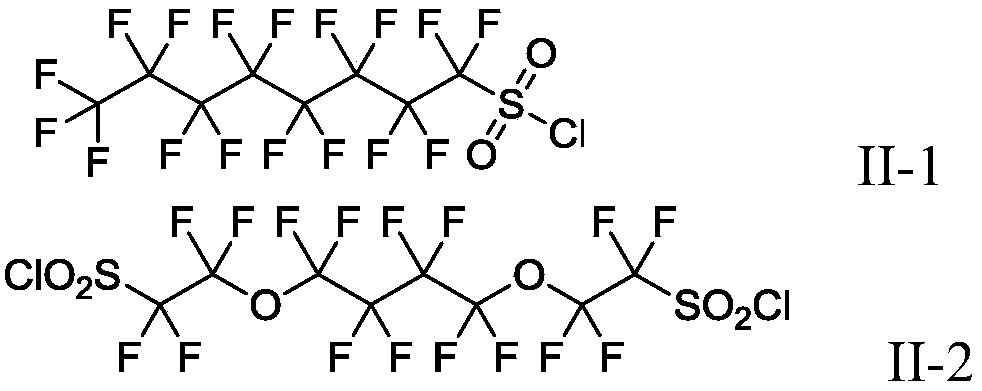
Preparation method of heptadecafluorooctyl iodoalkane
InactiveCN102992944AThe synthetic route is simpleRaw materials are easy to getPreparation by halogen replacementSulfonyl chlorideChemical synthesis
The invention discloses a preparation method of heptadecafluorooctyl iodoalkane, and belongs to the technical field of chemical synthesis. The preparation method comprises the following steps of: implementing a sulfonylation reaction on heptadecafluorooctyl potassium sulphonate at reacting temperature, to obtain heptadecafluorooctyl sulfonyl chloride after the reaction; implementing a bromination reaction on the heptadecafluorooctyl sulfonyl chloride at reacting temperature, to obtain heptadecafluorooctyl bromoalkane after the reaction; and implementing an iodination reaction on the heptadecafluorooctyl bromoalkane, to obtain heptadecafluorooctyl iodoalkane after the reaction. In comparison with the prior art, the preparation method of the heptadecafluorooctyl iodoalkane has the advantages of being simple in synthesis route, easily available in raw materials, moderate in processing conditions, lower in cost, and convenient for industrial production.
Owner:中国人民解放军防化学院 +1
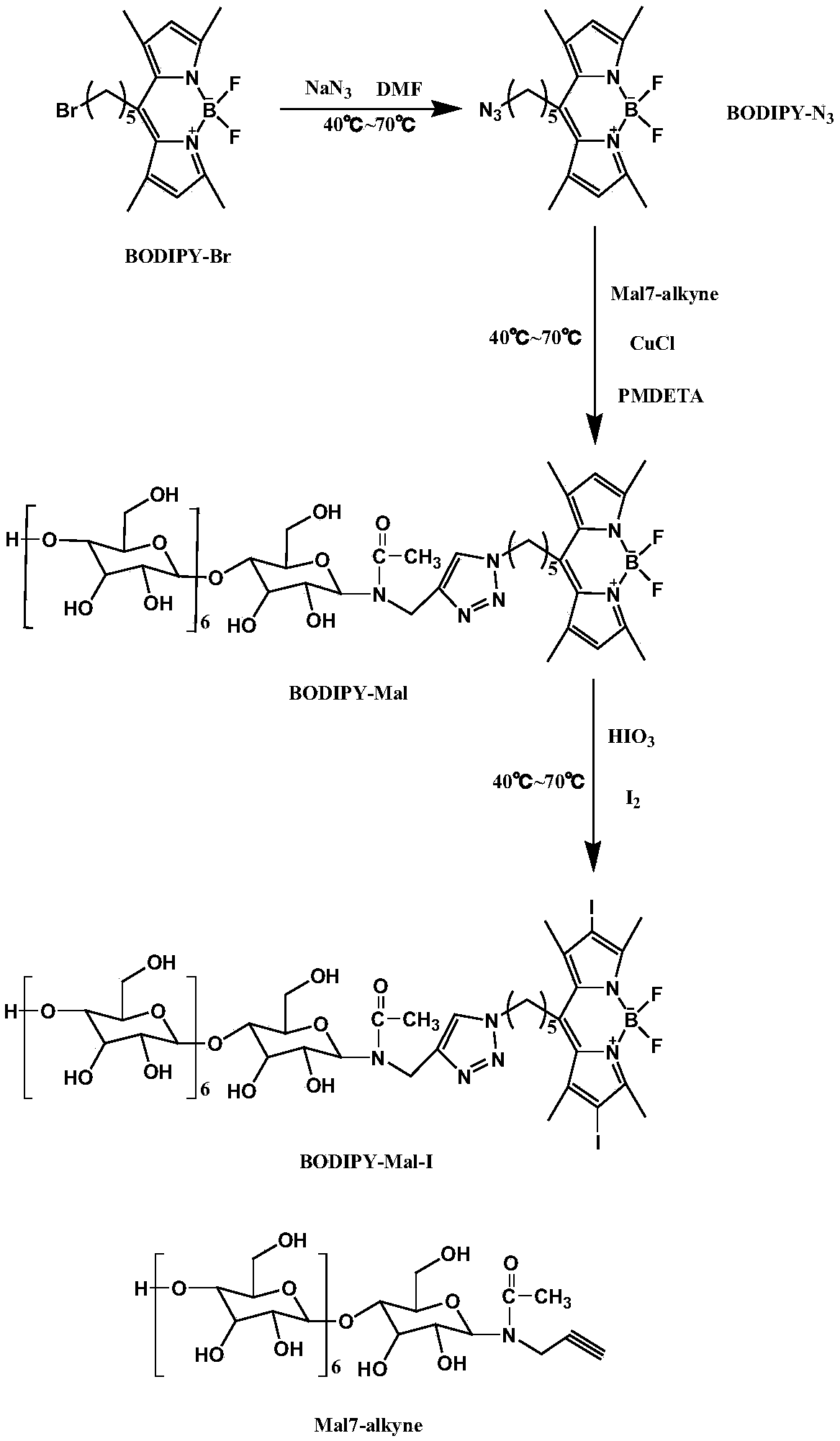
BODIPY-containing bactericidal material and preparation method and application thereof
ActiveCN108752401AComplete retention propertiesReserved natureAntibacterial agentsAntimycoticsSolubilityYeast
The invention discloses a BODIPY-containing bactericidal material and a preparation method and application thereof. The preparation method comprises that BODIPY-Br and sodium azide undergo a reactionto produce BODIPY-N3, BODIPY-N3 and acetylated maltoheptaose undergo a reaction under the catalytic action of a CuCl / PMDETA complex to produce BODIPY-Mal, and the BODIPY-Mal, iodic acid and iodine undergo an iodination reaction to produce the BODIPY-Mal-I bactericidal material. The BODIPY-Mal-I bactericidal material has good water solubility and completely retains the properties of BODIPY. The photodynamic power generated by BODIPY-Mal-I can effectively inactivate yeast cells and especially kill gram-positive bacteria. The preparation method has the advantages of simple processes, mild reaction conditions, low risk and low toxicity.
Owner:SOUTHWEST UNIVERSITY
Preparation methods of cefpirome intermediate and cefpirome
ActiveCN102391288AHigh purityHandling Recycling SimplifiedOrganic chemistryCyclopenteneCarboxylic acid
The invention relates to preparation methods of cefpirome intermediate and cefpirome; 7-amino cephalsporanic acid is used as a raw material; a silanization reaction, an iodination reaction, and a pyridine reaction are performed; the obtained product is added with an oxidant, and hydrochloric acid or is added into a mixed solvent of an organic solvent and water to prepare a halogen acid salt of the cefpirome intermediate (6R, 7R)-7-amino-3-[(2,3-cyclopentene-pyridine)methyl]ceph-3-ene-4-carboxylic acid. The invention also provides a method for preparing cefpirome sulfates by using the obtainedintermediate halogen acid salt. The cefpirome intermediate and cefpirome prepared by the methods have high yield, and low production cost; the operation is simple; the discharge of three wastes is less; treatment and recovery are easy, and the methods are applicable to industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveUS20060161028A1High purityCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:MITSUBISHI GAS CHEM CO INC
Process for production of iodine compounds and process for production of high-purity 5-iodo-2-methylbenzoic acid
InactiveCN1747910AHigh purity productHigh purityCarboxylic acid nitrile preparationMolecular sieve catalystsOrganic solventChemical products
Provided is a production method for an iodine compound in which iodine is reacted with a substrate in the presence of a porous material having a pore diameter of 500 nm or less or in the presence of the above porous material and an oxidizing agent and a production process for high purity 5-iodo-2-methylbenzoic acid comprising an iodination reaction step carried out by the above-mentioned, a crystal precipitation and separation step in which a product is precipitated by adding water or cooling and then separated and a purification step in which crystal separated is recrystallized using an organic solvent. According to the production method for an iodine compound described above, iodine can be introduced into various substrates at a high selectivity. Since expensive metals and specific reagents do not have to be used, it can readily be carried out in an industrially scale, and the product having a high purity can be obtained. Further, the process comprising the iodination reaction, separation and purification steps described above makes it possible to readily obtain at a high yield, 5-iodo-2-methylbenzoic acid having a high purity which is useful in uses for functional chemical products such as medicines. The process of the present invention comprising iodination reaction, separation and purification steps is characterized by that it is simple in terms of a procedure and that the purification load is smaller, and it is very advantageous in industrially carrying out.
Owner:日本精细化工株式会社
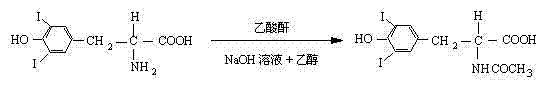
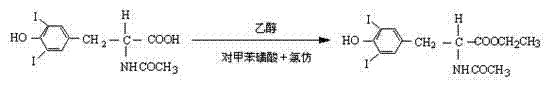
Improved L-thyroxine sodium synthesis method
ActiveCN102199103AIncrease productivityLower cost per unit of outputOrganic compound preparationCarboxylic acid amides preparationN-acetyl-L-tyrosine ethyl esterSynthesis methods
The invention relates to an improved L-thyroxine sodium synthesis method. The method comprises the following steps of: carrying out iodination reaction, N-acidylation reaction, esterification reaction, oxidative coupling reaction and hydrolysis reaction on L-tyrosine used as a raw material to obtain L-thyroxine, and salifying to obtain L-thyroxine sodium. In the oxidative coupling reaction,N-acetyl-3,5-diiodo-L-tyrosine ethyl ester is used as a starting material, manganese chloride or manganese nitrate is used as a catalyst, boric acid is used as a cocatalyst, ethanol is used as a solvent, the pH value of reaction liquid is 8-9, the reaction temperature is 40-50 DEG C, and oxygen is introduced to carry out oxidative coupling reaction for at least 80 hours at normal pressure to obtain N-acetyl-L-tyrosine ethyl ester; and the catalyst is added twice, the addition amount in the first time is at least 60wt% of the total mass of the catalyst, and the mass ratio of the catalyst to the starting material for the oxidative coupling reaction is 0.008-0.012. The improved synthesis method provided by the invention increases the yield and purity of N-acetyl-L-tyrosine ethyl ester and lowers product cost.
Owner:靖江市城中村投资建设有限公司
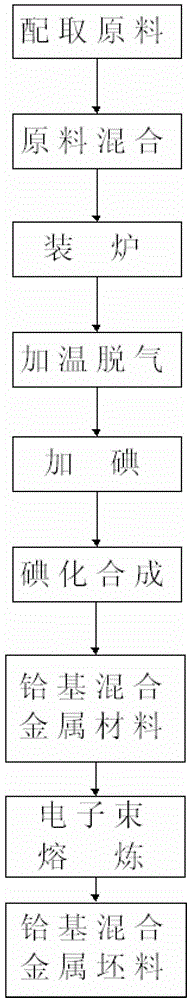
Hafnium-base mixed metal material and iodination preparation method thereof
The invention relates to a hafnium-base mixed metal material and an iodination preparation method thereof, and belongs to the field of synthesis and preparation of mixed metal materials. The hafnium-base mixed metal material is composed of a matrix metal hafnium and other metal compositions, and the other metal compositions comprise one or two or more of Zr, Ti, Al, B, Ba, Be, Ca, Ce, Co, Cs, Dy, Er, Fe, Gd, Ho, La, Mg, Mn, Mo, Nb, Ni, Pr, Sc, Sm, Sr, Ta, V, W, Y and Yb. The hafnium-base mixed metal material with the purity up to 99.95 wt% can be prepared through an iodination reaction, and continuous batch production can be realized, and the advantages of uniform product compositions, high production efficiency, relatively low production cost and the like are provided.
Owner:GENERAL RESEARCH INSTITUTE FOR NONFERROUS METALS BEIJNG
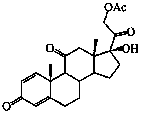
Preparation method of prednisone acetate
The invention discloses a preparation method of prednisone acetate, which comprises the following steps: by using dihydroxy progesterone dehydrogenate as a raw material, carrying out iodination reaction to obtain an iodination product, carrying out replacement reaction to obtain a substitution product, and finally, carrying out oxidation reaction to obtain the prednisone acetate. The dihydroxy progesterone dehydrogenate, which is used as the raw material for the first time, is sequentially subjected to iodination, replacement and oxidation reaction to obtain the high-purity prednisone acetate. The method has the advantages of short technical route, high product quality, high yield and low cost, and is suitable for large-scale industrial production.
Owner:ZHEJIANG XIANJU PHARMA
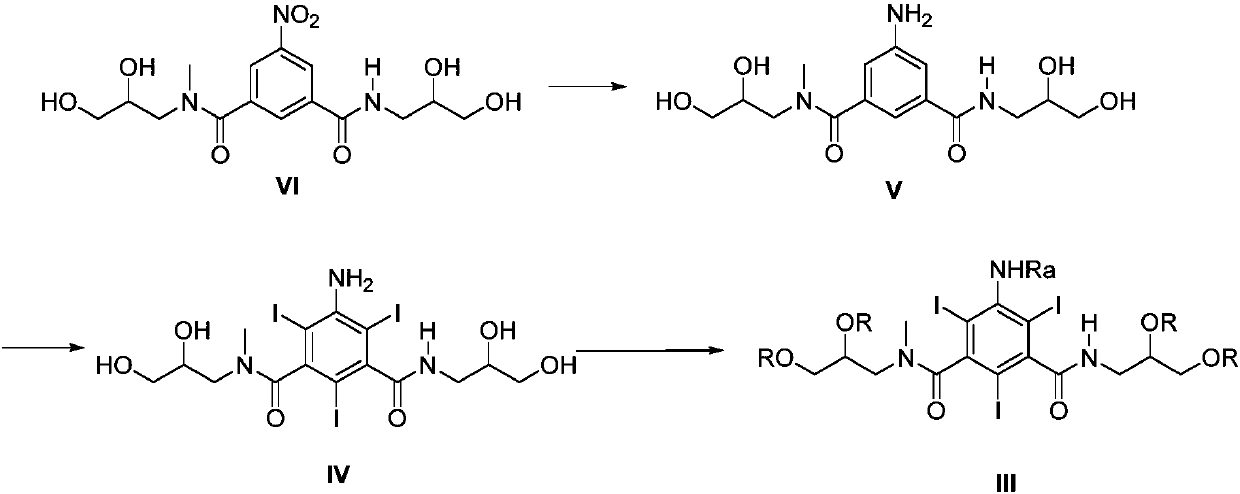
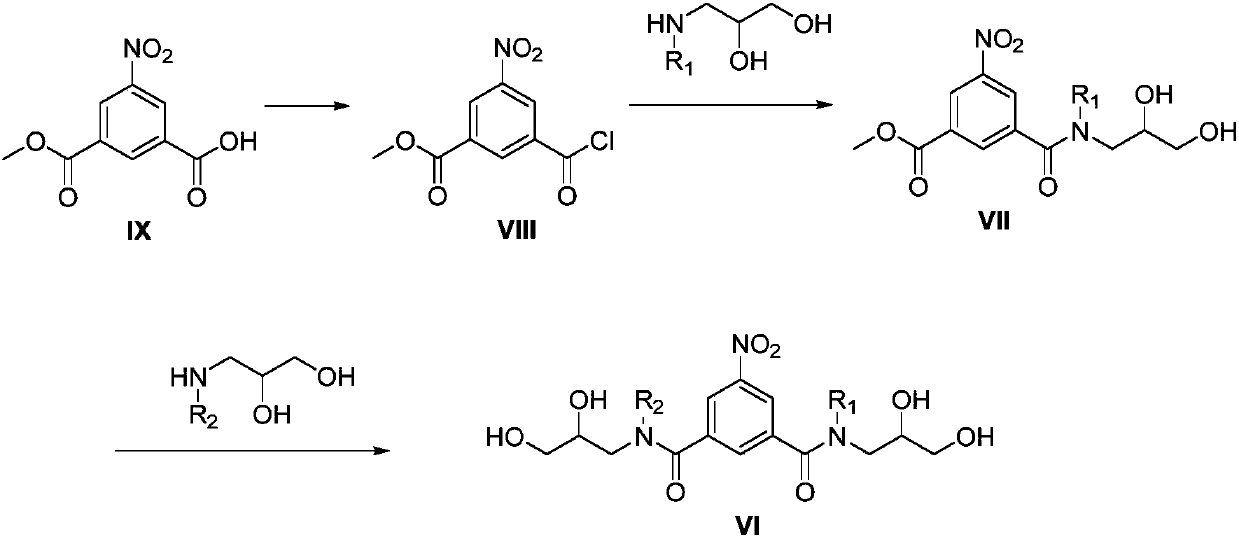
Preparation methods of iopromide and intermediate of iopromide
InactiveCN107778191AAvoid generatingReduce generationOrganic compound preparationCarboxylic acid amides preparationIopromideIodination reaction
The application of the invention relates to preparation methods of iopromide and an intermediate of the iopromide. The method specifically comprises the following steps: conducting a reduction reaction, an iodination reaction and an acylation reaction on a compound shown in the formula VI to prepare a compound shown in the formula III, and further preparing to obtain the iopromide. The method notonly avoids the generation of double acylation byproducts, but also effectively reduces the generation of byproducts in the preparation process, the intermediate is easy to separate and purify, and ahigh-purity product is obtained at a relatively high yield.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing loxilan intermediate
ActiveCN101948404AReduce pollutionReduce harmOrganic compound preparationCarboxylic acid amides preparationBenzoic acidAcetic anhydride
The invention discloses a method for preparing a loxilan intermediate. 5-nitro-N-(2-hydroxyethyl)-m-formamide benzoic acid serving as an initial raw material is subjected to methyl esterification reaction with methanol, amidation reaction with 3-amino-1,2-propylene glycol in methanol solution, iodination reaction with iodine monochlorde in aqueous solution after the product of amidation reaction is reduced with ferric acid, acetylation reaction with acetic anhydride and hydrolysis in aqueous ammonia to obtain 5-acetamido-N-(2,3-dihydroxypropyl)-N-(2-hydroxyethyl)-2,4,6-triiodo-1,3-phthalic amide. The target compound can be obtained by acylation and hydrolysis reaction after the iodination reaction; the use of the expensive iodine monochlorde is avoided at the initial stage of the reactionsteps, so the cost of the whole reaction is greatly reduced; and the reaction condition is mild and the purity of the product is high because the aqueous ammonia is used in the hydrolysis reaction.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Synthesis method and purification method of 5-iodo-2-methylbenzimidazole
InactiveCN102936223AGood atom economySimple and fast operationOrganic chemistryPurification methodsOrganic synthesis
The invention discloses a synthesis method and purification method of 5-iodo-2-methylbenzimidazole, relating to the field of organic synthesis. The method comprises the following steps: by using o-nitroaniline as a main raw material, simple substance iodine as an iodine source and oxydol as an oxidizer, carrying out iodination reaction in a polar solvent in the presence of sulfuric acid to obtain 4-iodo-2-nitroaniline; and in the polar solvent, carrying out hydrogenation reduction by using Raney nickel as a catalyst to obtain 4-iodo-1,2-phenylenediamine, and carrying out cyclization reaction by using slight acetic acid as a catalyst and ortho-triacetate as a cyclization reagent to obtain 5-iodo-2-methylbenzimidazole, and finally, carrying out decolorization and purification in an acetate generation mode. The synthesis method disclosed by the invention belongs to green synthesis, has the advantages of high atomic economical efficiency and less waste, and is simple to operate; the catalyst has the advantages of low price, small recovery loss and less waste, and is simple to operate; and the product decolorization and purification mode is simple and efficient, the product appears a creamy white powder solid, and the purity is higher than 99.5%.
Owner:JIANGSU ZHONGDAN PHARMA RES +1
Preparation method of cefalonium
ActiveCN104725403ARaw materials are easy to getEasy to operateOrganic chemistryIodination reactionSolvent
The invention relates to a preparation method of cefalonium. According to the preparation method, raw materials (cefalotin and pyrazinamide) react at a low temperature to obtain the product cefalonium. The preparation method particularly comprises the following steps: dissolving cefalotin acid into an organic acid, carrying out carboxyl protection by using a silanization protection reagent and then carrying out iodination reaction on reaction products and iodotrimethylsilane; then carrying out amination reaction on the reaction product from the former step and pyrazinamide; and finally carrying out deprotection by alcoholysis, regulating the pH value at a low temperature and crystalizing to obtain cefalonium. According to the preparation method of cefalonium, cefalotin acid is protected by the silanization protection reagent in an organic solvent and then reacts with iodotrimethylsilane; the reaction time is short, the reaction conditions are mild, the reaction is complete and no side reaction is almost generated; due to adoption of a mixed solvent crystallization method, the characteristics of high drying speed, light color and high yield can be achieved; in addition, the used solvent can be recycled and the amount of generated sewage can be reduced; therefore, the preparation method of cefalonium has remarkable economic and environmental benefits and facilitates industrial production.
Owner:QILU SYNVA PHARMA
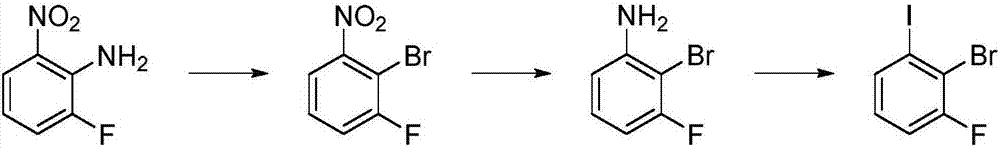
Preparation method of 1-fluoro-2-bromo-iodobenzene
ActiveCN108002976AReduce lossesImprove securityOrganic compound preparationAmino compound preparationBromineNitrobenzene
The invention provides a preparation method of 1-fluoro-2-bromo-iodobenzene. The method comprises the steps that 1-fluorine-2-amidogen-3-nitrobenzene is used as a raw material, 1-fluorine-2-bromine-3-nitrobenzene is obtained through a diazotization and bromination reaction, 1-fluoro-2-bromo-aminobenzene is obtained through a following reduction reaction, and then 1-fluoro-2-bromo-iodobenzene is obtained through a diazotization and iodination reaction. According to the preparation method, under the acidic condition, 1-fluoro-2-bromo-aminobenzene and sodium nitrite react in a solvent, hydrogen iodide or iodate is added to the mixed solution, and 1-fluoro-2-bromo-iodobenzene is obtained through a reaction. The preparation method of 1-fluoro-2-bromo-iodobenzene is high in safety, mild in reaction condition, low in cost, easy to operate and industrialize and high in utilization rate of iodine.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
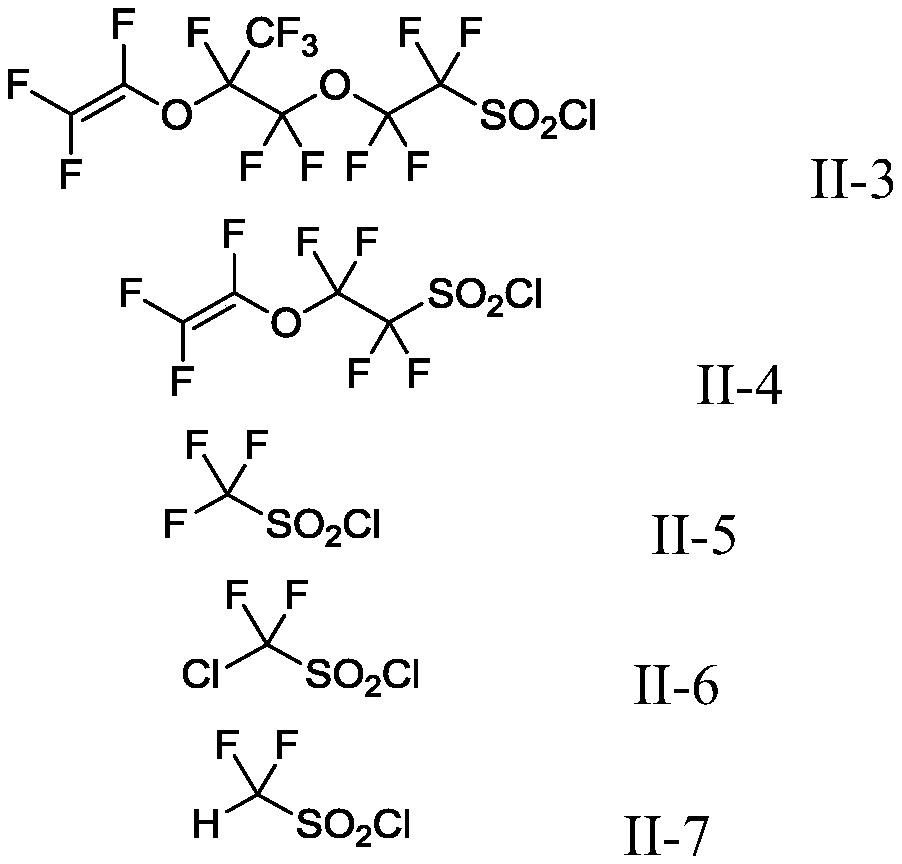
Production method of fluoroalkyl iodide
ActiveCN110759805AImprove toleranceEasy to manufactureOrganic compound preparationSulfonic acid preparationIodideIodination reaction
The invention discloses a production method of a fluoroalkyl iodide, and particularly discloses a production method of a fluoroalkyl iodide as shown in a formula (I) as shown in the specification. Theproduction method of the fluoroalkyl iodide as shown in the formula (I) as shown in the specification comprises the following step: in a first solvent, subjecting a compound as shown in a formula (II) as shown in the specification and an iodide to an iodination reaction as shown in the specification. The raw materials used in the method are easy to obtain, and prices are low; and the method is high in conversion rate and yield, and the tolerability to functional groups is high.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
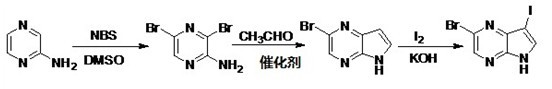
Preparation method of 3-iodo-5-bromo-4, 7-diazaindole
InactiveCN102627646ALow priceEasy to separateOrganic chemistryN-BromosuccinimidePotassium tert-butoxide
The invention discloses a preparation method of 3-iodo-5-bromo-4, 7-diazaindole. The preparation method comprises the following steps that 1), 2-aminopyrazine and N-bromosuccinimide undergo a bromination reaction to produce 2-amino-3, 5-dibromopyrazine; 2) 2-amino-3, 5-dibromopyrazine undergoes a synthesis reaction in the presence of acetaldehyde, potassium tert-butoxide and an N-heterocyclic carbine-Pd (NHC-Pd) compound as a catalyst to produce 5-bromo-4, 7-diazaindole; and 5-bromo-4, 7-diazaindole undergoes an iodination reaction to produce 3-iodo-5-bromo-4, 7-diazaindole. The preparation method has the advantages that raw material prices are low; a reaction process route is short; a reaction yield is high; and products can be separated easily.
Owner:STONE LAKE PHARMA TECH
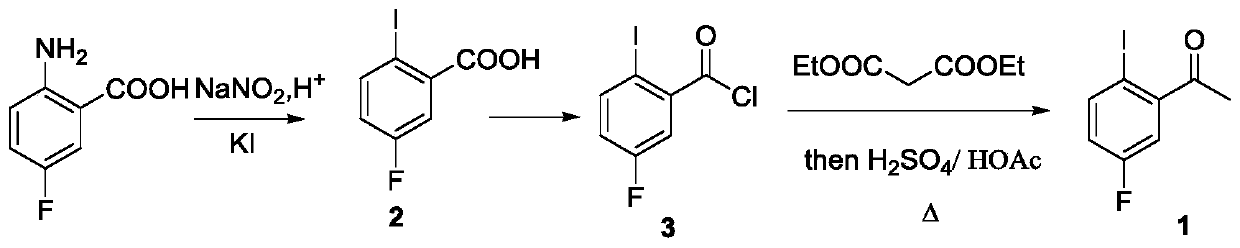
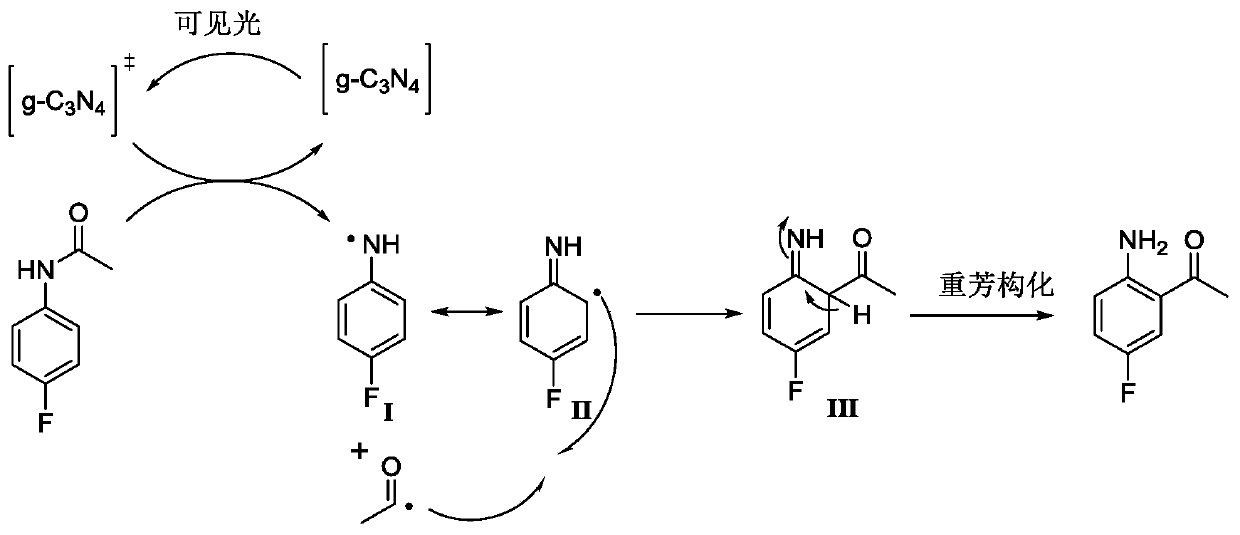
Preparation method of lorlatinib intermediate compound
InactiveCN110922315AReduce manufacturing costLow unit priceOrganic compound preparationCarbonyl compound separation/purificationPhotocatalytic reactionPtru catalyst
The invention relates to a preparation method of a lorlatinib intermediate compound. 4-fluoroacetanilide is taken as a raw material and carry out visible light Fries rearrangement in the presence of avisible light catalyst and visible light to obtain an intermediate product (2-amino-5-fluoroacetophenone); and the intermediate product can directly carry out diazotization-iodination reactions without purification to obtain the lorlatinib intermediate compound (3-fluoro-6-iodoacetophenone). The raw materials are cheap and easily available. The post treatment has simple steps. The using amounts of chemical reagents in rearrangement reactions are only catalytic amounts. The energy source of the reactions is the visible light. The preparation method is green and environmentally friendly. A continuous flowing type photocatalytic reaction system is formed. The purification method and post treatment are simple and convenient. No extra production cost or environmental protection cost is generated. The novel preparation method of 3-fluoro-6-iodoacetophenone can be applied to the industry and generates economical benefits.
Owner:CHANGZHOU INST OF TECH
Process for synthesizing diatrizoic acid by using solid-phase load method
ActiveCN103497120AShorten the production cycleIncrease productivityOrganic compound preparationCarboxylic acid amides preparationBenzoic acidAcetic anhydride
The invention discloses a process for synthesizing a diatrizoic acid by using a solid-phase method, and belongs to the technical field of drug synthesis. The problems that a purification process in the traditional synthesis technology is complicated, residual impurities are difficult to remove and the like are solved. The process for synthesizing the diatrizoic acid by using the solid-phase method comprises the following steps of bonding a 3, 5-diaminobenzoic acid on hydroxymethyl resin under the action of alkaline; performing iodine reaction on iodine monochloride and bonding zymolyte to obtain teriodide; performing acylation reaction on the teriodide and acetic anhydride to obtain diatrizoic acid bonding resin; and finally performing desorption on the diatrizoic acid bonding resin by the action of a trifluoroacetic acid to obtain the diatrizoic acid, and reusing the hydroxymethyl resin.
Owner:HUNAN XIANGYIKANG PHARMA
Preparation method of quaternary ammonium salt type cationic povidone-iodine antibacterial material
InactiveCN111205380ALarge specific surface areaAvoid churnBiocideAntifouling/underwater paintsFreeze-dryingPyrrolidinones
The invention relates to a preparation method of a quaternary ammonium salt type cationic povidone-iodine antibacterial material. The method comprises the following steps: (1) uniformly mixing vinyl pyrrolidone, a quaternary ammonium salt type cationic monomer, a hydrophobic comonomer and a silane coupling agent, adding an initiator, and carrying out ultrasonic mixing uniformly to obtain a mixed solution; (2) slowly dropwise adding the mixed solution into deionized water while stirring at a constant speed, and continuously reacting for 2-6 h at the temperature of 60-90 DEG C after drop-by-dropadding is finished so as to obtain a product A; (3) sequentially carrying out centrifugal separation, washing and freeze drying on the product A to obtain a quaternary ammonium salt type cationic polymer; and (4) under the protection of nitrogen, adding the quaternary ammonium salt type cationic polymer into an alcoholic solution of I2 for an iodination reaction to obtain a product B, performingwashing and suction filtration on the product B, and carrying out vacuum drying to obtain the product. The synthesis process is simple and efficient, and the obtained antibacterial material has broad-spectrum antibacterial activity and good biocompatibility and can be used for antibacterial research of products such as coatings, printing ink and dyes.
Owner:NORTHWEST NORMAL UNIVERSITY
Preparation method and intermediates of iopromide
ActiveCN106366016AEasy to separate and purifyNot easy to open and fall offOrganic compound preparationCarboxylic acid amides preparationIopromideIodination reaction
The present invention relates to a preparation method and intermediates of iopromide. The method specifically comprises: adopting a compound represented by a formula II as a starting raw material, and sequentially carrying out an acylation reaction, a lactonization reaction, a further acylation reaction, a reduction reaction, an iodination reaction, a re-acylation reaction and a final hydrolysis reaction to obtain the iopromide represented by a formula I, wherein a compound represented by a formula VII and a compound represented by a formula V are introduced as the intermediates so as to avoid the generation of the bismer by-product, the lactone ring is not easily subjected to ring opening removing during the iodination reaction process, and the introduced intermediates are easy to separate and purify, such that the high-purity iopromide can be prepared in the high-yield manner.
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Acetylthiocholine iodide as well as preparation method and application thereof
InactiveCN109879787AHigh purityEasy to operateOrganic chemistryMaterial analysis by observing effect on chemical indicatorORGANIC IODIDECLARITY
The invention provides acetylthiocholine iodide and a preparation method thereof. The preparation method comprises the following steps: step S1, dissolving a dimethylamino ethane compound in alkali toobtain an alkaline aqueous solution; step S2, adding a thioacetic acid compound into the alkaline aqueous solution to subject the thioacetic acid compound and the dimethylamino ethane compound to a thioreaction to generate (2-(dimethylamino)ethyl)ethanethioate; S3, separating the (2-(dimethylamino)ethyl)ethanethioate, and preparing an organic solution of the (2-(dimethylamino)ethyl)ethanethioate;and S4, dropwise adding an organic iodide into the organic solution of the (2-(dimethylamino)ethyl)ethanethioate for an iodination reaction so as to generate the acetylthiocholine iodide. According to the preparation method provided by the embodiment of the invention, the high-purity acetylthiocholine iodide can be obtained, and the titration content, the melting point, the clarity and other dataof the product are very excellent. The method is simple in operation, good in safety, suitable for industrial production, easy in raw material obtaining and low in cost.
Owner:安徽昊帆生物有限公司
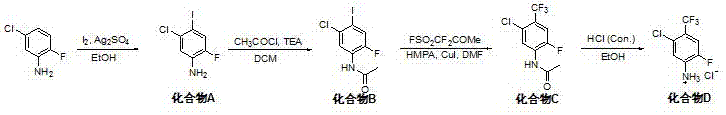
Novel synthesis method of aromatic 5-chloro-2-fluoro-4-(trifluoromethyl) aniline hydrochloride containing trifluoromethyl intermediate
InactiveCN107382742APositive progress effectInexpensive and easy to use raw materialsOrganic compound preparationCarboxylic acid amides preparationTrifluoromethylationAnhydrous ethanol
The invention discloses a novel synthesis method of aromatic 5-chloro-2-fluoro-4-(trifluoromethyl) aniline hydrochloride containing a trifluoromethyl intermediate. The method comprises steps as follows: 1) 5-chloro-2-fluoroaniline, elemental iodine and the like are subjected to an iodination reaction in an anhydrous ethanol solution; 2) with dichloromethane as a solvent, an obtained aromatic iodination product is subjected to an acetylation reaction; 3) the aromatic iodination product protected by acetyl, methyl fluorosulphonyldifluoroacetate and the like are subjected to a trifluoromethylation reaction at the temperature of 80 DEG C; 4) finally, 6 mol / L hydrochloric acid is used for a deacetylation reaction in ethanol, and a target product is obtained. The novel synthesis method has the advantages that the route design is novel, the product purity is good and operation is safe, simple and convenient; all reactions are stably conducted in the solvent, the process is easy to control, few crude product impurities exist, purification is easy, and the quality and yield of the product are increased. The total yield of the route is 66%, the product purity can reach 98.5%, and the novel synthesis method has higher research and development application value.
Owner:梁江丽
Synthesis method for 2,5-dibromo-iodobenzene
ActiveCN105753643AControl generationImprove iodination reactivityHalogenated hydrocarbon preparationChemical synthesisBenzene
The invention discloses a novel synthesis method for 2,5-dibromo-iodobenzene and belongs to the field of organic chemistry synthesis.The synthesis method includes the steps that with 1,4-dibromo-benzene being a starting raw material, trifluoroacetic acid and iodine are subjected to an iodination reaction to synthesize the target product 2,5-dibromo-iodobenzene.The method avoids low-temperature reaction, is easy to implement, generates a small number of by-products, is suitable for industrialized production and has good application prospects.2,5- dibromo-iodobenzene is an important fine chemical intermediate and is widely applied to the fields of synthesis medicine, pesticide, dye, plastics, functional polymer materials and others.
Owner:郑州金上化成新材料有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com